Evaluation of the Effect of a Safe Medication Strategy on Potentially Inappropriate Medications, Polypharmacy and Anticholinergic Burden for People with Dementia: An Intervention Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Participants
2.3. Data Collection
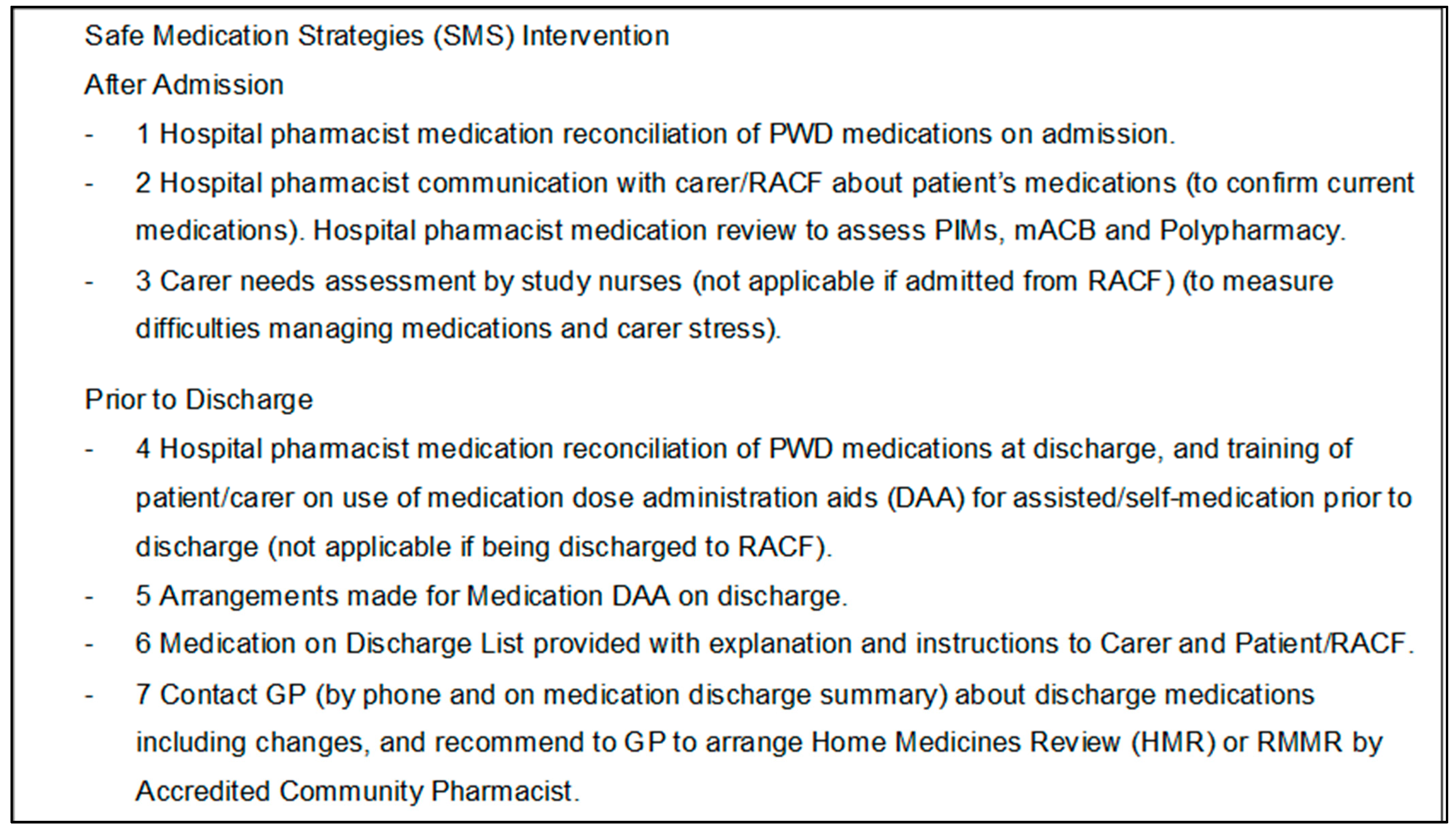
2.4. The Intervention
2.5. Statistical Analysis
2.6. Ethics Approval and Consent to Participate
3. Results
3.1. Medications, Polypharmacy, PIMs, and mACB Scores
3.2. PIMs Psychotropic and Sedative/Hypnotic Medication Categories
3.3. Pharmacist Medication Review and Reconciliation Conducted at Admission and Prior to Discharge as Part of the Intervention
3.4. Pharmacist Scoring of Prescribed Medications with Potential for Harm or Adverse Reaction or Prescribing Error per Participant at Admission
3.5. Pharmacist Scoring of Prescribed Medications with Potential for Harm or Adverse Reaction or Prescribing Error (Recommendation Level) at Admission
3.6. GP Acceptance of Community Pharmacist Recommendations at Three Months
4. Discussion
4.1. Clinical Implications
4.2. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Kable, A.; Fullerton, A.; Fraser, S.; Palazzi, K.; Hullick, C.; Oldmeadow, C.; Pond, D.; Searles, A.; Edmunds, K.; Attia, J. Comparison of Potentially Inappropriate Medications for People with Dementia at Admission and Discharge during an Unplanned Admission to Hospital: Results from the SMS Dementia Study. Healthcare 2019, 7, 8. [Google Scholar] [CrossRef]
- Masnoon, N.; Shakib, S.; Kalisch-Ellett, L.; Caughey, G. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017, 17, 230. [Google Scholar] [CrossRef]
