Efficacy, Safety and Economic Evaluation of Wolbigachul-Tang for Chronic Cough Due to Upper Airway Cough Syndrome (UACS): A Study Protocol for Randomized, Double-Blind, Active-Comparator Controlled, Parallel, Exploratory Clinical Trial
Abstract
:1. Introduction
2. Methods
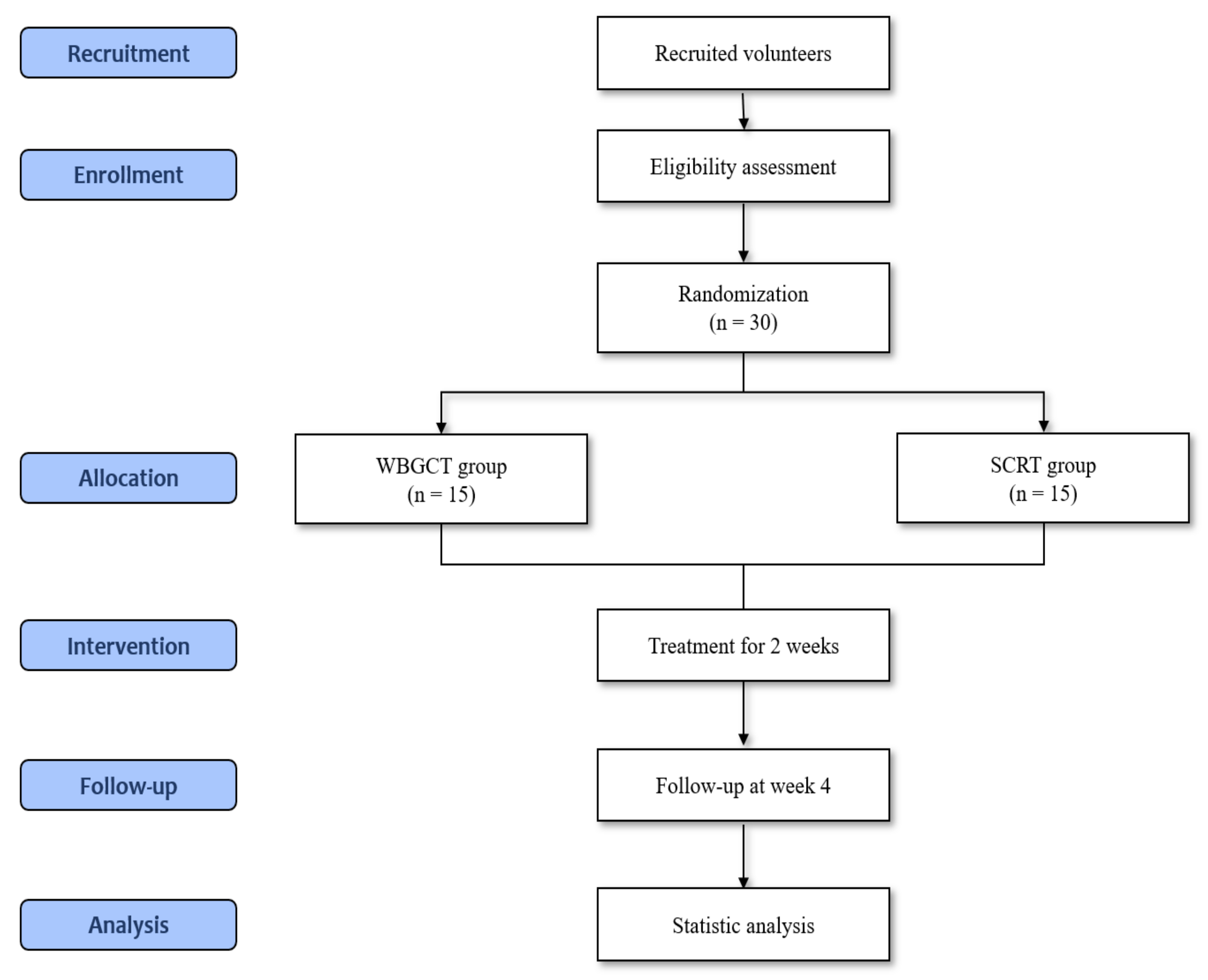
2.1. Study Design
