The Epidemiological Analysis of COVID-19 Outbreaks in Nursing Homes during the Period of Omicron Variant Predominance
Abstract
:1. Introduction
2. Methods
2.1. Study Design
2.2. Setting
2.3. Study Population
2.4. Epidemiological Survey
2.5. Study Variables
2.6. Data Sources and Measurement
2.7. Bias
2.8. Statistical Analysis
3. Results
3.1. Descriptive Data
3.2. Outcome Data
3.3. Main Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard with Vaccination Data; World Health Organization: Geneva, Switzerland, 2023; Available online: https://covid19.who.int/ (accessed on 16 September 2023).
- World Health Organization. Historical Working Definitions and Primary Actions for SARS-CoV-2 Variants; World Health Organization: Geneva, Switzerland, 2023; Available online: https://www.who.int/publications/m/item/historical-working-definitions-and-primary-actions-for-sars-cov-2-variants (accessed on 1 August 2023).
- Viana, R.; Moyo, S.; Amoako, D.G.; Tegally, H.; Scheepers, C.; Althaus, C.L.; Anyaneji, U.J.; Bester, P.A.; Boni, M.F.; Chand, M.; et al. Rapid epidemic expansion of the SARS-CoV-2 Omicron variant in southern Africa. Nature 2022, 603, 679–686. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Enhancing Response to Omicron SARS-CoV-2 Variant: Technical Brief and Priority Actions for Member States; World Health Organization: Geneva, Switzerland, 2022; Available online: https://www.who.int/publications/m/item/enhancing-readiness-for-omicron-(b.1.1.529)-technical-brief-and-priority-actions-for-member-states (accessed on 1 August 2023).
- McGarry, B.E.; Grabowski, D.C. Nursing Homes and COVID-19: A Crisis on Top of a Crisis. Ann. Am. Acad. Pol. Soc. Sci. 2021, 698, 137–162. [Google Scholar] [CrossRef]
- Aalto, U.L.; Pitkälä, K.H.; Andersen-Ranberg, K.; Bonin-Guillaume, S.; Cruz-Jentoft, A.J.; Eriksdotter, M.; Gordon, A.L.; Gosch, M.; Holmerova, I.; Kautiainen, H.; et al. COVID-19 pandemic and mortality in nursing homes across USA and Europe up to October 2021. Eur. Geriatr. Med. 2022, 13, 705–709. [Google Scholar] [CrossRef]
- Dyer, A.H.; Fallon, A.; Noonan, C.; Dolphin, H.; O’Farrelly, C.; Bourke, N.M.; O’Neill, D.; Kennelly, S.P. Managing the Impact of COVID-19 in Nursing Homes and Long-Term Care Facilities: An Update. J. Am. Med. Dir. Assoc. 2022, 23, 1590–1602. [Google Scholar] [CrossRef] [PubMed]
- Nealon, J.; Cowling, B.J. Omicron severity: Milder but not mild. Lancet 2022, 399, 412–413. [Google Scholar] [CrossRef] [PubMed]
- Parohan, M.; Yaghoubi, S.; Seraji, A.; Javanbakht, M.H.; Sarraf, P.; Djalali, M. Risk factors for mortality in patients with Coronavirus disease 2019 (COVID-19) infection: A systematic review and meta-analysis of observational studies. Aging Male 2020, 23, 1416–1424. [Google Scholar] [CrossRef] [PubMed]
- Flook, M.; Jackson, C.; Vasileiou, E.; Simpson, C.R.; Muckian, M.D.; Agrawal, U.; McCowan, C.; Jia, Y.; Murray, J.L.K.; Ritchie, L.D.; et al. Informing the public health response to COVID-19: A systematic review of risk factors for disease, severity, and mortality. BMC Infect. Dis. 2021, 21, 342. [Google Scholar] [CrossRef]
- da Costa, J.C.; Manso, M.C.; Gregório, S.; Leite, M.; Pinto, J.M. Barthel’s Index: A Better Predictor for COVID-19 Mortality Than Comorbidities. Tuberc. Respir. Dis. 2022, 85, 349–357. [Google Scholar] [CrossRef]
- CDC COVID-19 Response Team. Characteristics of Health Care Personnel with COVID-19—United States, February 12–April 9, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 477–481. [Google Scholar] [CrossRef]
- Hughes, M.M.; Groenewold, M.R.; Lessem, S.E.; Xu, K.; Ussery, E.N.; Wiegand, R.E.; Qin, X.; Do, T.; Thomas, D.; Tsai, S.; et al. Update: Characteristics of Health Care Personnel with COVID-19—United States, February 12–July 16, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1364–1368. [Google Scholar] [CrossRef]
- Ferland, L.; Carvalho, C.; Gomes Dias, J.; Lamb, F.; Adlhoch, C.; Suetens, C.; Beauté, J.; Kinross, P.; Plachouras, D.; Hannila-Handelberg, T.; et al. Risk of hospitalization and death for healthcare workers with COVID-19 in nine European countries, January 2020–January 2021. J. Hosp. Infect. 2022, 119, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Yang, S.C.; Jang, J.; Park, S.Y.; Kim, S.S.; Kim, C.; Kwon, D.; Lee, S.W. COVID-19 Cases and Deaths among Healthcare Personnel with the Progression of the Pandemic in Korea from March 2020 to February 2022. Trop. Med. Infect. Dis. 2023, 8, 308. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Interim Infection Prevention and Control Recommendations for Healthcare Personnel during the Coronavirus Disease 2019 (COVID-19) Pandemic. Available online: https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html (accessed on 20 September 2023).