- Clague, F.; Mercer, S.; McLean, G.; Reynish, E.; Guthrie, B. Comorbidity and polypharmacy in people with dementia: Insights from a large, population-based cross-sectional analysis of primary care data. Age Ageing 2017, 46, 33–39. [Google Scholar] [CrossRef]
- Renom-Guiteras, A.; Thurmann, P.; Miralles, R.; Klaaben-Mielke, R.; Thiem, U.; Stephan, A.; Bleijlevens, M.; Jolley, D.; Leino-Kilpi, H.; Hallberg, I.; et al. Potentially inappropriate medication among people with dementia in eight European countries. Age Ageing 2018, 47, 68–74. [Google Scholar] [CrossRef]
- Johnell, K. Inappropriate Drug Use in People with Cognitive Impairment and Dementia: A Systematic Review. Curr. Clin. Pharmacol. 2015, 10, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Redston, M.; Hilmer, S.; McLachlan, A.; Clough, A.; Gnjidic, D. Prevalence of Potentially Inappropriate Medication Use in Older Inpatients with and without Cognitive Impairment: A Systematic Review. J. Alzheimer’s Dis. 2018, 61, 1639–1652. [Google Scholar] [CrossRef] [PubMed]
- Australian Commission on Safety and Quality in Health Care. Reducing Inappropriate Use of Antipsychotics in People with Behavioural and Psychological Symptoms of Dementia (BPSD); NSQHS Standards; Australian Commission on Safety and Quality in Health Care: Sydney, Australia, 2018. [Google Scholar]
- Australian Medical Research Advisory Panel. Consultation on Australian Medical Research and Innovation Priorities for 2018–2021; Australian Medical Research Advisory Panel: Canberra, Australia, 2018. [Google Scholar]
- The Society of Hospital Pharmacists of Australia. Treatments Pharmacists and Consumers Should Question. Available online: http://www.choosingwisely.org.au/recommendations/shpa (accessed on 17 September 2020).
