Abstract
This study aimed to comprehensively summarize assistive technology devices for postural control and gait performance in stroke patients. In the study, we searched for randomized controlled trials (RCTs) published until 31 December 2022 in four electrical databases. The most frequently applied assistive technology devices involving postural stability and gait function for stroke patients were robot-assistive technology devices. Out of 1065 initially retrieved citations that met the inclusion criteria, 30 RCTs (12 studies for subacute patients and 18 studies for chronic patients) were included in this review based on eligibility criteria. The meta-analysis included ten RCTs (five studies for subacute patients and five for chronic patients) based on the inclusion criteria of the data analysis. After analyzing, the variables, only two parameters, the Berg balance scale (BBS) and the functional ambulation category (FAC), which had relevant data from at least three studies measuring postural control and gait function, were selected for the meta-analysis. The meta-analysis revealed significant differences in the experimental group compared to the control group for BBS in both subacute and chronic stroke patients and for the FAC in chronic stroke patients. Robot-assistive training was found to be superior to regular therapy in improving postural stability for subacute and chronic stroke patients but not gait function. This review suggests that robot-assistive technology devices should be considered in rehabilitative approaches for postural stability and gait function for subacute and chronic stroke patients.
1. Introduction
Gait impairment occurs immediately in more than 80% of patients post-stroke [1]. Notably, most stroke survivors can walk independently without physical assistance from another person; however, 50% of the overall prevalence includes some level of gait impairment [2,3,4]. Gait function after stroke is an essential indicator of functional independence and social integration. Therefore, the proper recovery of gait performance is a vital goal of rehabilitative approach programs for stroke survivors. Consequently, most therapeutic sessions from early stroke rehabilitation are directed at improving gait performance and mobility; however, only 30–50% of stroke survivors are able to participate in community walking [1,2,5].
Despite this, approximately 80% of stroke survivors are capable of independent walking after discharge; most of them show a decreased walking efficacy and abnormal gait cycles, such as a shorter stride length, reduced walking speed, shortened stance phase, and prolonged swing phase of the paretic side, and ultimately an amplified risk of falls and decreased quality of life [2,6]. The most commonly adopted assistive technology devices are walking aids because of gait disturbance, difficulties in daily activities, and social integration for stroke survivors. The assistive technology devices are to increase gait efficiency, reduce residual disability, lower the burden of care, and involve early intensive gait training for stroke survivors in clinical settings [3,7,8].
Mobility assistive devices for patients with a stroke offer the prevention of the fear of falling and risk of a fall, increasing the support surface, improving postural stability and safety, improving functional independence, and improving distance and pace, as well as adaptations around the home [9,10,11]. Mobility assistive devices encompass a range of devices, including walking sticks/aids/crutches that support dynamic balance, wheelchairs that rely solely on mobility, ankle–foot orthosis or slider shoes that improve impairment to specific body parts, and robots that support external skeletons and produce targeted movement [12,13,14,15,16]. Assistive technology devices were initially aimed at assisting patients in increasing repetition and training intensity, as well as maximizing effective intervention time. However, they have now evolved to also support therapy professionals in their work. Given the current situation of limited skilled therapists and a growing number of patients requiring mobility rehabilitation, the purpose and nature of assistive technology are shifting to alignment with technological advancements [17,18,19]. For instance, once the therapeutic effectiveness of body weight support training has been proven, robot-assistive gait training will be developed as a means to eliminate the need for manual assistance from therapists and to facilitate continuous body weight support training [19,20,21].
Of the most importance when choosing an assistive device is its effectiveness for patients with a stroke. A suitable assistive device must be selected carefully, considering the patient’s endurance, cognitive function, strength, and environmental needs. The suitable assistive devices according to the purpose of rehabilitation and the verification of their effect should be progressed in line with technological development [12,19]. On the other hand, there is a lack of research on the preferred aids and their impact on the recovery stage after a stroke. This review aimed to present clinical evidence regarding the most frequently utilized assistive devices and to provide a comprehensive summary of the therapeutic effects of assistive technology devices on postural control and gait function for stroke patients. Furthermore, a meta-analysis was conducted to establish evidence on the effects of commonly used assistive technology devices in improving postural control and gait function for stroke patients.
2. Materials and Methods
2.1. Review Design
This review’s protocol that followed the Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) guidelines was registered in PROSPERO (Registration Number: CRD42023428911) [22]. According to the protocol, two assessors (S and C) independently consulted with each other to clarify the eligibility of the studies, and their collective judgment determined whether the records were deemed appropriate for inclusion. A final decision on study inclusion was made, followed by data collection. The results of the two assessors were compared following the Peer Review of Electronic Search Strategies 2020 Guidelines and an evidence-based checklist. We then synthesized these studies quantitatively and qualitatively.
2.2. Eligibility Criteria
This study involved randomized controlled trials (RCTs) that investigated the therapeutic effects of assistive technology devices on postural control and gait performance. The inclusion criteria were as follows: (1) focus on assistive-technology-based training, (2) participants diagnosed with a stroke between 18 and 85 years of age without other neurological diseases, (3) the inclusion of patients in one of the three recovery periods (acute, ≤3 weeks; subacute, between 3 weeks and ≤6 months; and chronic stroke, >6 months), (4) the utilization of core outcome sets to measure postural control and gait performance, (5) involving human participants, (6) written in English, and (7) published as full reports. Included records were excluded if they were (1) unrelated to assistive technology device-based approaches, (2) focused on other neurological disorders except stroke, (3) not focused on postural stability and gait function training, (4) non-human studies, (5) non-original studies, such as news, notes, reviews, opinion pieces, letters, editorials, and comments, and (6) considered gray literature, such as unpublished dissertations, conference supplies, and research abstracts.
2.3. Information Sources and Search Strategy
All records published up to 31 December 2022 were searched in four electronic databases: PubMed, Medline, Embase, and ProQuest. The search strategy was a combination of the following medical subject heading and related terms: (Stroke OR Cerebrovascular accident OR Brain vascular accident) AND (Assistive technology OR Assistive device*) AND (Balance OR Postural control OR Postural stability OR Walking OR Gait OR Locomotion) AND (Randomized controlled trial* OR Randomized controlled trial*). This study also reviewed the reference lists of all the identified relevant publications.
2.4. Screening of Searched Studies
After searching and compiling the retrieved studies, duplicate studies were removed using a bibliographic information management program based on the studies’ digital object identifier, journal title, volume and issue, and pages. The list of searched studies was cross-referenced to identify and eliminate duplicates. The selection process of this study consisted of two stages: stage I involved screening based on titles and abstracts to eliminate irrelevant reports; stage II was based on full reports to determine inclusion.
Given the focus on stroke, assistive technology devices, postural stability, and gait function, two assessors independently searched and assessed the suitability of the selected studies. The studies were screened based on their titles and abstracts to determine whether they met the inclusion or exclusion criteria. In cases where it was challenging to select based solely on the abstract, the researcher retrieved and reviewed the full text of the article. If the selection process was ambiguous, the final decision was not based solely on the judgment of a single researcher.
To be included in the meta-analysis, the selected studies had individual participant data, including the number of participants, the values of the mean, and standard deviation, because the standard approach to a meta-analysis of continuous outcomes requires information on the mean and either the standard deviation (SD), variance, or standard error values for each group [23]. If the studies lacked individual participant data, we found an alternative method of deriving the missing values of the mean or SD from median and quartile values [24,25]. However, we excluded the records from the meta-analysis when it was impossible.