- Li, L. Challenges and Priorities in Responding to COVID-19 in Inpatient Psychiatry. Psychiatr. Serv. 2020, 71, 624–626. [Google Scholar] [CrossRef]
- Bojdani, E.; Rajagopalan, A.; Chen, A.; Gearin, P.; Olcott, W.; Shankar, V.; Cloutier, A.; Solomon, H.; Naqvi, N.Z.; Batty, N.; et al. COVID-19 Pandemic: Impact on psychiatric care in the United States. Psychiatry Res. 2020, 289, 113069. [Google Scholar] [CrossRef]
- Stratil, J.M.; Biallas, R.L.; Burns, J.; Arnold, L.; Geffert, K.; Kunzler, A.M.; Monsef, I.; Stadelmaier, J.; Wabnitz, K.; Litwin, T.; et al. Non-pharmacological measures implemented in the setting of long-term care facilities to prevent SARS-CoV-2 infections and their consequences: A rapid review. Cochrane Database Syst. Rev. 2021, 9, CD015085. [Google Scholar] [PubMed]
- Telford, C.T.; Onwubiko, U.; Holland, D.P.; Turner, K.; Prieto, J.; Smith, S.; Yoon, J.; Brown, W.; Chamberlain, A.; Gandhi, N.; et al. Preventing COVID-19 Outbreaks in Long-Term Care Facilities Through Preemptive Testing of Residents and Staff Members—Fulton County, Georgia, March–May 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1296–1299. [Google Scholar] [CrossRef] [PubMed]
- McGarry, B.E.; Gandhi, A.D.; Barnett, M.L. Covid-19 Surveillance Testing and Resident Outcomes in Nursing Homes. N. Engl. J. Med. 2023, 388, 1101–1110. [Google Scholar] [CrossRef]
- Shao, P.L.; Tu, H.C.; Gong, Y.N.; Shu, H.Y.; Kirby, R.; Hsu, L.Y.; Yeo, H.Y.; Kuo, H.Y.; Huang, Y.C.; Lin, Y.F.; et al. Emergence and Persistent Dominance of SARS-CoV-2 Omicron BA.2.3.7 Variant, Taiwan. Emerg. Infect. Dis. 2023, 29, 792–796. [Google Scholar] [CrossRef]
- Dykgraaf, S.H.; Matenge, S.; Desborough, J.; Sturgiss, E.; Dut, G.; Roberts, L.; McMillan, A.; Kidd, M. Protecting Nursing Homes and Long-Term Care Facilities From COVID-19: A Rapid Review of International Evidence. J. Am. Med. Dir. Assoc. 2021, 22, 1969–1988. [Google Scholar] [CrossRef]
- National Center for High-Performance Computing. COVID-19 Dashboard. Available online: https://covid-19.nchc.org.tw/2023_town_confirmed.php?mycity=%E5%8D%97%E6%8A%95%E7%B8%A3&mytown=%E5%9F%94%E9%87%8C%E9%8E%AE (accessed on 16 September 2023).
- Fryar, C.D.; Ostchega, Y.; Hales, C.M.; Zhang, G.; Kruszon-Moran, D. Hypertension Prevalence and Control Among Adults: United States, 2015–2016; NCHS Data Brief; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2017; pp. 1–8. [Google Scholar]
- Fagundes, D.F.; Costa, M.T.; Alves, B.; Carneiro, L.S.F.; Nascimento, O.J.M.; Leão, L.L.; Guimarães, A.L.S.; de Paula, A.M.B.; Monteiro-Junior, R.S. Dementia among older adults living in long-term care facilities: An epidemiological study. Dement. Neuropsychol. 2021, 15, 464–469. [Google Scholar] [CrossRef]