2.2. Participants
2.2.1. Inclusion/Exclusion Criteria
2.2.2. Sample Size
2.2.3. Recruitment
2.3. Interventions
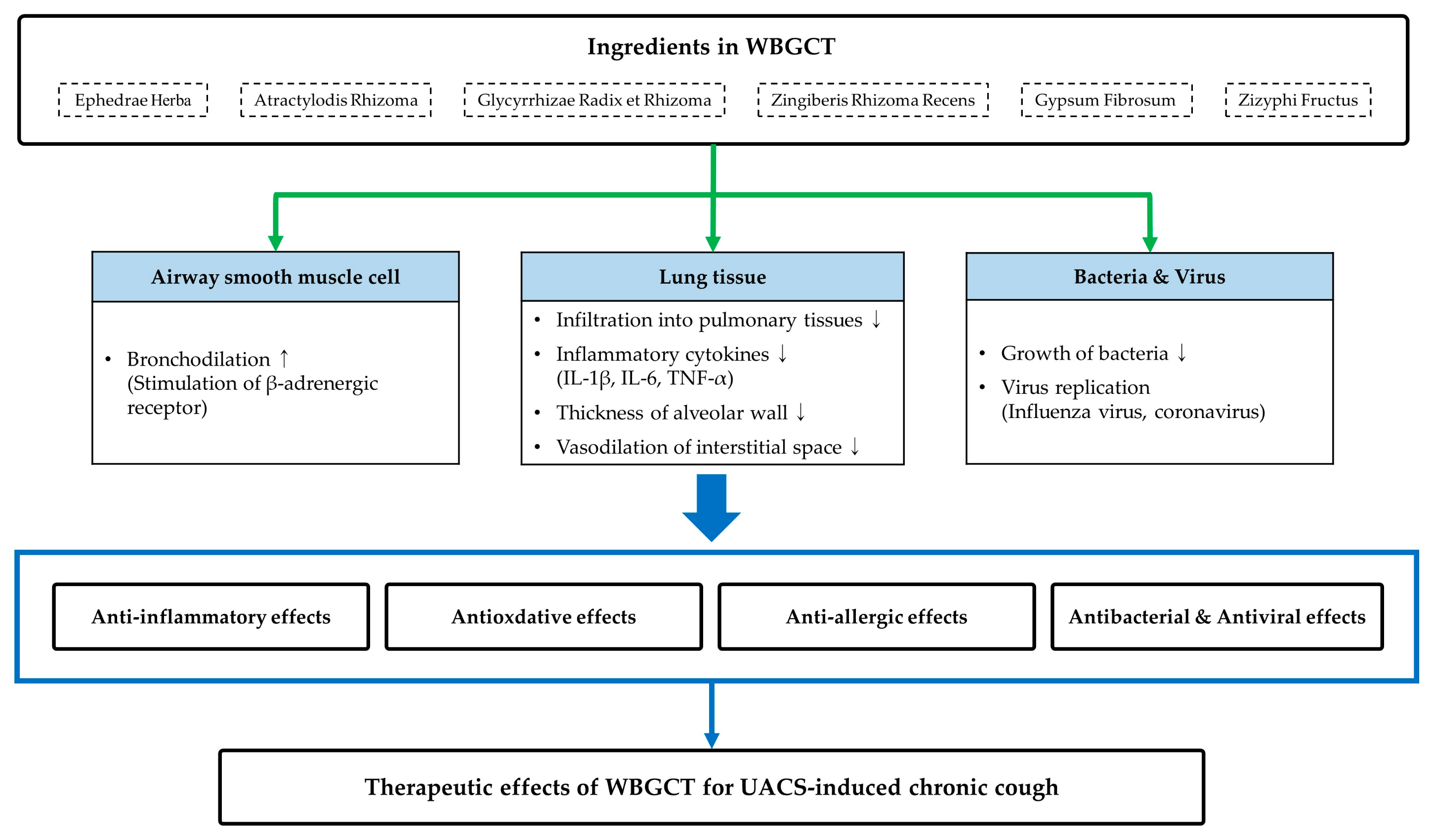
2.3.1. WBGCT Group
2.3.2. Control Group
2.4. Outcome Measures
2.4.1. Primary Outcome Measures: Cough Symptom Score (CSS)
2.4.2. Secondary Outcome Measures
- (1)
- Cough Visual Analog Scale (VAS).