2.5. Data Extraction
To summarize the evidence of assistive technology devices on postural stability and gait performance for stroke patients, this review extracted the following information from each selected study: the authors, year of publication, nation, population and age of participants, intervention details, device types, therapeutic intensity, comparison group, outcome, additional therapy provided, and summary of findings, if applicable.
To ensure quantitative consistency, we calculated the SD using the 95% confidence interval (CI) in selected studies that presented the mean and 95% CI. In cases where a study did not provide the means and SDs of the outcome measures, we calculated the values using the median and interquartile range from the selected studies that presented the median and quartile values [24,25,26]. A review protocol was established for data extraction to minimize selection bias. The protocol included selecting a sample and listing all items to be extracted from the primary RCTs. Two assessors independently performed the data extraction process. Any discrepancies or uncertainties were resolved through consultation with an expert to ensure consistency and accuracy. A final decision regarding the extracted data was made based on this collaborative process.
2.6. Data Analysis
The reviews were analyzed using a RevMan 5.4.1 program, which was provided at http://ims.cochrane.org/revman accessed on 19 May 2021. The mean and SD values were combined to calculate the mean difference and 95% CI to assess the effect estimates of the selected RCTs. Heterogeneity was assessed using the I2 statistic to ensure accurate interpretation and provide meaningful conclusions for clinical decision making. When at least three studies had relevant data and a sufficient homogeneity in the population, therapeutic interventions, and outcome measures, a meta-analysis was executed using a random-effects model [27]. An I2 > 40% threshold was used to determine statistical heterogeneity, and random effects’ models were employed in such cases [23]. Subgroup analyses examined the effects on postural stability and gait performance. If a study had one control group and two experimental groups with identical findings, the data from the experimental groups were pooled during the synthesis used by the RevMan program. Ten RCTs (five for the subacute phase and five for the chronic subacute phase) were investigated throughout the meta-analysis.
3. Results
3.1. Literature Characteristics of Included RCTs
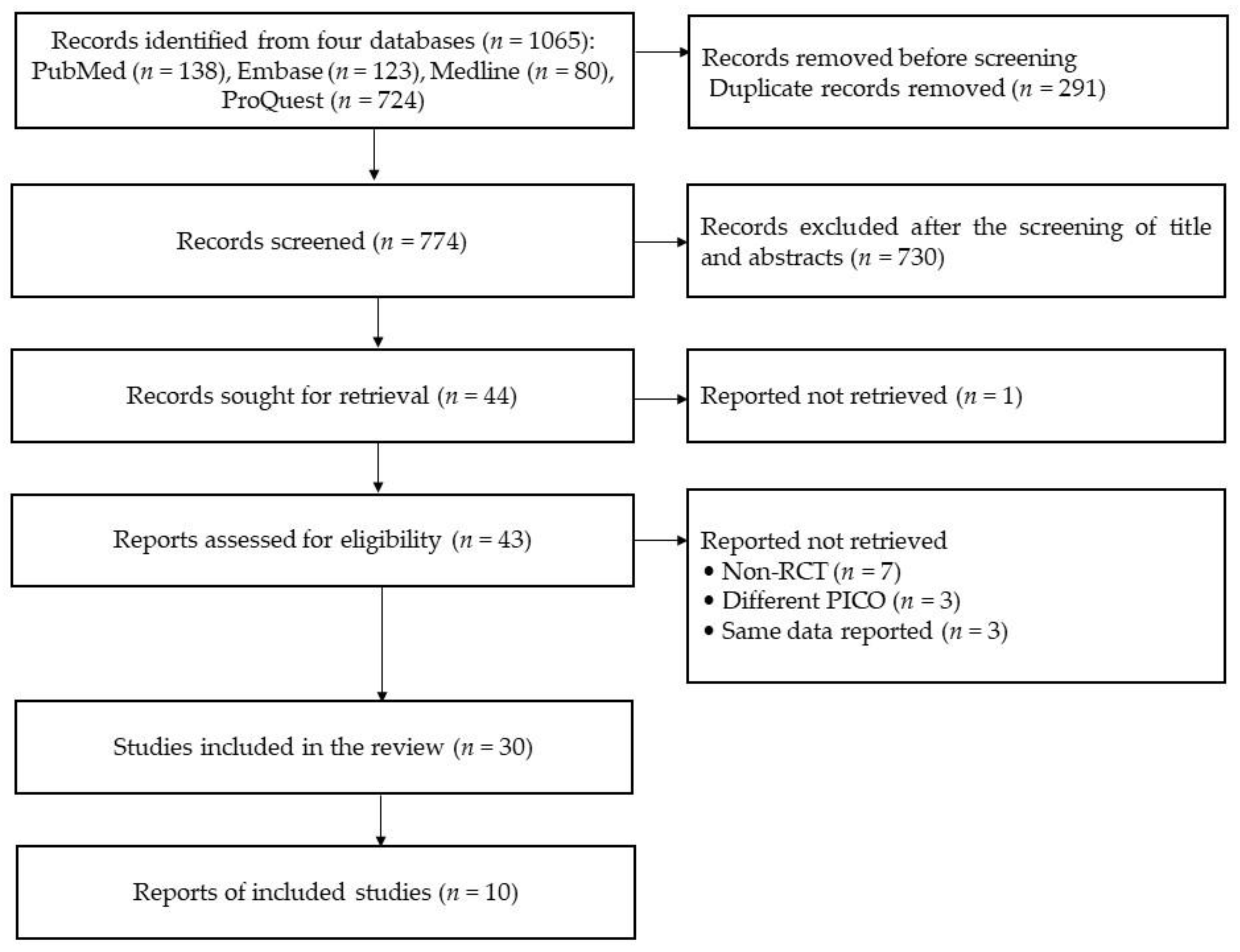
This study identified 1065 records from four databases, including PubMed (n=138), Embase (n = 123), Medline (n = 80), and ProQuest (n = 724). All of them included 291 duplicated records. After excluding the duplicated records, 774 records were screened based on the titles and abstracts and then the following 730 records were excluded: non-RCTs (n = 82), non-stroke studies (n = 194), and different PICO (n = 454). A total of 44 reports were sought for full-text retrieval, except for one record (not reporting participant’s information), leaving 43 reports assessed for eligibility. In total, 13 studies were excluded for the following reasons: being non-RCTs (n = 7), being different PICO (n = 3), and having the same data reported (n = 3). Finally, 30 studies were included in the qualitative synthesis, consisting of 12 and 18 RCTs in the subacute and chronic stages, respectively, based on patients’ recovery periods (see Figure 1).
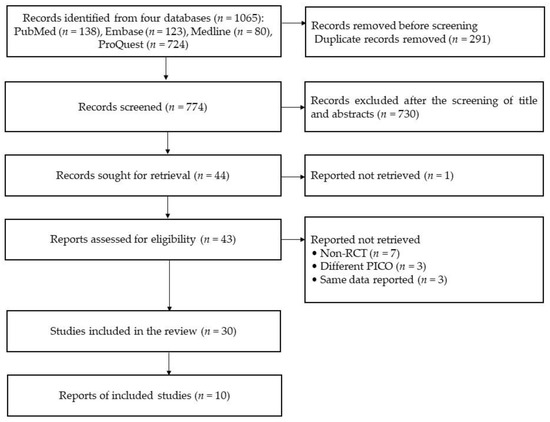
Figure 1.
The diagram of this systematic review (source from Page et al. [22]).