- Wang, L.; Li, X.; Wang, Z.; Bancks, M.P.; Carnethon, M.R.; Greenland, P.; Feng, Y.-Q.; Wang, H.; Zhong, V.W. Trends in Prevalence of Diabetes and Control of Risk Factors in Diabetes Among US Adults, 1999–2018. JAMA 2021, 326, 704–716. [Google Scholar] [CrossRef] [PubMed]
- Tilert, T.; Dillon, C.; Paulose-Ram, R.; Hnizdo, E.; Doney, B. Estimating the U.S. prevalence of chronic obstructive pulmonary disease using pre- and post-bronchodilator spirometry: The National Health and Nutrition Examination Survey (NHANES) 2007–2010. Respir. Res. 2013, 14, 103. [Google Scholar] [CrossRef] [PubMed]
- Gold, M.E.; Nanna, M.G.; Doerfler, S.M.; Schibler, T.; Wojdyla, D.; Peterson, E.D.; Navar, A.M. Prevalence, treatment, and control of severe hyperlipidemia. Am. J. Prev. Cardiol. 2020, 3, 100079. [Google Scholar] [CrossRef] [PubMed]
- Trevissón-Redondo, B.; López-López, D.; Pérez-Boal, E.; Marqués-Sánchez, P.; Liébana-Presa, C.; Navarro-Flores, E.; Jiménez-Fernández, R.; Corral-Liria, I.; Losa-Iglesias, M.; Becerro-de-Bengoa-Vallejo, R. Use of the Barthel Index to Assess Activities of Daily Living before and after SARS-COVID 19 Infection of Institutionalized Nursing Home Patients. Int. J. Environ. Res. Public Health 2021, 18, 7258. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Cazer, C.L.; Clarkberg, M.E.; Henderson, S.G.; Lee, S.E.; Meredith, G.R.; Osman, M.; Shmoys, D.B.; Frazier, P.I. Booster vaccination protection against SARS-CoV-2 infections in young adults during an Omicron BA.1-predominant period: A retrospective cohort study. PLoS Med. 2023, 20, e1004153. [Google Scholar] [CrossRef]
- Ulloa, A.C.; Buchan, S.A.; Daneman, N.; Brown, K.A. Estimates of SARS-CoV-2 Omicron Variant Severity in Ontario, Canada. JAMA 2022, 327, 1286–1288. [Google Scholar] [CrossRef]
- Wolter, N.; Jassat, W.; Walaza, S.; Welch, R.; Moultrie, H.; Groome, M.; Amoako, D.G.; Everatt, J.; Bhiman, J.N.; Scheepers, C.; et al. Early assessment of the clinical severity of the SARS-CoV-2 omicron variant in South Africa: A data linkage study. Lancet 2022, 399, 437–446. [Google Scholar] [CrossRef]
- Amodio, E.; Vella, G.; Restivo, V.; Casuccio, A.; Vitale, F.; on behalf of the COVID-19 Surveillance Working Group of the University of Palermo. Effectiveness of mRNA COVID-19 Vaccination on SARS-CoV-2 Infection and COVID-19 in Sicily over an Eight-Month Period. Vaccines 2022, 10, 426. [Google Scholar] [CrossRef]
- Shrotri, M.; Krutikov, M.; Nacer-Laidi, H.; Azmi, B.; Palmer, T.; Giddings, R.; Fuller, C.; Irwin-Singer, A.; Baynton, V.; Tut, G.; et al. Duration of vaccine effectiveness against SARS-CoV-2 infection, hospitalisation, and death in residents and staff of long-term care facilities in England (VIVALDI): A prospective cohort study. Lancet Healthy Longev. 2022, 3, e470–e480. [Google Scholar] [CrossRef]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Rickeard, T.; Gallagher, E.; Gower, C.; Kall, M.; Groves, N.; O’Connell, A.M.; et al. COVID-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N. Engl. J. Med. 2022, 386, 1532–1546. [Google Scholar] [CrossRef]