- Johnson, K.; Fashoyin, A.; Madden-Fuentes, R.; Muzyk, A.; Gagliardi, J.; Yanamadala, M. Discharge Plans for Geriatric Inpatients with Delirium: A Plan to Stop Antipsychotics? J. Am. Geriatr. Soc. 2017, 65, 2278–2281. [Google Scholar] [CrossRef] [PubMed]
- Fick, D.; Cooper, J.; Wade, W.; Waller, J.; Maclean, J.; Beers, M. Updating the Beers criteria for potentially inappropriate medication use in older adults. Arch. Intern. Med. 2003, 163, 2716–2724. [Google Scholar] [CrossRef]
- Chan, V.; Woo, B.; Sewell, D.; Allen, E.; Golshan, S.; Rice, V.; Minassian, A.; Daly, J. Reduction of suboptimal prescribing and clinical outcome for dementia patients in a senior behavioural health inpatient unit. Int. Psychogeriatr. 2009, 21, 195–199. [Google Scholar] [CrossRef]
- Martin, P.; Tamblyn, R.; Benedetti, A.; Ahmed, S.; Tannenbaum, C. Effect of a Pharmacist-Led Educational Intervention on Inappropriate Medication Prescriptions in Older Adults: The D-PRESCRIBE Randomized Clinical Trial. J. Am. Med. Assoc. 2018, 320, 1889–1898. [Google Scholar] [CrossRef]
- Stuhec, M.; Gorenc, K.; Zelko, E. Evaluation of a collaborative care approach between general practitioners and clinical pharmacists in primary care community settings in elderly patients on polypharmacy in Slovenia: A cohort retrospective study reveals positive evidence for implementation. BMC Health Serv. Res. 2019, 19, 118. [Google Scholar] [CrossRef]
- Lenander, C.; Bondesson, A.; Viberg, N.; Beckman, A.; Midlov, P. Effects of medication reviews on use of potentially inappropriate medications in elderly patients; a cross sectional study in Swedish primary care. BMC Health Serv. Res. 2018, 18, 9. [Google Scholar] [CrossRef]
- Mahlknecht, A.; Krisch, L.; Nestler, N.; Bauer, U.; Letz, N.; Zenz, D.; Schuler, J.; Fahrmann, L.; Hempel, G.; Flamm, M.; et al. Impact of training and structured medication review on medication appropriateness and patient-related outcomes in nursing homes: Results from the interventional study InTherAKT. BMC Geriatr. 2019, 19, 257. [Google Scholar] [CrossRef] [PubMed]
- Perez-Moreno, M.; Rodriguez-Camacho, J.; Calderon-Hernanz, B.; Comas-Diaz, B.; Tarradas-Torras, J. Clinical relevance of pharmacist intervention in an emergency department. Emerg. Med. J. 2017, 34, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Kable, A.; Hullick, C.; Palazzi, K.; Oldmeadow, C.; Searles, A.; Ling, R.; Pond, D.; Fullerton, A.; Fraser, S.; Bruce, R.; et al. Evaluation of a safe medication strategy intervention for people with dementia with an unplanned admission: Results from the Safe Medication Strategy Dementia Study. Australas. J. Ageing 2020, 40, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Aging Brain Program. Anticholinergic Cognitive Burden Scale. 2012. Available online: https://www.idhca.org/wp-content/uploads/2018/02/DESAI_ACB_scale_-_Legal_size_paper.pdf (accessed on 28 August 2023).
- Scottish Government Model of Care Polypharmacy Working Group. Polypharmacy Guidance; Scottish Government: Edinburgh, UK, 2015. [Google Scholar]
- Clinical Excellence Commission. Keeping Patients Safe: Medication Safety, Medication Reconciliation. Available online: https://www.cec.health.nsw.gov.au/keep-patients-safe/medication-safety/cmm/mec-rec (accessed on 28 August 2023).
- Australian Commission on Safety and Quality in Health Care. Medicines Use in Older People. Available online: https://www.safetyandquality.gov.au/our-work/healthcare-variation/fourth-atlas-2021/medicines-use-older-people (accessed on 9 October 2023).