3.2. Qualitative Synthesis of Selected RCTs to Review Postural Control and Gait Performance in Stroke Survivors
This review selected twelve RCTs involving patients with a subacute stroke [3,16,28,29,30,31,32,33,34,35,36,37]. The study involved 460 subacute stroke patients who received robot-assistive training, trunk stabilization exercise, or conventional walking training. They used ankle–foot orthosis (AFO), a compressor belt, or a robot gait training system (assist-as-needed principles and multiple degrees of freedom, an E-go device, an anklebot, a morning walk, a regent suit, an i-walker, and a Lokomat). The studies of the robot-assistive training provided 30 min to 2 h per session (10 sessions to 30 sessions), and the studies of the AFO provided 14 sessions in 26 weeks. The RCTs of the trunk stabilization exercise with a pelvic compressor belt provided 30 min per session (30 sessions). They used the joint angle, a sensory organization test (SOT), a 10 m walking test (10MWT), a 6 min walking test (6mWT), the Barthel index (BI), the Berg balance scale (BBS), the center of loading (COL), the functional ambulation category (FAC), FGA, the Fugl-Meyer assessment of motor functioning—lower extremity (FMA-LE), the modified Ashworth scale (MAS), the motricity index (MI), the limit of stability (LOS), the Rivermead mobility index (RMI), a timed up-and-go test (TUG), a stairs test, Tinetti’s scale, spatiotemporal parameters in a gait analysis (gait cycle duration, single-support period, step length and width, cadence, and gait speed), and others in a gait analysis (moment, power, pelvic tilt, thoracic tilt, ground reaction times, and the center of pressure progression). Four RCTs reported more benefits of robot-assistive training than comparison therapy [31,33,34,35], and only one RCT reported more positive effects of AFO provision than comparison therapy (Table 1 and Table 2) [30].

Table 1.
A qualitative synthesis of the selected studies in the review of robotics for subacute patients.

Table 2.
A qualitative synthesis of the selected studies in the review of other technology for subacute patients.
The review selected 18 RCTs involving patients with a chronic stroke (Table 3 and Table 4) [38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55]. They involved 639 chronic stroke patients. These RCT records included 12 types of robot-assisted training using Lokomat, Ekso, RoboGait, an electrical walker, a wearable hip-assistance robot, a trunk stability rehabilitation robot trainer, Exowalk, a GEAR system, and a Bionic leg device to improve the gait function for stroke patients [38,39,42,44,46,47,48,49,50,51,52,54]. The robot-assisted training involved 30 min to 60 min per session and 12 to 40 sessions. The RCTs with robot-assisted training used the activities-specific balance confidence scale (ABC), BBS, 10MWT, 6mWT, RMI, TUG, MAS, FAC, K-MBI, MI, FMA-LE, five-time sit-to-stand test (5XSST), California functional evaluation 40 (CAFÉ 40), Emory functional ambulation profile (EFAP), fall efficacy scale (FES), functional reach test (FRT), global rating of change (GRC), GQI, gait quality index (GQI), Rivermead visual gait assessment (RVGA), 36-item short-form survey (SF-36), medical outcome study 8-item short-form health survey (SF-8), Romberg test, stroke impact scale (SIS), visual analog scale (VAS), electromyography, electroencephalography, and spatiotemporal parameters in a gait analysis. Seven RCTs reported more benefits of robot-assistive training than comparison therapy [38,39,42,46,50,51].

Table 3.
A qualitative synthesis of the selected studies in the review of robotics for chronic patients.

Table 4.
A qualitative synthesis of the selected studies in the review of other technology for chronic patients.
They also included six other types of training, including a toe spreader, an ankle movement system, postural insoles, a foot drop stimulator, non-elastic taping, and an ankle stretcher to improve the gait performance for stroke patients [40,41,43,45,53,55]. They also measured MAS, BBS, proprioception, the range of motion, strength, FMA-LE, TUG, FES, 6mWT, FRT, SIS, FAC, RMI, 10MWT, MI, MBI, SOT, and spatiotemporal parameters in a gait analysis. However, they did not include any assistive technology devices to improve postural control (only for patients with a chronic stroke). Four RCTs reported more positive effects of therapeutic intervention than comparison therapy (Table 2) [41,43,45,55].
3.3. Effectiveness of Assistive Technology Device on Postural Control and Gait Performance in the RCT Studies
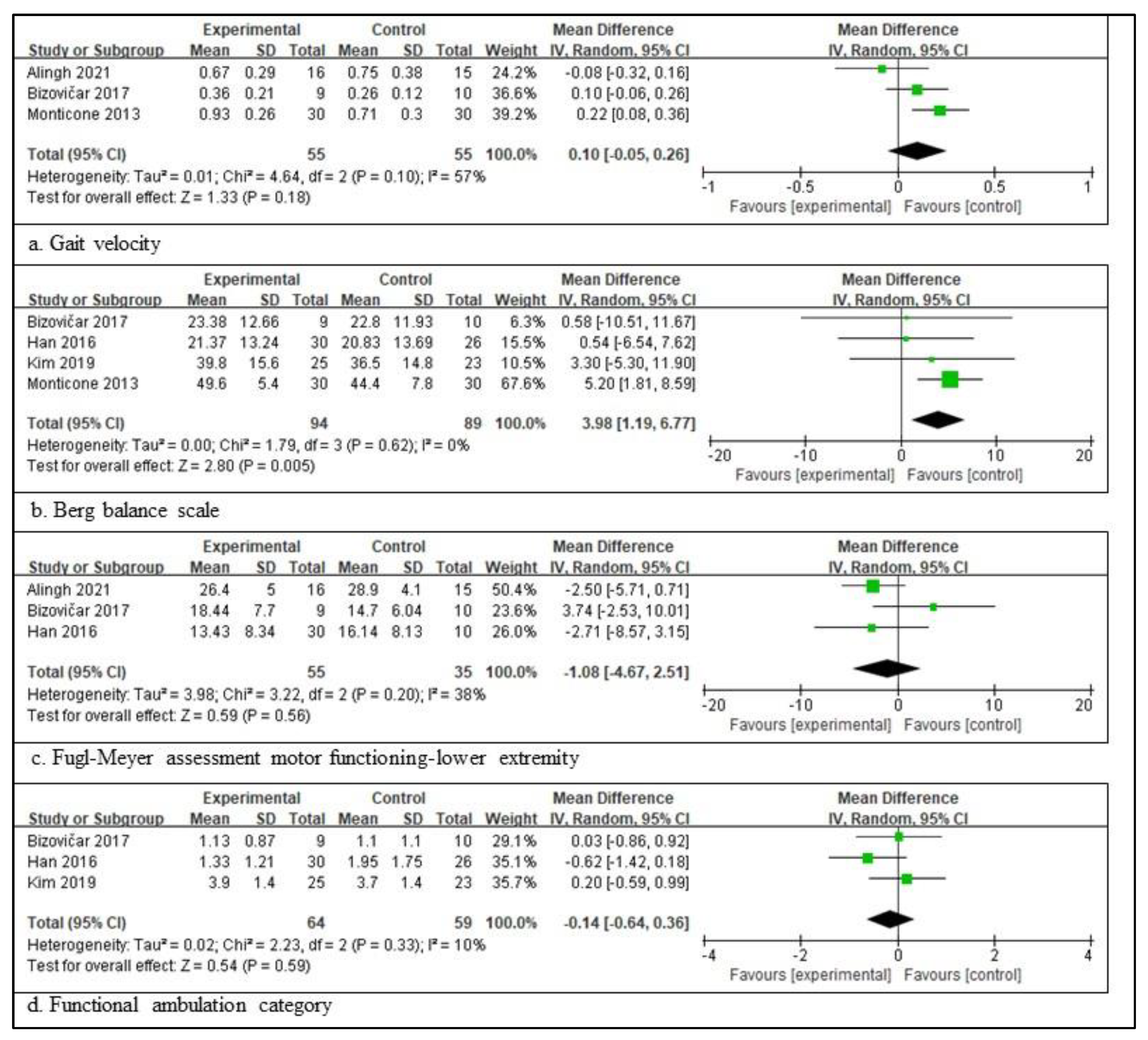
In patients with a subacute stroke, three RCTs involving 110 patients, providing the participants’ information, assessed the gait velocity [3,28,34]; four RCTs involving 183 patients, providing the participants’ information, assessed the BBS [3,32,33]. Three RCTs involving 90 patients, providing the participants’ information, assessed the FMA-LE score [3,28,32], and three RCTs involving 123 patients, providing the participants’ information, assessed the FAC score [3,32,33]. The scores for BBS are significantly different between the experimental and control groups. The total mean difference (95% CI) values were 3.98 (1.19, 6.77), and the heterogeneity values were Tau2 = 0.00, Chi2 = 1.79, df = 3 (p = 0.62), and I2 = 0% for BBS. The test for the overall effect yielded Z = 2.80 (p = 0.005) for BBS. However, the other three measures did not differ significantly between the experimental and control groups. The total mean difference (95% CI) values were as follows: 0.10 (−0.05, 0.26) for gait velocity, −1.18 (−4.67, 2.51) for FMA-LE, and −0.14 (−0.64, 0.36) for the FAC score. The heterogeneity values were as follows: Tau2 = 0.01, Chi2 = 4.64, df = 2 (p = 0.10), and I2 = 57% for gait velocity; Tau2 = 3.98, Chi2 = 3.22, df = 2 (p = 0.20), and I2 = 38% for FMA-LE; and Tau2 = 0.02, Chi2 = 2.23, df = 2 (p = 0.33), and I2 = 10% for the FAC score. The test for the overall effect yielded Z = 1.33 (p = 0.18) for gait velocity, Z = 0.59 (p = 0.56) for FMA-LE, and Z = 0.54 (p = 0.59) for the FAC score. A random-effects model was selected because of significant heterogeneity (Figure 2).