- Brown, K.A.; Jones, A.; Daneman, N.; Chan, A.K.; Schwartz, K.L.; Garber, G.E.; Costa, A.P.; Stall, N.M. Association Between Nursing Home Crowding and COVID-19 Infection and Mortality in Ontario, Canada. JAMA Intern. Med. 2021, 181, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Leece, P.; Whelan, M.; Costa, A.P.; Daneman, N.; Johnstone, J.; McGeer, A.; Rochon, P.; Schwartz, K.L.; Brown, K.A. Nursing home crowding and its association with outbreak-associated respiratory infection in Ontario, Canada before the COVID-19 pandemic (2014-19): A retrospective cohort study. Lancet Healthy Longev. 2023, 4, e107–e114. [Google Scholar] [CrossRef] [PubMed]
- Zoghbi, M.; Haddad, C.; Khansa, W.; Karam, E.; Chamoun, A.; Hachem, D. COVID-19 outbreak in a psychiatric hospital: What makes it worse? Ann. Gen. Psychiatry 2022, 21, 26. [Google Scholar] [CrossRef] [PubMed]
- Kosar, C.M.; White, E.M.; Feifer, R.A.; Blackman, C.; Gravenstein, S.; Panagiotou, O.A.; McConeghy, K.; Mor, V. COVID-19 Mortality Rates Among Nursing Home Residents Declined From March To November 2020. Health Aff. 2021, 40, 655–663. [Google Scholar] [CrossRef]
- Shang, W.; Kang, L.; Cao, G.; Wang, Y.; Gao, P.; Liu, J.; Liu, M. Percentage of Asymptomatic Infections among SARS-CoV-2 Omicron Variant-Positive Individuals: A Systematic Review and Meta-Analysis. Vaccines 2022, 10, 1049. [Google Scholar] [CrossRef]
- Dinnes, J.; Sharma, P.; Berhane, S.; van Wyk, S.S.; Nyaaba, N.; Domen, J.; Taylor, M.; Cunningham, J.; Davenport, C.; Dittrich, S.; et al. Rapid, point-of-care antigen tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst. Rev. 2022, 7, CD013705. [Google Scholar]
- Yang, H.; Rigsby, M.; Zhu, X.; Lee, C.; Ory, M. COVID-19 in Long-Term Care Facilities: A Rapid Review of Infection Correlates and Impacts on Mental Health and Behaviors. Herd 2022, 15, 277–294. [Google Scholar] [CrossRef]
- Horita, N.; Fukumoto, T. Global case fatality rate from COVID-19 has decreased by 96.8% during 2.5 years of the pandemic. J. Med. Virol. 2023, 95, e28231. [Google Scholar] [CrossRef]
- Mehta, H.B.; Li, S.; Goodwin, J.S. Risk Factors Associated with SARS-CoV-2 Infections, Hospitalization, and Mortality among US Nursing Home Residents. JAMA Netw. Open 2021, 4, e216315. [Google Scholar] [CrossRef]
- Cazzoletti, L.; Zanolin, M.E.; Tocco Tussardi, I.; Alemayohu, M.A.; Zanetel, E.; Visentin, D.; Fabbri, L.; Giordani, M.; Ruscitti, G.; Benetollo, P.P.; et al. Risk Factors Associated with Nursing Home COVID-19 Outbreaks: A Retrospective Cohort Study. Int. J. Environ. Res. Public Health 2021, 18, 8434. [Google Scholar] [CrossRef]