- Harris, P.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, J.; Conde, G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- Sharma, H. Statistical significance or clinical significance? A researcher’s dilemma for appropriate interpretation of results. Saudi J. Anaesth. 2021, 15, 431–434. [Google Scholar] [CrossRef]
- Hubbard, R.; Peel, N.; Scott, I.; Martin, J.; Smith, A.; Pillans, P.; Poudel, A.; Gray, L. Polypharmacy among inpatients aged 70 years or older in Australia. Med. J. Aust. 2015, 202, 373–378. [Google Scholar] [CrossRef]
- Hilmer, S. The dilemma of polypharmacy. Aust. Prescr. 2008, 31, 2–3. [Google Scholar] [CrossRef]
- Bala, S.; Jamieson, H.; Nishtala, P. Determinants of prescribing potentially inappropriate medications in a nationwide cohort of community dwellers with dementia receiving a comprehensive geriatric assessment. Int. J. Geriatr. Psychiatry 2019, 34, 153–161. [Google Scholar] [CrossRef]
- Alzheimer’s Australia. The Use of Restraints and Psychotropic Medications in People with Dementia; Alzheimer’s Australia Inc.: Shenton Park, Australia, 2014. [Google Scholar]
- Briggs, S.; Pearce, R.; Dilworth, S.; Higgins, I.; Hullick, C.; Attia, J. Clinical pharmacist review: A randomised controlled trial. Emerg. Med. Australas. 2015, 27, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Viva Communications. Medicines Safety Now a National Priority; Viva Communications: Pasig City, Philippines, 2019. [Google Scholar]
- Von Elm, E.; Altman, D.; Egger, M.; Pocock, S.; Gotzsche, P.; Vandenbroucke, J. The Strengthening and Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Available online: https://www.equator-network.org/reporting-guidelines/strobe/ (accessed on 17 September 2020).
| Phase 1 | Phase 2 | |||||
|---|---|---|---|---|---|---|
| Characteristic | Class/Statistic | Control (n = 117) | Intervention (n = 161) | Control (n = 172) | Intervention (n = 178) | Total (n = 628) |
| Gender | Male | 66 (56%) | 73 (45%) | 98 (57%) | 73 (41%) | 310 (49%) |
| Female | 51 (44%) | 88 (55%) | 74 (43%) | 105 (59%) | 318 (51%) | |
| Age | Mean (SD) | 83 (7) | 86 (7) | 82 (8) | 84 (8) | 84 (8) |
| Aboriginal and/or TSI | Yes | 2 (1.7%) | 1 (0.6%) | 10 (5.8%) | 3 (1.7%) | 16 (2.6%) |
| Missing | 1 | 0 | 0 | 1 | 2 | |
| CCI | Mean (SD) | 2 (2) | 2 (2) | 2 (2) | 2 (2) | 2 (2) |
| CCI group | 0 | 23 (20%) | 33 (21%) | 35 (20%) | 49 (28%) | 140 (22%) |
| 1–2 | 62 (53%) | 89 (56%) | 87 (51%) | 89 (51%) | 327 (52%) | |
| 3–4 | 22 (19%) | 22 (14%) | 34 (20%) | 26 (15%) | 104 (17%) | |
| 5+ | 10 (8.5%) | 16 (10%) | 15 (8.8%) | 12 (6.8%) | 53 (8.5%) | |
| Missing | 0 | 1 | 1 | 2 | 4 | |