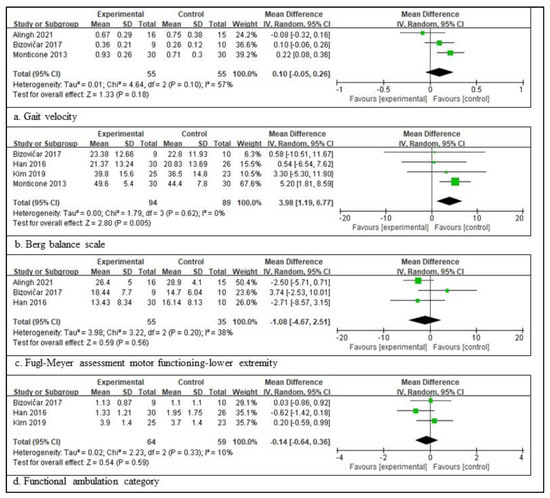
Figure 2.
Outcome measures to examine the assistive technology devices for postural stability and gait function for subacute stroke [3,28,31,32,33,34]. The size of the square is proportional to the weight of the study with the pooled estimate, and the line in the middle of the square is the confidence interval for each study. The green color of the block means that the data are continuous. The placement of the center of the diamond on the x-axis represents the point estimate, and the width of the diamond represents the 95% CI around the point estimate of the pooled effect [23].
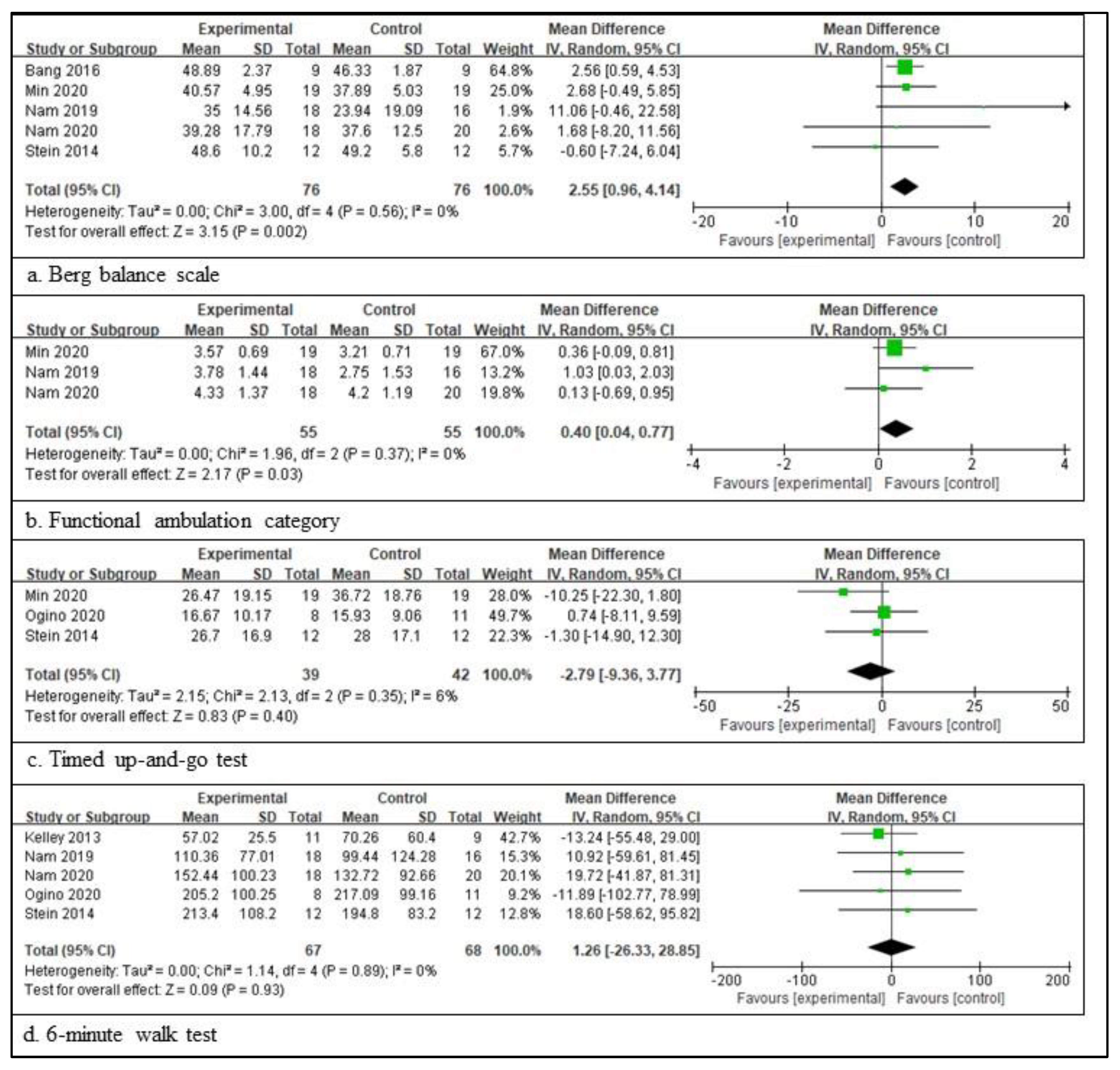
In patients with a chronic stroke, five RCTs involving 152 patients provided the participants’ information and assessed the BBS score [38,47,48,49,52], three RCTs involving 81 patients provided the participants’ information and assessed the TUG score [47,50,52], five RCTs involving 135 patients provided the participants’ information and assessed the 6mWT score [44,48,49,50,52], and three RCTs involving 110 patients provided the participants’ information and assessed the FAC score [47,48,49]. The scores for BBS are significantly different between the experimental and control groups. The total mean difference (95% CI) values were 2.55 (0.96, 4.14), and the heterogeneity values were Tau2 = 0.00, Chi2 = 3.00, df = 5 (p = 0.56), and I2 = 0% for BBS. The test for the overall effect yielded Z = 3.15 (p = 0.002) for BBS. The scores for FAC were also significantly different between the two groups. The total mean difference (95% CI) values were 0.40 (0.04, 0.77), and the heterogeneity values were Tau2 = 0.00, Chi2 = 1.96, df = 2 (p = 0.37), and I2 = 0% for the FAC score. The test for the overall effect yielded Z = 2.17 (p = 0.03) for the FAC score. However, the other two measures did not differ significantly between the experimental and control groups. The total mean difference (95% CI) values were −2.79 (−9.36, 3.77) for TUG and 1.26 (−26.33, 28.85) for 6mWT. The heterogeneity values were Tau2 = 2.15, Chi2 = 2.13, df = 2 (p = 0.35), and I2 = 6% for gait velocity, as well as Tau2 = 0.00, Chi2 = 1.14, df = 4 (p = 0.89), and I2 = 0% for 6mWT. The test for the overall effect yielded Z = 0.83 (p = 0.40) for TUG and Z = 0.09 (p = 0.93) for 6mWT. A random-effects model was selected because of significant heterogeneity (Figure 3).