- Levin, A.T.; Jylhävä, J.; Religa, D.; Shallcross, L. COVID-19 prevalence and mortality in longer-term care facilities. Eur. J. Epidemiol. 2022, 37, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Veronese, N.; Custodero, C.; Cella, A.; Demurtas, J.; Zora, S.; Maggi, S.; Barbagallo, M.; Sabbà, C.; Ferrucci, L.; Pilotto, A. Prevalence of multidimensional frailty and pre-frailty in older people in different settings: A systematic review and meta-analysis. Ageing Res. Rev. 2021, 72, 101498. [Google Scholar] [CrossRef] [PubMed]
- Panagiotou, O.A.; Kosar, C.M.; White, E.M.; Bantis, L.E.; Yang, X.; Santostefano, C.M.; Feifer, R.A.; Blackman, C.; Rudolph, J.L.; Gravenstein, S.; et al. Risk Factors Associated with All-Cause 30-Day Mortality in Nursing Home Residents with COVID-19. JAMA Intern. Med. 2021, 181, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Adjei, S.; Hong, K.; Molinari, N.M.; Bull-Otterson, L.; Ajani, U.A.; Gundlapalli, A.V.; Harris, A.M.; Hsu, J.; Kadri, S.S.; Starnes, J.; et al. Mortality Risk Among Patients Hospitalized Primarily for COVID-19 during the Omicron and Delta Variant Pandemic Periods—United States, April 2020–June 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 1182–1189. [Google Scholar] [CrossRef]
- Shallcross, L.; Burke, D.; Abbott, O.; Donaldson, A.; Hallatt, G.; Hayward, A.; Hopkins, S.; Krutikov, M.; Sharp, K.; Wardman, L.; et al. Factors associated with SARS-CoV-2 infection and outbreaks in long-term care facilities in England: A national cross-sectional survey. Lancet Healthy Longev. 2021, 2, e129–e142. [Google Scholar] [CrossRef] [PubMed]
- Blain, H.; Rolland, Y.; Tuaillon, E.; Giacosa, N.; Albrand, M.; Jaussent, A.; Benetos, A.; Miot, S.; Bousquet, J. Efficacy of a Test-Retest Strategy in Residents and Health Care Personnel of a Nursing Home Facing a COVID-19 Outbreak. J. Am. Med. Dir. Assoc. 2020, 21, 933–936. [Google Scholar] [CrossRef]
- Arons, M.M.; Hatfield, K.M.; Reddy, S.C.; Kimball, A.; James, A.; Jacobs, J.R.; Taylor, J.; Spicer, K.; Bardossy, A.C.; Oakley, L.P.; et al. Presymptomatic SARS-CoV-2 Infections and Transmission in a Skilled Nursing Facility. N. Engl. J. Med. 2020, 382, 2081–2090. [Google Scholar] [CrossRef]
- Sacco, G.; Foucault, G.; Briere, O.; Annweiler, C. COVID-19 in seniors: Findings and lessons from mass screening in a nursing home. Maturitas 2020, 141, 46–52. [Google Scholar] [CrossRef]
- Malikov, K.; Huang, Q.; Shi, S.; Stall, N.M.; Tuite, A.R.; Hillmer, M.P. Temporal Associations between Community Incidence of COVID-19 and Nursing Home Outbreaks in Ontario, Canada. J. Am. Med. Dir. Assoc. 2021, 22, 260–262. [Google Scholar] [CrossRef]