| Admitted from | Home | 97 (83%) | 125 (78%) | 149 (87%) | 152 (85%) | 523 (83%) |
| RACF | 20 (17%) | 36 (22%) | 23 (13%) | 26 (15%) | 105 (17%) | |
| Discharge | Home (GP) | 68 (59%) | 80 (50%) | 98 (57%) | 100 (56%) | 346 (55%) |
| RACF | 27 (23%) | 57 (36%) | 42 (24%) | 52 (29%) | 178 (29%) | |
| Transitions program | 1 (0.9%) | 2 (1.3%) | 3 (1.7%) | 6 (1.0%) | ||
| Transfer to other Acute Care Facility | 15 (13%) | 14 (8.8%) | 14 (8.1%) | 6 (3.4%) | 49 (7.9%) | |
| Rehabilitation Facility | 7 (4.1%) | 4 (2.2%) | 11 (1.8%) | |||
| Died during index admission | 3 (2.6%) | 6 (3.8%) | 8 (4.7%) | 15 (8.5%) | 32 (5.1%) | |
| Missing | 3 | 2 | 0 | 1 | 6 | |
| Phase 1 | Phase 2 | ||||||
|---|---|---|---|---|---|---|---|
| Characteristic | Timepoint | Class/Statistic | Control (n = 117) | Intervention (n = 161) | Control (n = 172) | Intervention (n = 178) | Total (n = 628) |
| Number of medications | Admission | Mean (SD) | 12 (5) | 11 (4) | 14 (5) | 13 (5) | 13 (5) |
| Median (min, max) | 12 (2, 27) | 11 (1, 21) | 13 (1, 28) | 14 (1, 33) | 12 (1, 33) | ||
| Missing | 1 | 0 | 0 | 0 | 1 | ||
| Discharge | Mean (SD) | 10 (4) | 9 (4) | 10 (4) | 9 (4) | 9 (4) | |
| Median (min, max) | 10 (2, 20) | 8 (1, 20) | 10 (2, 25) | 9 (1, 28) | 9 (1, 28) | ||
| Missing | 10 | 10 | 21 | 20 | 61 | ||
| 3 month | Mean (SD) | 11 (4) | 9 (4) | 10 (4) | 9 (4) | 9 (4) | |
| Median (min, max) | 10 (2, 22) | 9 (2, 20) | 9 (2, 21) | 8 (1, 28) | 9 (1, 28) | ||
| Missing | 31 | 50 | 43 | 49 | 173 | ||
| Polypharmacy | Admission | Yes | 110 (95%) | 150 (93%) | 169 (98%) | 168 (94%) | 597 (95%) |
| Missing | 1 | 0 | 0 | 0 | 1 | ||
| Discharge | Yes | 99 (93%) | 133 (88%) | 139 (92%) | 141 (89%) | 512 (90%) | |
| Missing | 10 | 10 | 21 | 20 | 61 | ||
| 3 month | Yes | 83 (97%) | 103 (93%) | 117 (91%) | 111 (86%) | 414 (91%) | |
| Missing | 31 | 50 | 43 | 49 | 173 | ||
| PIMs prescribed | Admission | Yes | 116 (100%) | 154 (96%) | 171 (99%) | 176 (99%) | 617 (98%) |
| Missing | 1 | 0 | 0 | 0 | 1 | ||
| Discharge | Yes | 106 (99%) | 138 (91%) | 148 (98%) | 148 (94%) | 540 (95%) | |
| Missing | 10 | 10 | 21 | 20 | 61 | ||
| 3 month | Yes | 85 (99%) | 107 (96%) | 124 (96%) | 118 (91%) | 434 (95%) | |
| Missing | 31 | 50 | 43 | 49 | 173 | ||
| Number of PIMs | Admission | Mean (SD) | 4 (2) | 4 (2) | 5 (2) | 4 (2) | 4 (2) |
| Median (min, max) | 4 (1, 10) | 3 (0, 11) | 5 (0, 17) | 4 (0, 11) | 4 (0, 17) | ||
| Missing | 1 | 0 | 0 | 0 | 1 | ||
| Discharge | Mean (SD) | 4 (2) | 3 (2) | 4 (2) | 3 (2) | 3 (2) | |
| Median (min, max) | 4 (0, 9) | 3 (0, 8) | 4 (0, 10) | 3 (0, 8) | 3 (0, 10) | ||