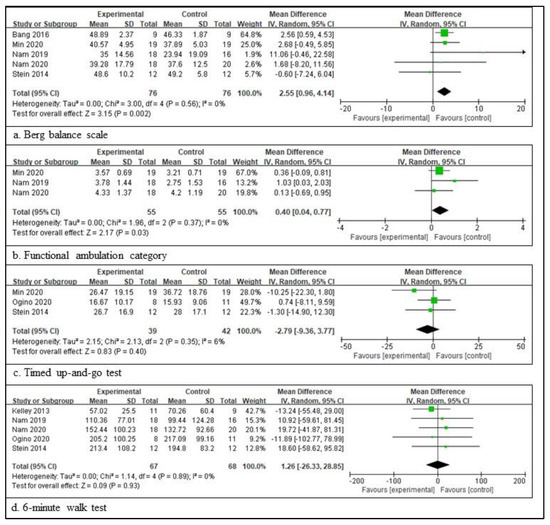
Figure 3.
Outcome measures to examine the assistive technology devices for postural stability and gait function for chronic stroke [38,44,47,48,49,50,52]. The meaning of the figure (squares and diamonds) is the same as that of Figure 2.
4. Discussion
This review aimed to present clinical evidence regarding the most frequently utilized assistive devices and to provide a comprehensive summary of the therapeutic effects of assistive technology devices on postural control and gait function for stroke patients. The main results of this review are as follows: First, the most frequently applied assistive technology devices for postural stability and gait function were robotic technology for subacute and chronic stroke patients. Other assistive technology devices were pelvic compressor belts and AFO for subacute stroke patients and a toe spreader, an ankle movement system, postural insoles, non-elastic taping, and an ankle stretcher for chronic stroke patients. Second, assistive technology training with robotics significantly benefits postural stability compared with conventional therapy in subacute patients [3,28,32] and chronic patients [47,48,49,50,52]. However, assistive technology training with robotics demonstrates a significant positive benefit on gait function compared with conventional therapy in subacute patients [3,28,32,34,56] and in chronic patients [44,47,50,52], even though chronic stroke patients showed a decreased assistive degree measured with FAC [47,48,49]. Third, the BBS score significantly differed between the experimental and control groups in subacute and chronic patients. However, gait velocity, FAC, and FMA-LE for subacute stroke patients and TUG and 6mWT for chronic stroke patients were not significantly different in the experimental group compared to those of the control group.
Robot-assistive devices are classified into two different types, exoskeletons and end-effectors [57,58]. The exoskeleton type is an external skeleton attached to the lower limbs and actuated by motors to create stepping movements [59]. The RCTs in this review conducted the exoskeletons to support standing posture and dynamic balance during stepping movement for subacute stroke patients [3,16,28,32,34,35] and for chronic stroke patients [38,39,42,44,46,47,48,49,50,51,52,54]. The exoskeleton robots are classified into three types: robotics with a body-weight-supported system [16,28,32]; a whole-body suit type covered with a vest, shorts, knee caps, and foot straps [34]; and a wheeled walker type [3,35]. According to the studies, the walker study by Morone et al. [35] and the whole-body suit study by Monticone et al. [34] were more effective than conventional therapy. However, other studies with exoskeletons did not report more therapeutic effects than conventional therapy for subacute stroke patients [3,16,28,32]. The end-effector type freely guides stepping movements throughout the proximal part of the lower extremity while fixing the distal part of the lower extremity [60]. The RCTs in this review conducted the end effectors to support the above ankle and to facilitate the below ankle movements [31,33]. The end-effector type of a robot-assistive device was a volitional ankle movement control system [31] and a seating-type robot system [33]. The two RCTs reported that robot-assistive training showed more therapeutic effects than conventional therapy. Therefore, regardless of the type of robot-assisted device, qualitative synthesis cannot prove whether it is more effective than conventional therapy for postural stability and gait function in subacute stroke patients.
The review performed quantitative synthesis based on the participant’s information. In this review, four variables were quantitatively synthesized in the RCTs of subacute stroke patients. As a result of the synthesis, the BBS, which evaluates static and dynamic postural balance through 14 predetermined tasks regarding mobility, showed significant therapeutic benefits after robot-assistive training compared with conventional therapy [3,32,33,34]. Postural stability means an even weight bearing on both feet. It decreases due to weight-bearing asymmetry, muscle weakness, bothered perception, devastated ankle proprioception, cognitive impairment, and visual dependency after a stroke, which restricts mobility and functional independence [61]. An improvement in postural stability means that the prerequisites for improving the mobility and functional independence of subacute stroke patients have been fulfilled by robot-assistive training.
The RCTs for chronic stroke patients also used the exoskeleton type [38,39,42,44,48,49,50,51,54], single-segment supporter [46,47], and end-effector type [52] of robot-assistive training. The RCTs reported positive effects compared with conventional therapy; five RCTs used an exoskeleton type [38,39,42,50,51], and two RCTs used a single-segment supporter [46,47]. In quantitative synthesis, two variables, BBS [38,47,48,49,52] and FAC [47,48,49], showed significant positive effects after robot-assistive training for chronic stroke patients. Based on BBS, robot-assistive training was beneficial for postural stability in chronic and subacute patients. Positive efficacy was not proved in other variables; however, for TUG and 6mWT, it is also essential to show an effect in FAC. The result of FAC synthesis will be a signal to prove that robot-assistive training is effective in improving the gait function of chronic stroke patients. Robotics technology developed around exoskeletons and end effectors has been used for treating patients with a stroke for the past 20 years. However, technology development continues to prove its effectiveness and replace human efforts [50,62]. In this review, the response that some research is effective is probably the result of continuing research with robot-assistive technology.
AFO is the most common orthosis used for stroke patients. Notably, few studies have individually investigated the effects of AFO; however, efforts to prove the effects and develop new devices are continuing [41,45]. Two RCTs for chronic stroke patients reported that the assistive device showed benefits compared with conventional therapy [41,45], but the RCTs did not involve a meta-analysis. In the review, two RCTs involved ankle or toe spreaders [40,55], one RCT involved postural insoles [43], and one RCT involved non-elastic taping [53]. An ankle spreader and postural insoles showed positive effects, but a toe spreader and non-elastic taping did not. It is not desirable to conclude the effectiveness of assistive devices from one or two RCTs. The studies suggest that future research about assistive devices should be continued.