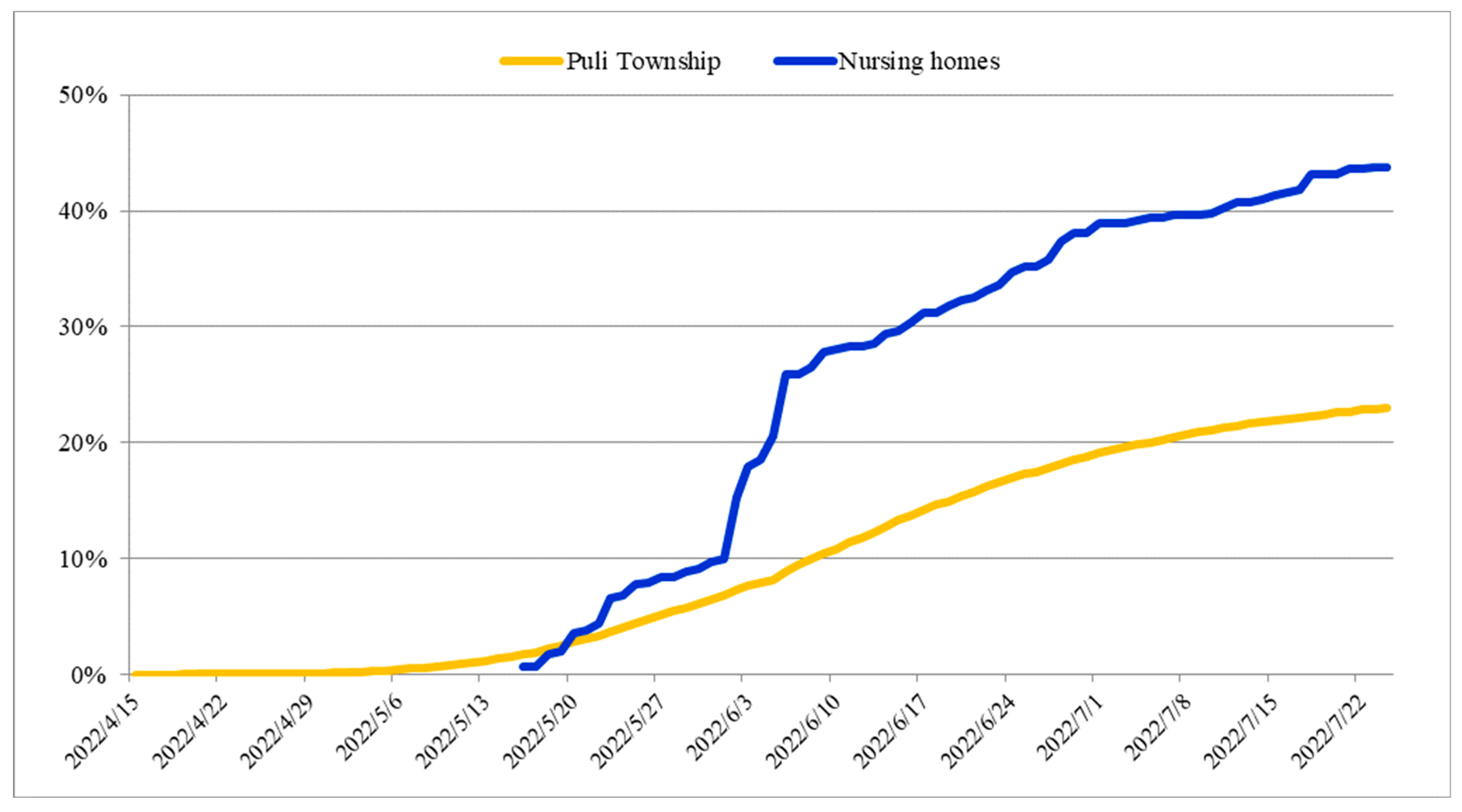

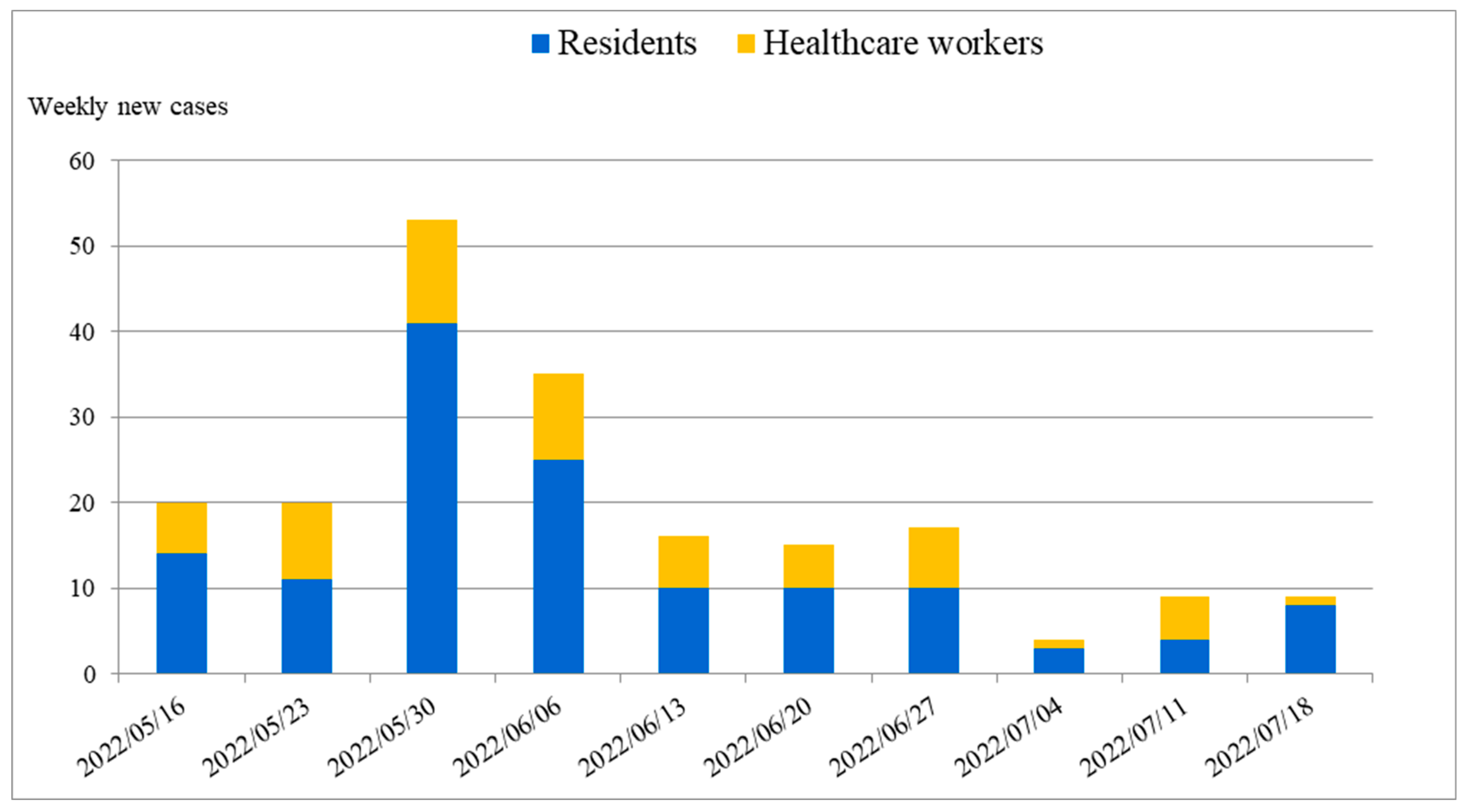
| Unit B | Unit F | Unit P | Total | |
|---|---|---|---|---|
| Space for all residents | ||||
| Number of residents | 87 | 184 | 56 | 327 |
| Total space (m2) | 3289.6 | 5485.5 | 2374.7 | 11,149.8 |
| Average space (m2) per resident | 37.8 | 29.8 | 42.4 | 34.1 |
| Space for residents dining together | ||||
| No. of residents dining together | 11 | 22 | 56 | 89 |
| Space of public dining area (m2) | 334.7 | 712.7 | 230.9 | 1278.2 |
| Average public dining space (m2) per resident | 30.4 | 32.4 | 4.1 | 14.4 |
| Unit B N = 87 | Unit F n = 184 | Unit P n = 56 | Total n = 327 | p | |
|---|---|---|---|---|---|
| Gender | 0.023 | ||||
| Male | 78 (89.7%) | 142 (77.2%) | 41 (73.2%) | 261 (79.8%) | |
| Female | 9 (10.3%) | 42 (22.8%) | 15 (26.8%) | 66 (20.2%) | |
| Age (mean ± SD) | 76.5 ± 17.3 | 77.5 ± 15.3 | 74.5 ± 14.9 | 76.2 ± 15.4 | 0.587 |
| Consent to a DNR order | 52 (59.8%) | 110 (59.8%) | 9 (16.1%) | 171 (52.3%) | <0.001 |
| Medical supports | |||||
| Tube feeding | 57 (65.5%) | 81 (44%) | 0 (0%) | 138 (42.2%) | <0.001 |
| Artificial airway | 10 (11.5%) | 4 (2.2%) | 0 (0%) | 14 (4.3%) | <0.001 |
| Dependency of ADL | <0.001 | ||||
| Total dependency | 68 (78.2%) | 130 (70.7%) | 5 (8.9%) | 203 (62.1%) | <0.001 |
| Severe dependency | 10 (11.5%) | 34 (18.5%) | 23 (41.1%) | 67 (20.5%) | <0.001 |
| Moderate dependency | 5 (5.7%) | 15 (8.2%) | 18 (32.1%) | 38 (11.6%) | <0.001 |
| Mild dependency | 2 (2.3%) | 4 (2.2%) | 5 (8.9%) | 11 (3.4%) | NA |
| Independent | 2 (2.3%) | 1 (0.5%) | 5 (8.9%) | 8 (2.4%) | NA |
| Underlying diseases | |||||
| Hypertension | 47 (54%) | 97 (52.7%) | 33 (58.9%) | 177 (54.1%) | 0.716 |
| Dementia | 33 (37.9%) | 63 (34.2%) | 41 (73.2%) | 137 (41.9%) | <0.001 |
| Cerebral vascular disease | 43 (49.4%) | 74 (40.2%) | 19 (33.9%) | 136 (41.6%) | 0.158 |
| Diabetes mellitus | 38 (43.7%) | 56 (30.4%) | 15 (26.8%) | 109 (33.3%) | 0.051 |
| Chronic kidney disease | 22 (25.3%) | 31 (16.8%) | 11 (19.6%) | 64 (19.6%) | 0.263 |
| Chronic pulmonary disease | 16 (18.4%) | 30 (16.3%) | 11 (19.6%) | 57 (17.4%) | 0.815 |
| Heart failure | 17 (19.5%) | 23 (12.5%) | 4 (7.1%) | 44 (13.5%) | 0.090 |
| Hyperlipidemia | 14 (16.1%) | 19 (10.3%) | 8 (14.3%) | 41 (12.5%) | 0.372 |
| Coronary artery disease | 17 (19.5%) | 20 (10.9%) | 2 (3.6%) | 39 (11.9%) | 0.012 |
| Advanced malignancy | 6 (6.9%) | 15 (8.2%) | 3 (5.4%) | 24 (7.3%) | 0.878 |
| End-stage renal disease | 4 (4.6%) | 6 (3.3%) | 2 (3.6%) | 12 (3.7%) | 0.919 |
| Chronic liver disease | 0 (0%) | 5 (2.7%) | 2 (3.6%) | 7 (2.1%) | 0.235 |
| Registered Nurses (n = 28) | Nursing Assistants (n = 59) | Social Workers (n = 3) | Housekeepers and Porters (n = 7) | Clerks (n = 4) | Total (n = 101) | |