- (2)
- Nasal Discharge Score (NDS)
- (3)
- Questionnaire of Clinical Symptoms of Cough and Sputum (QCSCS)
- (4)
- Leicester Cough Questionnaire-Korean version (LCQ-K)
- (5)
- Integrative Medicine Outcome Scale (IMOS) & Integrative Medicine Patient Satisfaction Scale (IMPSS)
- (6)
- 5-level EuroQol 5-Dimensional Questionnaire (EQ-5D-5L)
2.4.3. Exploratory Outcome Measures: Pattern Identification for Chronic Cough Questionnaire (PICCQ)
2.5. Safety Assessment
2.6. Economic Evaluation
2.7. Assignment of Interventions
2.7.1. Allocation
2.7.2. Blinding
2.8. Data Management and Monitoring
2.9. Statistical Analysis
3. Ethics and Dissemination
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pratter, M.R.; Brightling, C.E.; Boulet, L.P.; Irwin, R.S. An empiric integrative approach to the management of cough: ACCP evidence-based clinical practice guidelines. Chest 2006, 129, 222S–231S. [Google Scholar] [CrossRef] [PubMed]
- Morice, A.; Fontana, G.; Belvisi, M.; Birring, S.; Chung, K.; Dicpinigaitis, P.V.; Kastelik, J.; McGarvey, L.; Smith, J.; Tatar, M. ERS guidelines on the assessment of cough. Eur. Respir. J. 2007, 29, 1256–1276. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.J.; Kim, C.W. Understanding the impact of chronic cough on the quality of life in the general population. Allergy Asthma Immunol. Res. 2020, 12, 906–909. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.F.; Pavord, I.D. Prevalence, pathogenesis, and causes of chronic cough. Lancet 2008, 371, 1364–1374. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.; Chen, R.; Lin, J.; Huang, K.; Shen, H.; Kong, L.; Zhou, X.; Luo, Z.; Yang, L.; Wen, F. A prospective, multicenter survey on causes of chronic cough in China. Chest 2013, 143, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Qiu, Z.H.; Wei, W.L.; Liu, B.; Xu, X.H.; Lü, H.J.; Qiu, Z.M. Discrepancy between presumptive and definite causes of chronic cough. Chin. Med. J. (Engl.) 2011, 124, 4138–4143. [Google Scholar]
- Macedo, P.; Saleh, H.; Torrego, A.; Arbery, J.; MacKay, I.; Durham, S.R.; Chung, K.F. Postnasal drip and chronic cough: An open interventional study. Respir. Med. 2009, 103, 1700–1705. [Google Scholar] [CrossRef]
- French, C.T.; Fletcher, K.E.; Irwin, R.S. Gender differences in health-related quality of life in patients complaining of chronic cough. Chest 2004, 125, 482–488. [Google Scholar] [CrossRef]
- Pratter, M.R. Chronic upper airway cough syndrome secondary to rhinosinus diseases (previously referred to as postnasal drip syndrome): ACCP evidence-based clinical practice guidelines. Chest 2006, 129, 63S–71S. [Google Scholar] [CrossRef]
- Irwin, R.S.; French, C.L.; Chang, A.B.; Altman, K.W.; Adams, T.M.; Azoulay, E.; Barker, A.F.; Birring, S.S.; Blackhall, F.; Bolser, D.C. Classification of cough as a symptom in adults and management algorithms: CHEST guideline and expert panel report. Chest 2018, 153, 196–209. [Google Scholar] [CrossRef]
- Li, L.; Liu, R.; Peng, C.; Chen, X.; Li, J. Pharmacogenomics for the efficacy and side effects of antihistamines. Exp. Dermatol. 2022, 31, 993–1004. [Google Scholar] [CrossRef] [PubMed]