Gait function from daily living to community ambulation has major implications for health conditions. For stroke survivors, it is a vital predictor for an individual’s independence, social integration, and quality of life [1]. Assistive technology devices, specifically robotics, have benefits regarding postural stability and gait function to solve a clinical problem that often remains compromised in terms of daily mobility and community ambulation for stroke survivors. In the future, assistive technology, from simple walking aids to a wide variety of other high technologies like robotics, will be investigated for the potential to reduce residual disability, slow functional declines and lower health care costs, and decrease the burden of care regarding postural stability and gait function for stroke survivors. The same variables were used among the RCTs included in the review in this study; however, a meta-analysis could not be performed due to a lack of participant information. Some studies described results only in graphs, not Arabic numerals, and some studies provided outcome measures only as changeable values from pre-training to post-training. In the future, researchers must provide participant information in essential numerals for readers and the citation of future studies when conducting research.
5. Conclusions
This review aimed to present clinical evidence regarding the most frequently utilized assistive devices and to provide a comprehensive summary of the therapeutic effects of assistive technology devices on postural stability and gait function in patients with a stroke. The findings of this review indicated that the most frequently conducted assistive technology device for patients with a subacute and chronic stroke was rehabilitative robotics for postural stability and gait. The robot-assistive training showed beneficial therapeutic effects for postural stability in subacute and chronic stroke patients compared with conventional therapy. The robot-assistive training showed the potential to improve the gait function in chronic stroke patients compared with conventional therapy. The robot-assistive technology devices can be attributed to assisting experts, facilitating more movement repetitions and physical capacities, lowering human resources, and encouraging the participants within a given timeframe. This review suggests that robot-assistive technology devices will be used in rehabilitative approaches for postural stability and gait function in subacute and chronic stroke patients.
Author Contributions
Conceptualization: S.H. and C.-S.S.; methodology: S.H. and C.-S.S.; software: S.H. and C.-S.S.; validation: S.H. and C.-S.S.; formal analysis: S.H. and C.-S.S.; investigation: S.H. and C.-S.S.; resources: S.H. and C.-S.S.; data curation: S.H. and C.-S.S.; writing—original draft preparation: S.H. and C.-S.S.; writing—review and editing: S.H. and C.-S.S.; visualization: S.H. and C.-S.S.; supervision: S.H. and C.-S.S.; project administration: C.-S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We would like to express our gratitude to Hyunjin Song, a librarian at the Seoul Library of Baekseok University, for her support in searching and selecting this review as a scientific approach to a systematic review with a meta-analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, S.; Francisco, G.E.; Zhou, P. Post-Stroke Hemiplegic Gait: New Perspective and Insights. Front. Physiol. 2018, 9, 1021. [Google Scholar] [CrossRef] [PubMed]
- Selves, C.; Stoquart, G.; Lejeune, T. Gait Rehabilitation after Stroke: Review of the Evidence of Predictors, Clinical Outcomes and Timing for Interventions. Acta Neurol. Belg. 2020, 120, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Bizovičar, N.; Matjačić, Z.; Stanonik, I.; Goljar, N. Overground Gait Training Using a Motorized Assistive Device in Patients with Severe Disabilities after Stroke. Int. J. Rehabil. Res. 2017, 40, 46–52. [Google Scholar] [CrossRef]
- Mohan, D.M.; Khandoker, A.H.; Wasti, S.A.; Ismail Ibrahim Ismail Alali, S.; Jelinek, H.F.; Khalaf, K. Assessment Methods of Post-Stroke Gait: A Scoping Review of Technology-Driven Approaches to Gait Characterization and Analysis. Front. Neurol. 2021, 12, 650024. [Google Scholar] [CrossRef]
- Cirstea, C.M. Gait Rehabilitation after Stroke. Stroke 2020, 51, 2892–2894. [Google Scholar] [CrossRef]
- Perry, J.; Burnfield, J. Gait Analysis: Normal and Pathological Function, 2nd ed.; Slack Incorporated: West Deptford, NJ, USA, 2010; ISBN 9781556427664. [Google Scholar]
- Karakkattil, P.S.; Trudelle-Jackson, E.; Medley, A.; Swank, C. Effects of Two Different Types of Ankle–Foot Orthoses on Gait Outcomes in Patients with Subacute Stroke: A Randomized Crossover Trial. Clin. Rehabil. 2020, 34, 1094–1102. [Google Scholar] [CrossRef]
- Huizenga, D.; Rashford, L.; Darcy, B.; Lundin, E.; Medas, R.; Shultz, S.T.; DuBose, E.; Reed, K.B. Wearable Gait Device for Stroke Gait Rehabilitation at Home. Top. Stroke Rehabil. 2021, 28, 443–455. [Google Scholar] [CrossRef]
- Caro, C.C.; Costa, J.D.; Cruz, D.M.C.d. The Use of Mobility Assistive Devices and the Functional Independence in Stroke Patients. Cad. Bras. Ter. Ocup. 2018, 26, 558–568. [Google Scholar] [CrossRef]
- Tyson, S.F.; Rogerson, L. Assistive Walking Devices in Nonambulant Patients Undergoing Rehabilitation after Stroke: The Effects on Functional Mobility, Walking Impairments, and Patients’ Opinion. Arch. Phys. Med. Rehabil. 2009, 90, 475–479. [Google Scholar] [CrossRef]
- Morris, L.; Cramp, M.; Turton, A. User Perspectives on the Future of Mobility Assistive Devices: Understanding Users’ Assistive Device Experiences and Needs. J. Rehabil. Assist. Technol. Eng. 2022, 9, 205566832211147. [Google Scholar] [CrossRef]
- Jutai, J.; Coulson, S.; Teasell, R.; Bayley, M.; Garland, J.; Mayo, N.; Wood-Dauphinee, S. Mobility Assistive Device Utilization in a Prospective Study of Patients with First-Ever Stroke. Arch. Phys. Med. Rehabil. 2007, 88, 1268–1275. [Google Scholar] [CrossRef]