| Missing | 10 | 10 | 21 | 20 | 61 | ||
| 3 month | Mean (SD) | 4 (2) | 3 (2) | 4 (2) | 3 (2) | 4 (2) | |
| Median (min, max) | 4 (0, 10) | 3 (0, 8) | 4 (0, 9) | 3 (0, 9) | 3 (0, 10) | ||
| Missing | 31 | 50 | 43 | 49 | 173 | ||
| MACB total | Admission | Mean (SD) | 3 (2) | 2 (2) | 3 (2) | 3 (2) | 3 (2) |
| Median (min, max) | 3 (0, 9) | 2 (0, 15) | 3 (0, 15) | 3 (0, 10) | 2 (0, 15) | ||
| Missing | 1 | 0 | 0 | 0 | 1 | ||
| Discharge | Mean (SD) | 3 (2) | 2 (2) | 3 (2) | 2 (2) | 2 (2) | |
| Median (min, max) | 2 (0, 10) | 2 (0, 10) | 2 (0, 9) | 2 (0, 8) | 2 (0, 10) | ||
| Missing | 10 | 10 | 21 | 20 | 61 | ||
| 3 month | Mean (SD) | 3 (2) | 2 (2) | 3 (2) | 2 (1) | 2 (2) | |
| Median (min, max) | 3 (0, 10) | 2 (0, 7) | 2 (0, 10) | 2 (0, 5) | 2 (0, 10) | ||
| Missing | 31 | 50 | 43 | 49 | 173 | ||
| Phase 1 | Phase 2 | ||||||
|---|---|---|---|---|---|---|---|
| Characteristic | Timepoint | Class/Statistic | Control (n = 117) | Intervention (n = 161) | Control (n = 172) | Intervention (n = 178) | Total (n = 628) |
| Psychotropic medication | Admission | No | 63 (54%) | 102 (63%) | 99 (58%) | 90 (51%) | 354 (56%) |
| Yes | 53 (46%) | 59 (37%) | 73 (42%) | 88 (49%) | 273 (44%) | ||
| Missing | 1 | 0 | 0 | 0 | 1 | ||
| Discharge | No | 60 (56%) | 92 (61%) | 86 (57%) | 89 (56%) | 327 (58%) | |
| Yes | 47 (44%) | 59 (39%) | 65 (43%) | 69 (44%) | 240 (42%) | ||
| Missing | 10 | 10 | 21 | 20 | 61 | ||
| 3 month | No | 44 (51%) | 62 (56%) | 68 (53%) | 70 (54%) | 244 (54%) | |
| Yes | 42 (49%) | 49 (44%) | 61 (47%) | 59 (46%) | 211 (46%) | ||
| Missing | 31 | 50 | 43 | 49 | 173 | ||
| Sedative/hypnotic medication | Admission | No | 97 (84%) | 141 (88%) | 140 (81%) | 148 (83%) | 526 (84%) |
| Yes | 19 (16%) | 20 (12%) | 32 (19%) | 30 (17%) | 101 (16%) | ||
| Missing | 1 | 0 | 0 | 0 | 1 | ||
| Discharge | No | 99 (93%) | 140 (93%) | 139 (92%) | 153 (97%) | 531 (94%) | |
| Yes | 8 (7.5%) | 11 (7.3%) | 12 (7.9%) | 5 (3.2%) | 36 (6.3%) | ||
| Missing | 10 | 10 | 21 | 20 | 61 | ||
| 3 month | No | 77 (90%) | 95 (86%) | 116 (90%) | 122 (95%) | 410 (90%) | |
| Yes | 9 (10%) | 16 (14%) | 13 (10%) | 7 (5.4%) | 45 (9.9%) | ||
| Missing | 31 | 50 | 43 | 49 | 173 | ||
| Phase 1 | Phase 2 | ||||||
|---|---|---|---|---|---|---|---|
| Characteristic | Timepoint | Class/Statistic | Control (n = 117) | Intervention (n = 161) | Control (n = 172) | Intervention (n = 178) | Total (n = 628) |
| Medication reconciliation | Admission | No | 75 (65%) | 111 (69%) | 101 (59%) | 1 (0.6%) | 288 (46%) |
| Yes | 41 (35%) | 49 (31%) | 71 (41%) | 173 (97%) | 334 (53%) | ||
| Unable to deliver | 4 (2.2%) | 4 (0.6%) | |||||