- Bolser, D.C. Older-generation antihistamines and cough due to upper airway cough syndrome (UACS): Efficacy and mechanism. Lung 2008, 186, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Zhao, J.; Wu, J. Curative Effect of Shufeng Jiedu Capsules Combined with Conventional Treatment on Upper Airway Cough Syndrome. China Pharm. 2018, 21, 856–858. [Google Scholar]
- Jiang, H.; Liu, W.; Li, G.; Fan, T.; Mao, B. Chinese medicinal herbs in the treatment of upper airway cough syndrome: A systematic review of randomized, controlled trials. Altern. Ther. Health Med. 2016, 22, 38–51. [Google Scholar] [PubMed]
- Abe, K.; Takagi, K. Efficacy of decocted “Eppikahangeto” and Extract Drug of “Eppikajutsuto” added to “Hangekoubokuto” to Bronchial Asthma in Children. Kampo Med. 1991, 42, 271–281. [Google Scholar] [CrossRef]
- Mori, H.; Shimazaki, Y.; Kurata, H.; Hiroshi, T.; Masasuke, T. Comparative study of Kampo preparations sho-seiryu-to and eppika-jutsu-to for nasal allergy and allergic conjunctivitis. Ther. Res. 1997, 18, 3093–3099. [Google Scholar]
- Minamizawa, K.; Goto, H.; Shimada, Y.; Terasawa, K.; Haji, A. Effects of eppikahangeto, a Kampo formula, and Ephedrae herba against citric acid-induced laryngeal cough in guinea pigs. J. Pharmacol. Sci. 2006, 101, 118–125. [Google Scholar] [CrossRef]
- Im, S.K.; Sul, J.U.; Kim, S.J.; Jeong, J.M.; Seon, Y.G.; Choi, J.B. The Study on Anti-Oxidant and Anti-Inflammatory Effects of Wolbigachul-tang. J. Korean Med. Rehabil. 2023, 33, 1–12. [Google Scholar] [CrossRef]
- Kwak, H.Y.; Kim, J.H.; Seon, J.I.; Lim, S.K.; Kwon, Y.J.; Kim, D.H.; Lee, U.I.; Kang, J.W.; Lee, J.D.; Choi, D.Y. The effects and adverse events of Gamiwolbigachultang on the changes of body composition and musculoskeletal pain in 28 overweight patients: A retrospective observational study. J. Acupunct. Res. 2011, 28, 103–110. [Google Scholar]
- Wang, S.D.; Lin, L.J.; Chen, C.L.; Lee, S.C.; Lin, C.C.; Wang, J.Y.; Kao, S.T. Xiao-Qing-Long-Tang attenuates allergic airway inflammation and remodeling in repetitive Dermatogoides pteronyssinus challenged chronic asthmatic mice model. J. Ethnopharmacol. 2012, 142, 531–538. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, J.; Liu, H.; Lin, Z.; Luo, Q.; Li, Y.; Ruan, Y.; Zhou, S. Efficacy and safety of the Chinese herbal medicine Xiao-qing-long-tang for allergic rhinitis: A systematic review and meta-analysis of randomized controlled trials. J. Ethnopharmacol. 2022, 297, 115169. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Lim, C.Y.; Kim, B.Y.; Cho, S.I. So-Cheong-Ryong-Tang, a herbal medicine, modulates inflammatory cell infiltration and prevents airway remodeling via regulation of interleukin-17 and GM-CSF in allergic asthma in mice. Pharmacogn. Mag. 2014, 10 (Suppl. S3), S506–S511. [Google Scholar] [PubMed]
- Mo, J.H.; Lee, S.E.; Wee, J.H.; Lee, J.E.; Rhee, C.S.; Lee, C.H.; Kim, D.Y. Anti-allergic effects of So-Cheong-Ryong-Tang, a traditional Korean herbal medicine, in an allergic rhinitis mouse model. Eur. Arch. Otorhinolaryngol. 2013, 270, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Hong, S.U.; Kim, H.T.; Seo, H.S.; Kim, K.S.; Ko, S.G.; Choi, I.H. A multicenter study on the efficacy and safety of So-Cheong-Ryong-Tang for perennial allergic rhinitis. Complement. Ther. Med. 2019, 45, 50–56. [Google Scholar] [CrossRef]