- S⊘rensen, H.V.; Lendal, S.; Schultz-Larsen, K.; Uhrskov, T. Stroke Rehabilitation: Assistive Technology Devices and Environmental Modifications Following Primary Rehabilitation in Hospital—A Therapeutic Perspective. Assist. Technol. 2003, 15, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Taki, S.; Imura, T.; Iwamoto, Y.; Imada, N.; Tanaka, R.; Araki, H.; Araki, O. Effects of Exoskeletal Lower Limb Robot Training on the Activities of Daily Living in Stroke Patients: Retrospective Pre-Post Comparison Using Propensity Score Matched Analysis. J. Stroke Cerebrovasc. Dis. 2020, 29, 105176. [Google Scholar] [CrossRef] [PubMed]
- Forrester, L.W.; Roy, A.; Hafer-Macko, C.; Krebs, H.I.; Macko, R.F. Task-Specific Ankle Robotics Gait Training after Stroke: A Randomized Pilot Study. J. Neuroeng. Rehabil. 2016, 13, 51. [Google Scholar] [CrossRef] [PubMed]
- Van Nunen, M.P.M.; Gerrits, K.H.L.; Konijnenbelt, M.; Janssen, T.W.J.; De Haan, A. Recovery of Walking Ability Using a Robotic Device in Subacute Stroke Patients: A Randomized Controlled Study. Disabil. Rehabil. Assist. Technol. 2015, 10, 141–148. [Google Scholar] [CrossRef]
- Mate, K.K.V.; Abou-Sharkh, A.; Mansoubi, M.; Alosaimi, A.; Dawes, H.; Michael, W.; Stanwood, O.; Harding, S.; Gorenko, D.; Mayo, N.E. Evidence for the Efficacy of Commercially Available Wearable Biofeedback Gait Devices: Consumer-Centered Review. JMIR Rehabil. Assist. Technol. 2023, 10, e40680. [Google Scholar] [CrossRef]
- Eng, J.J.; Tang, P.-F. Gait Training Strategies to Optimize Walking Ability in People with Stroke: A Synthesis of the Evidence. Expert Rev. Neurother. 2007, 7, 1417–1436. [Google Scholar] [CrossRef]
- Mikolajczyk, T.; Ciobanu, I.; Badea, D.I.; Iliescu, A.; Pizzamiglio, S.; Schauer, T.; Seel, T.; Seiciu, P.L.; Turner, D.L.; Berteanu, M. Advanced Technology for Gait Rehabilitation: An Overview. Adv. Mech. Eng. 2018, 10, 168781401878362. [Google Scholar] [CrossRef]
- Stevenson, A.J.; Mrachacz-Kersting, N.; van Asseldonk, E.; Turner, D.L.; Spaich, E.G. Spinal Plasticity in Robot-Mediated Therapy for the Lower Limbs. J. Neuroeng. Rehabil. 2015, 12, 81. [Google Scholar] [CrossRef]
- Jezernik, S.; Colombo, G.; Keller, T.; Frueh, H.; Morari, M. Robotic Orthosis Lokomat: A Rehabilitation and Research Tool. Neuromodulation Technol. Neural Interface 2003, 6, 108–115. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Higgins, J.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.; Welck, V. Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; John Wiley & Sons: Chichester, UK, 2019. [Google Scholar]
- Luo, D.; Wan, X.; Liu, J.; Tong, T. Optimally Estimating the Sample Mean from the Sample Size, Median, Mid-Range, and/or Mid-Quartile Range. Stat. Methods Med. Res. 2018, 27, 1785–1805. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the Sample Mean and Standard Deviation from the Sample Size, Median, Range and/or Interquartile Range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef] [PubMed]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the Mean and Variance from the Median, Range, and the Size of a Sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Nedergård, H.; Arumugam, A.; Sandlund, M.; Bråndal, A.; Häger, C.K. Effect of Robotic-Assisted Gait Training on Objective Biomechanical Measures of Gait in Persons Post-Stroke: A Systematic Review and Meta-Analysis. J. Neuroeng. Rehabil. 2021, 18, 64. [Google Scholar] [CrossRef]
- Alingh, J.F.; Fleerkotte, B.M.; Groen, B.E.; Rietman, J.S.; Weerdesteyn, V.; van Asseldonk, E.H.F.; Geurts, A.C.H.; Buurke, J.H. Effect of Assist-as-Needed Robotic Gait Training on the Gait Pattern Post Stroke: A Randomized Controlled Trial. J. Neuroeng. Rehabil. 2021, 18, 26. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Kim, N.H.; Son, S.M.; Cha, Y.J. Effects of Trunk Stabilization Exercise While Wearing a Pelvic Compression Belt on Walking and Balancing Abilities in Patients with Stroke. Am. J. Phys. Med. Rehabil. 2020, 99, 1048–1055. [Google Scholar] [CrossRef] [PubMed]
- De Sèze, M.P.; Bonhomme, C.; Daviet, J.C.; Burguete, E.; MacHat, H.; Rousseaux, M.; Mazaux, J.M. Effect of Early Compensation of Distal Motor Deficiency by the Chignon Ankle-Foot Orthosis on Gait in Hemiplegic Patients: A Randomized Pilot Study. Clin. Rehabil. 2011, 25, 989–998. [Google Scholar] [CrossRef]
- Forrester, L.W.; Roy, A.; Krywonis, A.; Kehs, G.; Krebs, H.I.; Macko, R.F. Modular Ankle Robotics Training in Early Subacute Stroke: A Randomized Controlled Pilot Study. Neurorehabil. Neural Repair 2014, 28, 678–687. [Google Scholar] [CrossRef]
- Han, E.Y.; Im, S.H.; Kim, B.R.; Seo, M.J.; Kim, M.O. Robot-Assisted Gait Training Improves Brachial-Ankle Pulse Wave Velocity and Peak Aerobic Capacity in Subacute Stroke Patients with Totally Dependent Ambulation Randomized Controlled Trial. Medicine 2016, 95, e5078. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, D.Y.; Chun, M.H.; Kim, S.W.; Jeon, H.R.; Hwang, C.H.; Choi, J.K.; Bae, S. Effects of Robot-(Morning Walk®) Assisted Gait Training for Patients after Stroke: A Randomized Controlled Trial. Clin. Rehabil. 2019, 33, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Monticone, M.; Ambrosini, E.; Ferrante, S.; Colombo, R. “Regent Suit” Training Improves Recovery of Motor and Daily Living Activities in Subjects with Subacute Stroke: A Randomized Controlled Trial. Clin. Rehabil. 2013, 27, 792–802. [Google Scholar] [CrossRef] [PubMed]
- Morone, G.; Annicchiarico, R.; Iosa, M.; Federici, A.; Paolucci, S.; Cortés, U.; Caltagirone, C. Overground Walking Training with the I-Walker, a Robotic Servo-Assistive Device, Enhances Balance in Patients with Subacute Stroke: A Randomized Controlled Trial. J. Neuroeng. Rehabil. 2016, 13, 47. [Google Scholar] [CrossRef] [PubMed]
- Nikamp, C.D.M.; Buurke, J.H.; van der Palen, J.; Hermens, H.J.; Rietman, J.S. Six-Month Effects of Early or Delayed Provision of an Ankle-Foot Orthosis in Patients with (Sub)Acute Stroke: A Randomized Controlled Trial. Clin. Rehabil. 2017, 31, 1616–1624. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Tanaka, S.; Motojima, N. Comparison of Ankle–Foot Orthoses with Plantar Flexion Stop and Plantar Flexion Resistance in the Gait of Stroke Patients: A Randomized Controlled Trial. Prosthet. Orthot. Int. 2018, 42, 544–553. [Google Scholar] [CrossRef]
- Bang, D.H.; Shin, W.S. Effects of Robot-Assisted Gait Training on Spatiotemporal Gait Parameters and Balance in Patients with Chronic Stroke: A Randomized Controlled Pilot Trial. Neurorehabilitation 2016, 38, 343–349. [Google Scholar] [CrossRef]