| Missing | 1 | 1 | 0 | 0 | 2 | ||
| Medication reconciliation | Discharge | No | 109 (99.1%) | 111 (74%) | 156 (95%) | 4 (2.5%) | 380 (65%) |
| Yes | 1 (0.9%) | 38 (26%) | 8 (4.9%) | 139 (85%) | 186 (32%) | ||
| Unable to deliver | 20 (12%) | 20 (3.4%) | |||||
| Missing | 7 | 12 | 8 | 15 | 42 | ||
| Phase 1 | Phase 2 | |||||
|---|---|---|---|---|---|---|
| Characteristic | Class/Statistic | Control (n = 117) | Intervention (n = 161) | Control (n = 172) | Intervention (n = 178) | Total (n = 628) |
| At least 1 medication recommendation | No | 82 (70%) | 135 (84%) | 111 (65%) | 12 (6.7%) | 340 (54%) |
| Yes | 35 (30%) | 26 (16%) | 61 (35%) | 166 (93%) | 288 (46%) | |
| Number of recommendations | mean (SD) | 1 (2) | 0 (1) | 1 (2) | 6 (4) | 2 (3) |
| Median (min, max) | 0 (0, 10) | 0 (0, 11) | 0 (0, 10) | 6 (0, 26) | 0 (0, 26) | |
| At least 1 Significant (Severity) medication recommendation | No | 88 (75%) | 143 (89%) | 122 (71%) | 49 (28%) | 402 (64%) |
| Yes | 29 (25%) | 18 (11%) | 50 (29%) | 129 (72%) | 226 (36%) | |
| Number of (Significant Severity) recommendations | mean (SD) | 1 (1) | 0 (1) | 1 (1) | 2 (2) | 1 (1) |
| Median (min, max) | 0 (0, 6) | 0 (0, 4) | 0 (0, 6) | 2 (0, 8) | 0 (0, 8) | |
| At least 1 medication recommendation—not due to error (severity 5) | No | 103 (88%) | 153 (95%) | 138 (80%) | 27 (15%) | 421 (67%) |
| Yes | 14 (12%) | 8 (5.0%) | 34 (20%) | 151 (85%) | 207 (33%) | |
| Number of medications not due to error | mean (SD) | 0 (1) | 0 (1) | 0 (1) | 3 (3) | 1 (2) |
| Median (min, max) | 0 (0, 5) | 0 (0, 11) | 0 (0, 9) | 3 (0, 23) | 0 (0, 23) | |
| At least 1 Relevant (Impact) medication recommendation | No | 87 (74%) | 143 (89%) | 116 (67%) | 18 (10%) | 364 (58%) |
| Yes | 30 (26%) | 18 (11%) | 56 (33%) | 160 (90%) | 264 (42%) | |
| Number of Relevant (Impact) recommendations | mean (SD) | 1 (1) | 0 (1) | 1 (2) | 4 (3) | 2 (3) |
| Median (min, max) | 0 (0, 5) | 0 (0, 4) | 0 (0, 9) | 4 (0, 24) | 0 (0, 24) | |
| Phase 1 | Phase 2 | |||||
|---|---|---|---|---|---|---|
| Characteristic | Class/Statistic | Control (n = 211) | Intervention (n = 196) | Control (n = 316) | Intervention (n = 1066) | Total (n = 1789) |
| Significant (Severity) medication recommendation | No | 46 (36%) | 29 (49%) | 102 (50%) | 725 (69%) | 902 (62%) |
| Yes | 83 (64%) | 30 (51%) | 103 (50%) | 329 (31%) | 545 (38%) | |
| Missing | 82 | 137 | 111 | 12 | 342 | |
| Severity of the prescription error | 2. Serious | 10 (7.8%) | 10 (17%) | 12 (5.9%) | 51 (4.8%) | 83 (5.7%) |
| 3. Significant | 73 (57%) | 20 (34%) | 91 (44%) | 278 (26%) | 462 (32%) | |