- Lee, M.Y.; Seo, C.S.; Kim, J.Y.; Shin, H.K. Evaluation of a water extract of So-Cheong-Ryong-Tang for acute toxicity and genotoxicity using in vitro and in vivo tests. BMC Complement. Altern. Med. 2015, 15, 235. [Google Scholar] [CrossRef]
- Julious, S.A. Sample size of 12 per group rule of thumb for a pilot study. Pharm. Stat. 2005, 4, 287–291. [Google Scholar] [CrossRef]
- Hsu, J.; Stone, R.; Logan-Sinclair, R.; Worsdell, M.; Busst, C.; Chung, K. Coughing frequency in patients with persistent cough: Assessment using a 24 hour ambulatory recorder. Eur. Respir. J. 1994, 7, 1246–1253. [Google Scholar] [CrossRef]
- Irwin, R.S. Assessing cough severity and efficacy of therapy in clinical research: ACCP evidence-based clinical practice guidelines. Chest 2006, 129, 232S–237S. [Google Scholar] [CrossRef]
- Decalmer, S.C.; Webster, D.; Kelsall, A.A.; McGuinness, K.; Woodcock, A.A.; Smith, J.A. Chronic cough: How do cough reflex sensitivity and subjective assessments correlate with objective cough counts during ambulatory monitoring? Thorax 2007, 62, 329–334. [Google Scholar] [CrossRef]
- Kwon, J.W.; Moon, J.Y.; Kim, S.H.; Song, W.J.; Kim, M.H.; Kang, M.G.; Lim, K.H.; Lee, S.H.; Lee, S.M.; Lee, J.Y. Korean version of the Cough Symptom Score: Clinical utility and validity for chronic cough. Korean J. Intern. Med. 2017, 32, 910. [Google Scholar] [CrossRef]
- Martin Nguyen, A.; Bacci, E.D.; Vernon, M.; Birring, S.S.; Rosa, C.L.; Muccino, D.; Schelfhout, J. Validation of a visual analog scale for assessing cough severity in patients with chronic cough. Ther. Adv. Respir. Dis. 2021, 15, 17534666211049743. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Food and Drug Safety, Herbal Medicine Evaluation Department. The Traditional Korean Medicine Clinical Practice Guidlines for Antitussives and Expectorants; Ministry of Food and Drug Safety: Seoul, Republic of Korea, 2007; pp. 5–15.
- Baek, H.J.; Lee, B.J.; Jung, S.K.; Jung, H.J. Retrospective study of patients with cough treated with eunhwayeongyo-tang. J. Intern. Korean Med. 2016, 37, 961–977. [Google Scholar] [CrossRef]
- Birring, S.; Prudon, B.; Carr, A.; Singh, S.; Morgan, M.; Pavord, I. Development of a symptom specific health status measure for patients with chronic cough: Leicester Cough Questionnaire (LCQ). Thorax 2003, 58, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Han, J.M.; Jung, I.C.; Kang, W.C.; Kim, S.S.; Yeo, Y.; Park, Y.C. Reliability and validity of leicester cough questionnaire Korean version. Chron. Respir. Dis. 2014, 11, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Matthys, H.; Kamin, W. Positioning of the Bronchitis Severity Score (BSS) for standardised use in clinical studies. Curr. Med. Res. Opin. 2013, 29, 1383–1390. [Google Scholar] [CrossRef]
- Kim, S.H.; Ahn, J.H.; Ock, M.S.; Shin, S.J.; Park, J.Y.; Luo, N.; Jo, M.W. The EQ-5D-5L valuation study in Korea. Qual. Life Res. 2016, 25, 1845–1852. [Google Scholar] [CrossRef]
- Kim, K.I.; Shin, S.W.; Lee, N.L.; Lee, B.J.; Jung, H.J.; Jung, S.K.; Lee, J.H. Preliminary study for development of pattern identification tool of chronic cough. J. Intern. Korean Med. 2015, 36, 22–39. [Google Scholar]