|---|---|---|---|---|---|---|
| Gender | ||||||
| Female | 27 (96.4%) | 50 (84.7%) | 1 (33.3%) | 3 (42.9%) | 4 (100%) | 85 (84.2%) |
| Male | 1 (3.6) | 9 (15.3%) | 2 (66.7%) | 4 (57.1%) | 0 (0%) | 16 (15.8%) |
| Age | 39.2 ± 9.2 | 42.5 ± 12.6 | 40.3 ± 12.7 | 58 ± 5.6 | 34 ± 3.9 | 42.3 ± 11.9 |
| Underlying diseases | ||||||
| Diabetes mellitus | 1 (3.6%) | 4 (6.8%) | 0 (0%) | 2 (28.6%) | 0 (0%) | 7 (6.9%) |
| Hypertension | 3 (10.7%) | 11 (18.6%) | 0 (0%) | 2 (28.6%) | 0 (0%) | 16 (15.8%) |
| Chronic liver disease | 0 | 2 | 0 | 0 | 0 | 2 (2.0%) |
| Cancer | 1 (3.6%) | 0 | 0 | 0 | 0 | 1 (1%) |
| Congestive heart failure | 0 | 0 | 0 | 0 | 0 | 0 (0%) |
| COPD | 0 | 1 | 0 | 0 | 0 | 1 (1%) |
| Hyperlipidemia | NA | NA | NA | NA | NA | NA |
| Chronic kidney disease | NA | NA | NA | NA | NA | NA |
| Unit B | Unit F | Unit P | Total | p | |
|---|---|---|---|---|---|
| Residents | |||||
| Number of residents | 87 | 184 | 56 | 327 | |
| Vaccination rate | 53 (60.9%) | 127 (69.0%) | 50 (89.3%) | 230 (70.3%) | 0.001 |
| Average days between a booster dose and COVID-19 infection | 80.9 ± 29.1 | 97.7 ± 26.1 | 134.5 ± 22.8 | 101.8 ± 32.0 | NA |
| Asymptomatic residents with positive COVID-19 test | 7 (8.0%) | 25 (13.6%) | 22 (39.3%) | 54 (16.5%) | <0.001 |
| Infection rate | 22 (25.3%) | 63 (34.2%) | 51 (91.1%) | 136 (41.6%) | <0.001 |
| Hospitalization | 13 (59.1%) | 18 (28.6%) | 13 (25.5%) | 44 (32.4%) | 0.013 |
| Case fatality rate | 2 (9.1%) | 9 (14.3%) | 3 (5.9%) | 14 (10.3%) | 0.355 |
| COVID-19-related mortality rate | 2 (2.3%) | 9 (4.9%) | 3 (5.4%) | 14 (4.3%) | 0.567 |
| Healthcare workers | |||||
| Number of HCWs | 38 | 66 | 25 | 129 | |
| Vaccination rate | 36 (94.7%) | 61 (92.4%) | 23 (92.0%) | 120 (93.0%) | 1.000 |
| Average days between a booster dose and COVID-19 infection | 110.5 ± 35.6 | 114.8 ± 61.6 | 125.7 ± 18.3 | 115.6 ± 48.7 | NA |
| Asymptomatic HCWs with positive COVID-19 test | 1 (2.6%) | 7 (10.6%) | 7 (28.0%) | 15 (11.6%) | 0.01 |
| Infection rate | 16 (42.1%) | 32 (48.5%) | 14 (56.0%) | 62 (48.1%) | 0.549 |
| Hospitalization | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| COVID-19-related mortality rate | 0 (%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Infected n = 136 | Not Infected n = 191 | Univariate HR (95% CI) | p Value | Multivariate HR (95% CI) | p Value | |
|---|---|---|---|---|---|---|
| Age (mean ± SD) | 76.2 ± 15.4 | 79.7 ± 13.8 | 0.99 (0.98–1.00) | 0.056 | 0.99 (0.98–1.00) | 0.149 |
| Gender | ||||||
| Male | 119 (87.5%) | 142 (74.3%) | 2.12 (1.27–3.52) | 0.004 | 2.46 (1.47–4.11) | 0.001 |
| Female | 17 (12.5%) | 49 (25.7%) | 1.00 (reference) | 1.00 (reference) | ||
| Dependency of ADL | ||||||
| Total dependency | 59 (43.4%) | 144 (75.4%) | 1.00 (reference) | 1.00 (reference) | ||