| 4. Least | 19 (15%) | 7 (12%) | 20 (9.8%) | 104 (9.9%) | 150 (10%) | |
| 5. No error | 27 (21%) | 22 (37%) | 82 (40%) | 621 (59%) | 752 (52%) | |
| Missing | 82 | 137 | 111 | 12 | 342 | |
| Relevant (Impact) medication recommendation | No | 56 (43%) | 32 (52%) | 63 (31%) | 324 (31%) | 475 (33%) |
| Yes | 73 (57%) | 29 (48%) | 142 (69%) | 730 (69%) | 974 (67%) | |
| Missing | 82 | 135 | 111 | 12 | 340 | |
| Impact of the service provided by the pharmacist | 1. Extremely significant | 3 (0.3%) | 3 (0.2%) | |||
| 2. Highly significant | 5 (3.9%) | 11 (18%) | 11 (5.4%) | 99 (9.4%) | 126 (8.7%) | |
| 3. Significant | 68 (53%) | 18 (30%) | 131 (64%) | 628 (60%) | 845 (58%) | |
| 4. Little significant | 15 (12%) | 6 (9.8%) | 10 (4.9%) | 35 (3.3%) | 66 (4.6%) | |
| 5. Insignificant | 40 (31%) | 26 (43%) | 53 (26%) | 289 (27%) | 408 (28%) | |
| 6. Injurious Intervention | 1 (0.8%) | 1 (0.1%) | ||||
| Missing | 82 | 135 | 111 | 12 | 340 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kable, A.; Fraser, S.; Fullerton, A.; Hullick, C.; Palazzi, K.; Oldmeadow, C.; Pond, C.D.; Searles, A.; Ling, R.; Bruce, R.; et al. Evaluation of the Effect of a Safe Medication Strategy on Potentially Inappropriate Medications, Polypharmacy and Anticholinergic Burden for People with Dementia: An Intervention Study. Healthcare 2023, 11, 2771. https://doi.org/10.3390/healthcare11202771
Kable A, Fraser S, Fullerton A, Hullick C, Palazzi K, Oldmeadow C, Pond CD, Searles A, Ling R, Bruce R, et al. Evaluation of the Effect of a Safe Medication Strategy on Potentially Inappropriate Medications, Polypharmacy and Anticholinergic Burden for People with Dementia: An Intervention Study. Healthcare. 2023; 11(20):2771. https://doi.org/10.3390/healthcare11202771
Chicago/Turabian StyleKable, Ashley, Samantha Fraser, Anne Fullerton, Carolyn Hullick, Kerrin Palazzi, Christopher Oldmeadow, Constance Dimity Pond, Andrew Searles, Rod Ling, Remia Bruce, and et al. 2023. "Evaluation of the Effect of a Safe Medication Strategy on Potentially Inappropriate Medications, Polypharmacy and Anticholinergic Burden for People with Dementia: An Intervention Study" Healthcare 11, no. 20: 2771. https://doi.org/10.3390/healthcare11202771
APA StyleKable, A., Fraser, S., Fullerton, A., Hullick, C., Palazzi, K., Oldmeadow, C., Pond, C. D., Searles, A., Ling, R., Bruce, R., Murdoch, W., & Attia, J. (2023). Evaluation of the Effect of a Safe Medication Strategy on Potentially Inappropriate Medications, Polypharmacy and Anticholinergic Burden for People with Dementia: An Intervention Study. Healthcare, 11(20), 2771. https://doi.org/10.3390/healthcare11202771