- McGarvey, L.; Heaney, L.; Lawson, J.; Johnston, B.; Scally, C.; Ennis, M.; Shepherd, D.; MacMahon, J. Evaluation and outcome of patients with chronic non-productive cough using a comprehensive diagnostic protocol. Thorax 1998, 53, 738–743. [Google Scholar] [CrossRef]
- Smyrnios, N.A.; Irwin, R.S.; Curley, F.J. Chronic cough with a history of excessive sputum production: The spectrum and frequency of causes, key components of the diagnostic evaluation, and outcome of specific therapy. Chest 1995, 108, 991–997. [Google Scholar] [CrossRef]
- Matsui, T.; Yamashita, H.; Mori, M.; Tanaka, H.; Inagaki, N. Eppikajutsuto protects against food allergy induced by ovalbumin in a murine model. Int. Arch. Allergy Immunol. 2017, 173, 71–83. [Google Scholar] [CrossRef]
- Lucanska, M.; Hajtman, A.; Calkovsky, V.; Peter, K.; Pecova, R. Upper airway cough syndrome in pathogenesis of chronic cough. Physiol. Res. 2020, 69, S35–S42. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Lou, X.; Jin, Z.; Yu, L.; Deng, L.; Wan, H. Mahuang decoction mitigates airway inflammation and regulates IL-21/STAT3 signaling pathway in rat asthma model. J. Ethnopharmacol. 2018, 224, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.R. The history of Ephedra (ma-huang). J. R. Coll. Physicians Edinb. 2011, 41, 78–84. [Google Scholar] [CrossRef]
- Zheng, Q.; Mu, X.; Pan, S.; Luan, R.; Zhao, P. Ephedrae herba: A comprehensive review of its traditional uses, phytochemistry, pharmacology, and toxicology. J. Ethnopharmacol. 2023, 307, 116153. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhao, Z.; Chang, L.; Cao, Y.; Wang, S.; Kang, C.; Wang, H.; Zhou, L.; Huang, L.; Guo, L. Atractylodis Rhizoma: A review of its traditional uses, phytochemistry, pharmacology, toxicology and quality control. J. Ethnopharmacol. 2021, 266, 113415. [Google Scholar] [CrossRef]
- Cheng, Y.; Mai, J.Y.; Hou, T.L.; Ping, J.; Chen, J.J. Antiviral activities of atractylon from Atractylodis Rhizoma. Mol. Med. Rep. 2016, 14, 3704–3710. [Google Scholar] [CrossRef]
- Wang, H.; Wang, M.; Li, X. Evaluation of the antipyretic activity of Gypsum Fibrosum and its constituents. Asian J. Tradit. Med. 2009, 4, 82–84. [Google Scholar]
- Im, G.H.; Jo, J.J.; An, H.J.; Yu, Y.C.; Kim, S.U.; Kim, H.M.; Kim, J.S. Effect of Gypsum Fibrosum on Interleukin-4 secretion of mice splenocytes. Korea J. Herbol. 2002, 17, 139. [Google Scholar]
- Li, F.; Liu, B.; Li, T.; Wu, Q.; Xu, Z.; Gu, Y.; Li, W.; Wang, P.; Ma, T.; Lei, H. Review of constituents and biological activities of triterpene saponins from Glycyrrhizae Radix et Rhizoma and its solubilization characteristics. Molecules 2020, 25, 3904. [Google Scholar] [CrossRef]
- Li, X.; Ao, M.; Zhang, C.; Fan, S.; Chen, Z.; Yu, L. Zingiberis Rhizoma Recens: A review of its traditional uses, phytochemistry, pharmacology, and toxicology. Evid. Based Complement. Alternat. Med. 2021, 2021, 6668990. [Google Scholar] [CrossRef]
- Byun, J.S.; Yang, S.Y.; Jeong, I.C.; Hong, K.E.; Kang, W.C.; Yeo, Y.; Park, Y.C. Effects of So-cheong-ryong-tang and Yeon-gyo-pae-dok-san on the common cold: Randomized, double blind, placebo controlled trial. J. Ethnopharmacol. 2011, 133, 642–646. [Google Scholar] [CrossRef] [PubMed]