| Severe dependency | 43 (31.6%) | 24 (12.6%) | 2.70 (1.82–4.01) | <0.001 | 2.20 (1.40–3.47) | 0.001 |
| Other dependencies | 34 (25.0%) | 23 (12.0%) | 2.42 (1.59–3.70) | <0.001 | 1.93 (1.18–3.17) | 0.009 |
| Tube feeding | ||||||
| No | 98 (72.1%) | 92 (48.2%) | 1.00 (reference) | 1.00 (reference) | ||
| Yes | 38 (27.9%) | 99 (51.8%) | 0.48 (0.33–0.69) | <0.001 | 0.65 (0.41–1.02) | 0.063 |
| Dementia | ||||||
| No | 69 (50.7%) | 121 (63.4%) | 1.00 (reference) | 1.00 (reference) | ||
| Yes | 67 (49.3%) | 70 (36.6%) | 1.46 (1.04–2.05) | 0.027 | 1.61 (1.14–2.27) | 0.007 |
| Death n = 15 | Survival n = 121 | Univariate HR (95% CI) | p Value | Multivariate Adjusted-for-Age HR (95% CI) | p Value | |
|---|---|---|---|---|---|---|
| Age (mean ± SD) | 84.9 ± 11.2 | 75.1 ± 15.6 | 1.05 (1.01–1.10) | 0.026 | ||
| Consent to a DNR order | ||||||
| No | 4 (26.7%) | 70 (57.9%) | 1 (reference) | 1 (reference) | ||
| Yes | 11 (73.3%) | 51 (42.1%) | 3.41 (1.09–10.71) | 0.036 | 2.65 (0.83–8.49) | 0.100 |
| Pneumonia | ||||||
| No | 3 (20.0%) | 98 (81.0%) | 1 (reference) | 1 (reference) | ||
| Yes | 12 (80.0%) | 23 (19.0%) | 13.62 (3.84–48.33) | <0.001 | 11.03 (3.02–40.31) | <0.001 |
| Hospitalization | ||||||
| No | 3 (20.0%) | 88 (72.7%) | 1 (reference) | 1 (reference) | ||
| Yes | 12 (80.0%) | 33 (27.3%) | 9.11 (2.57–32.31) | 0.001 | 7.18 (1.97–26.25) | 0.003 |
| Intensive care | ||||||
| No | 10 (66.7%) | 120 (99.2%) | 1 (reference) | 1 (reference) | ||
| Yes | 5 (33.3%) | 1 (0.8%) | 12.36 (4.19–36.44) | <0.001 | 8.67 (2.79–26.89) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, J.C.-H.; Chang, Y.-Y.; Hsu, C.-Y.; Chen, H.-J.; Chan, F.-T.; Shi, Z.-Y. The Epidemiological Analysis of COVID-19 Outbreaks in Nursing Homes during the Period of Omicron Variant Predominance. Healthcare 2023, 11, 2868. https://doi.org/10.3390/healthcare11212868
Tsai JC-H, Chang Y-Y, Hsu C-Y, Chen H-J, Chan F-T, Shi Z-Y. The Epidemiological Analysis of COVID-19 Outbreaks in Nursing Homes during the Period of Omicron Variant Predominance. Healthcare. 2023; 11(21):2868. https://doi.org/10.3390/healthcare11212868
Chicago/Turabian StyleTsai, Jeffrey Che-Hung, Ying-Ying Chang, Chiann-Yi Hsu, Hui-Ju Chen, Feng-Tse Chan, and Zhi-Yuan Shi. 2023. "The Epidemiological Analysis of COVID-19 Outbreaks in Nursing Homes during the Period of Omicron Variant Predominance" Healthcare 11, no. 21: 2868. https://doi.org/10.3390/healthcare11212868
APA StyleTsai, J. C.-H., Chang, Y.-Y., Hsu, C.-Y., Chen, H.-J., Chan, F.-T., & Shi, Z.-Y. (2023). The Epidemiological Analysis of COVID-19 Outbreaks in Nursing Homes during the Period of Omicron Variant Predominance. Healthcare, 11(21), 2868. https://doi.org/10.3390/healthcare11212868