- Calabrò, R.S.; Naro, A.; Russo, M.; Bramanti, P.; Carioti, L.; Balletta, T.; Buda, A.; Manuli, A.; Filoni, S.; Bramanti, A. Shaping Neuroplasticity by Using Powered Exoskeletons in Patients with Stroke: A Randomized Clinical Trial. J. Neuroeng. Rehabil. 2018, 15, 35. [Google Scholar] [CrossRef]
- Chiong, Y.; Tay, S.S.; Lim, P.A.C.; Tan, D.M.L. The Effects of Toe Spreader in People with Overactive Toe Flexors Post Stroke: A Randomized Controlled Pilot Study. Clin. Rehabil. 2013, 27, 90–95. [Google Scholar] [CrossRef]
- Cho, J.E.; Shin, J.H.; Kim, H. Does Electrical Stimulation Synchronized with Ankle Movements Better Improve Ankle Proprioception and Gait Kinematics in Chronic Stroke? A Randomized Controlled Study. Neurorehabilitation 2022, 51, 259–269. [Google Scholar] [CrossRef]
- Erbil, D.; Tugba, G.; Murat, T.H.; Melike, A.; Merve, A.; Cagla, K.; Mehmetali, Ç.C.; Akay, Ö.; Nigar, D. Effects of Robot-Assisted Gait Training in Chronic Stroke Patients Treated by Botulinum Toxin-a: A Pivotal Study. Physiother. Res. Int. 2018, 23, e1718. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.A.B.; Cimolin, V.; Neto, H.P.; Grecco, L.A.C.; Lazzari, R.D.; Dumont, A.J.L.; Galli, M.; Oliveira, C.S. Effect of Postural Insoles on Gait Pattern in Individuals with Hemiparesis: A Randomized Controlled Clinical Trial. J. Bodyw. Mov. Ther. 2018, 22, 792–797. [Google Scholar] [CrossRef]
- Kelley, C.P.; Childress, J.; Boake, C.; Noser, E.A. Over-Ground and Robotic-Assisted Locomotor Training in Adults with Chronic Stroke: A Blinded Randomized Clinical Trial. Disabil. Rehabil. Assist. Technol. 2013, 8, 161–168. [Google Scholar] [CrossRef]
- Kluding, P.M.; Dunning, K.; O’Dell, M.W.; Wu, S.S.; Ginosian, J.; Feld, J.; McBride, K. Foot Drop Stimulation versus Ankle Foot Orthosis after Stroke: 30-Week Outcomes. Stroke 2013, 44, 1660–1669. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Lee, S.H.; Seo, K.; Lee, M.; Chang, W.H.; Choi, B.O.; Ryu, G.H.; Kim, Y.H. Training for Walking Efficiency with a Wearable Hip-Assist Robot in Patients with Stroke a Pilot Randomized Controlled Trial. Stroke 2019, 50, 3545–3552. [Google Scholar] [CrossRef] [PubMed]
- Min, J.H.; Seong, H.Y.; Ko, S.H.; Jo, W.R.; Sohn, H.J.; Ahn, Y.H.; Son, J.H.; Seo, H.Y.; Son, Y.R.; Mun, S.J.; et al. Effects of Trunk Stabilization Training Robot on Postural Control and Gait in Patients with Chronic Stroke: A Randomized Controlled Trial. Int. J. Rehabil. Res. 2020, 43, 159–166. [Google Scholar] [CrossRef]
- Nam, Y.G.; Lee, J.W.; Park, J.W.; Lee, H.J.; Nam, K.Y.; Park, J.H.; Yu, C.S.; Choi, M.R.; Kwon, B.S. Effects of Electromechanical Exoskeleton-Assisted Gait Training on Walking Ability of Stroke Patients: A Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2019, 100, 26–31. [Google Scholar] [CrossRef]
- Nam, Y.G.; Park, J.W.; Lee, H.J.; Nam, K.Y.; Choi, M.R.; Yu, C.S.; Zhu, L.; Zhang, X.; Lee, J.W.; Kwon, B.S. Further Effects of Electromechanically Assisted Gait Trainer (Exowalk®) in Patients with Chronic Stroke: A Randomized Controlled Trial. J. Rehabil. Med. 2020, 52, jrm00097. [Google Scholar] [CrossRef] [PubMed]
- Ogino, T.; Kanata, Y.; Uegaki, R.; Yamaguchi, T.; Morisaki, K.; Nakano, S.; Domen, K. Effects of Gait Exercise Assist Robot (GEAR) on Subjects with Chronic Stroke: A Randomized Controlled Pilot Trial. J. Stroke Cerebrovasc. Dis. 2020, 29, 104886. [Google Scholar] [CrossRef]
- Rodrigues, T.A.; Goroso, D.G.; Westgate, P.M.; Carrico, C.; Batistella, L.R.; Sawaki, L. Slow Versus Fast Robot-Assisted Locomotor Training after Severe Stroke: A Randomized Controlled Trial. Am. J. Phys. Med. Rehabil. 2017, 96, S165–S170. [Google Scholar] [CrossRef]
- Stein, J.; Bishop, L.; Stein, D.J.; Wong, C.K. Gait Training with a Robotic Leg Brace after Stroke: A Randomized Controlled Pilot Study. Am. J. Phys. Med. Rehabil. 2014, 93, 987–994. [Google Scholar] [CrossRef]
- Wang, R.Y.; Lin, C.Y.; Chen, J.L.; Lee, C.S.; Chen, Y.J.; Yang, Y.R. Adjunct Non-Elastic Hip Taping Improves Gait Stability in Cane-Assisted Individuals with Chronic Stroke: A Randomized Controlled Trial. J. Clin. Med. 2022, 11, 1553. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Landry, J.M.; Kim, J.; Schmit, B.D.; Yen, S.C.; Macdonald, J. Robotic Resistance/Assistance Training Improves Locomotor Function in Individuals Poststroke: A Randomized Controlled Study. Arch. Phys. Med. Rehabil. 2014, 95, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Yoo, D.; Son, Y.; Kim, D.H.; Seo, K.H.; Lee, B.C. Technology-Assisted Ankle Rehabilitation Improves Balance and Gait Performance in Stroke Survivors: A Randomized Controlled Study with 1-Month Follow-Up. IEEE Trans. Neural Syst. Rehabil. Eng. 2018, 26, 2315–2323. [Google Scholar] [CrossRef]
- Kim, H.Y.; Shin, J.H.; Yang, S.P.; Shin, M.A.; Lee, S.H. Robot-Assisted Gait Training for Balance and Lower Extremity Function in Patients with Infratentorial Stroke: A Single-Blinded Randomized Controlled Trial. J. Neuroeng. Rehabil. 2019, 16, 99. [Google Scholar] [CrossRef]
- Mehrholz, J.; Thomas, S.; Werner, C.; Kugler, J.; Pohl, M.; Elsner, B. Electromechanical-Assisted Training for Walking after Stroke. Cochrane Database Syst. Rev. 2017, 5, CD006185. [Google Scholar] [CrossRef] [PubMed]
- Bruni, M.F.; Melegari, C.; De Cola, M.C.; Bramanti, A.; Bramanti, P.; Calabrò, R.S. What Does Best Evidence Tell Us about Robotic Gait Rehabilitation in Stroke Patients: A Systematic Review and Meta-Analysis. J. Clin. Neurosci. 2018, 48, 11–17. [Google Scholar] [CrossRef]
- Hesse, S.; Waldner, A.; Tomelleri, C. Innovative Gait Robot for the Repetitive Practice of Floor Walking and Stair Climbing up and down in Stroke Patients. J. Neuroeng. Rehabil. 2010, 7, 30. [Google Scholar] [CrossRef]
- Schmidt, H.; Hesse, S.; Bernhardt, R.; Krüger, J. HapticWalker---A Novel Haptic Foot Device. ACM Trans. Appl. Percept. 2005, 2, 166–180. [Google Scholar] [CrossRef]
- Halmi, Z.; Stone, T.W.; Dinya, E.; Málly, J. Postural Instability Years after Stroke. J. Stroke Cerebrovasc. Dis. 2020, 29, 105038. [Google Scholar] [CrossRef]
- Krebs, H.I.; Ferraro, M.; Buerger, S.P.; Newbery, M.J.; Makiyama, A.; Sandmann, M.; Lynch, D.; Volpe, B.T.; Hogan, N. Rehabilitation Robotics: Pilot Trial of a Spatial Extension for MIT-Manus. J. Neuroeng. Rehabil. 2004, 1, 5. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).