| Study Period | ||||
|---|---|---|---|---|
| Enrollment | Allocation | Post- Allocation | Follow-Up | |
| Visit | 1 | 2 | 3 | 4 |
| Timepoint | −2 Weeks | 0 | Week 2 ± 4 Days | Week 4 ± 4 Days |
| Enrollment | ||||
| Informed consent | X | |||
| Eligibility screen | X | |||
| Allocation | X | |||
| Demographic characteristics | X | |||
| Social and economic characteristics | X | |||
| Medical history | X | |||
| Chest X-ray, EKG | X | |||
| Vital signs | X | X | X | X |
| Pulmonary Function Test | X | |||
| Paranasal Sinuses X-ray | X | |||
| Interventions | ||||
| WBGCT |  | |||
| SCRT |  | |||
| Assessments | ||||
| Clinical laboratory test 1 | X | X | ||
| Pregnancy Diagnosis Test | X | |||
| CSS | X | X | X | X |
| Cough VAS | X | X | X | |
| NDS | X | X | X | |
| Cough and sputum severity standard | X | X | X | |
| LCQ-K | X | X | X | |
| IMOS & IMPSS | X | X | ||
| EQ-5D-5L | X | X | X | |
| PICCQ | X | |||
| Adverse reaction assessment | X | X | X | |
| Compliance test | X | X | ||
| Latin Name | Amount (g) |
|---|---|
| Wolbigachul-tang | |
| Ephedrae Herba | 3.75 |
| Zizyphi Fructus | 0.67 |
| Gypsum Fibrosum | 1.25 |
| Atractylodis Rhizoma | 2.50 |
| Glycyrrhizae Radix et Rhizoma | 1.25 |
| Zingiberis Rhizoma Recens | 0.83 |
| Socheongryong-tang | |
| Ephedrae Herba | 0.30 |
| Paeoniae Radix | 0.51 |
| Pinelliae Tuber | 0.47 |
| Schisandrae Fructus | 0.55 |
| Cinnamomi Ramulus | 0.04 |
| Asiasari Radix et Rhizoma | 0.18 |
| Glycyrrhizae Radix et Rhizoma | 0.34 |
| Zingiberis Rhizoma | 0.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woo, S.-C.; Lyu, Y.R.; Lee, S.W.; Kwon, O.-J.; Choi, Y.-E.; Yang, C.; Park, Y.C. Efficacy, Safety and Economic Evaluation of Wolbigachul-Tang for Chronic Cough Due to Upper Airway Cough Syndrome (UACS): A Study Protocol for Randomized, Double-Blind, Active-Comparator Controlled, Parallel, Exploratory Clinical Trial. Healthcare 2023, 11, 2733. https://doi.org/10.3390/healthcare11202733
Woo S-C, Lyu YR, Lee SW, Kwon O-J, Choi Y-E, Yang C, Park YC. Efficacy, Safety and Economic Evaluation of Wolbigachul-Tang for Chronic Cough Due to Upper Airway Cough Syndrome (UACS): A Study Protocol for Randomized, Double-Blind, Active-Comparator Controlled, Parallel, Exploratory Clinical Trial. Healthcare. 2023; 11(20):2733. https://doi.org/10.3390/healthcare11202733
Chicago/Turabian StyleWoo, Seong-Cheon, Yee Ran Lyu, Su Won Lee, O-Jin Kwon, Young-Eun Choi, Changsop Yang, and Yang Chun Park. 2023. "Efficacy, Safety and Economic Evaluation of Wolbigachul-Tang for Chronic Cough Due to Upper Airway Cough Syndrome (UACS): A Study Protocol for Randomized, Double-Blind, Active-Comparator Controlled, Parallel, Exploratory Clinical Trial" Healthcare 11, no. 20: 2733. https://doi.org/10.3390/healthcare11202733
APA StyleWoo, S.-C., Lyu, Y. R., Lee, S. W., Kwon, O.-J., Choi, Y.-E., Yang, C., & Park, Y. C. (2023). Efficacy, Safety and Economic Evaluation of Wolbigachul-Tang for Chronic Cough Due to Upper Airway Cough Syndrome (UACS): A Study Protocol for Randomized, Double-Blind, Active-Comparator Controlled, Parallel, Exploratory Clinical Trial. Healthcare, 11(20), 2733. https://doi.org/10.3390/healthcare11202733







