Long-Term Follow-Up of Health-Related Quality of Life and Short-Term Intervention with CFTR Modulator Therapy in Adults with Cystic Fibrosis: Evaluation of Changes over Several Years with or without 33 Weeks of CFTR Modulator Therapy
Abstract
:1. Introduction
2. Methods
2.1. Patients and Study Design
2.1.1. Measurements
2.1.2. Anthropometric Characteristics and Lung Function
2.2. Health-Related Quality of Life
2.3. Statistics
3. Results
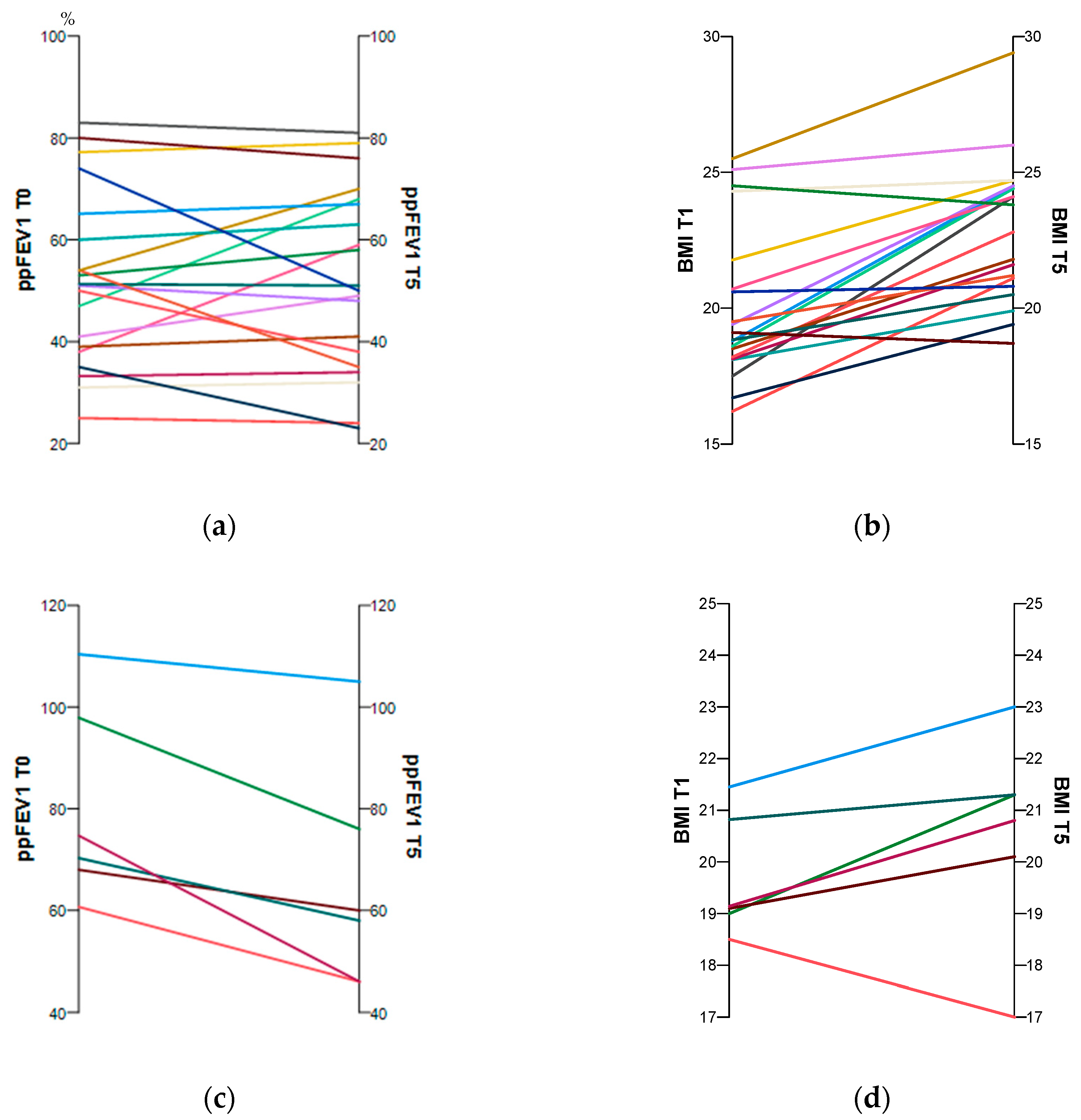
3.1. Anthropometric Characteristics and Lung Function with and without ETI Therapy
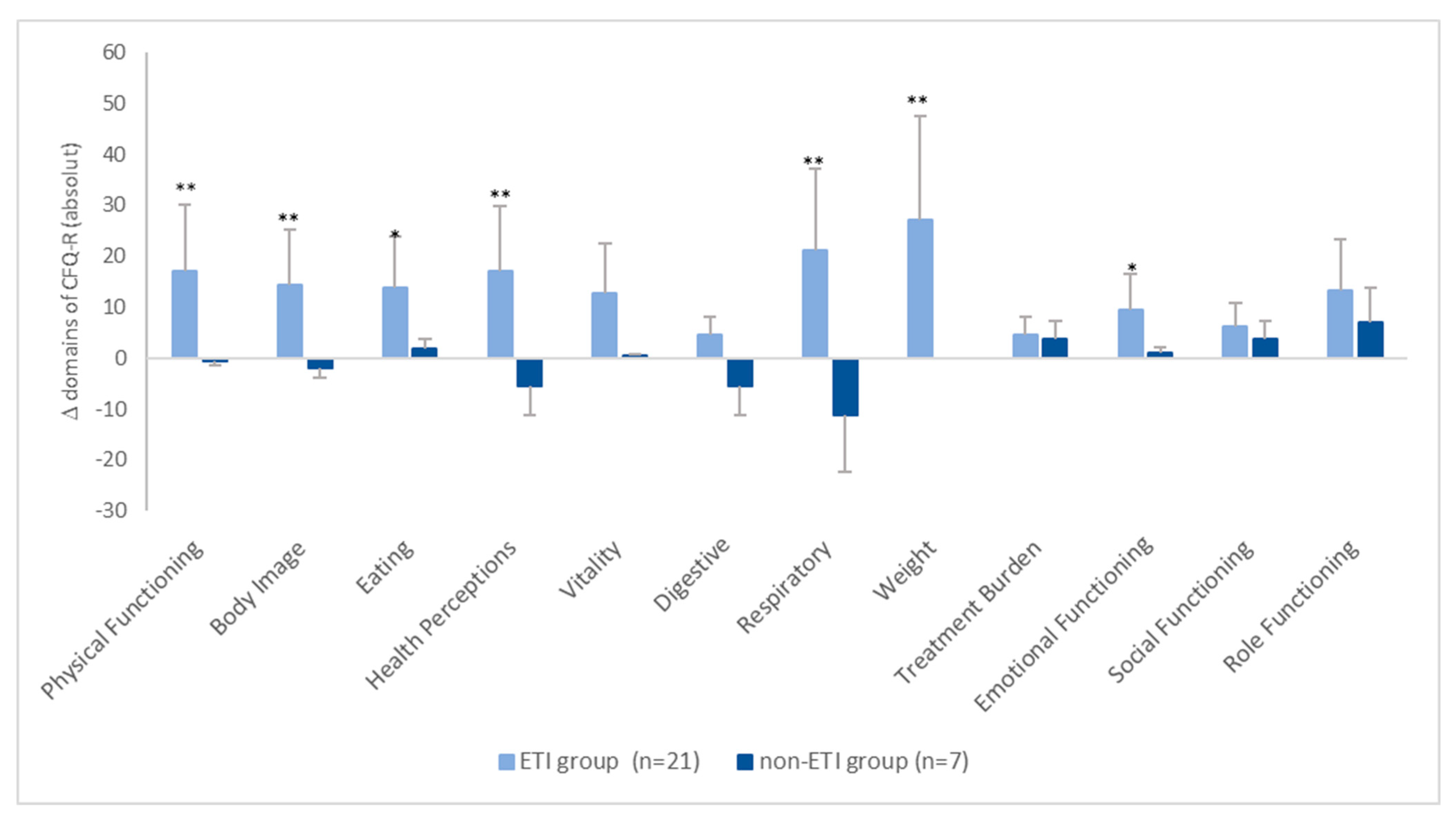
3.2. Health-Related Quality of Life
3.3. Correlation of HRQoL Domains with Clinical Outcome Parameters at Baseline (T0) and Follow-Up (T5) and Their Changes over Time
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shteinberg, M.; Haq, I.J.; Polineni, D.; Davies, J.C. Cystic fibrosis. Lancet 2021, 397, 2195–2211. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, K.M.; Collaco, J.M. Cystic Fibrosis. Pediatr. Rev. 2021, 42, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Quittner, A.L.; Saez-Flores, E.; Barton, J.D. The psychological burden of cystic fibrosis. Curr. Opin. Pulm. Med. 2016, 22, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Quittner, A.L.; Goldbeck, L.; Abbott, J.; Duff, A.; Lambrecht, P.; Solé, A.; Tibosch, M.M.; Brucefors, A.B.; Yüksel, H.; Catastini, P.; et al. Prevalence of depression and anxiety in patients with cystic fibrosis and parent caregivers: Results of The International Depression Epidemiological Study across nine countries. Thorax 2014, 69, 1090–1097. [Google Scholar] [CrossRef]
- Sutharsan, S.; McKone, E.F.; Downey, D.G.; Duckers, J.; MacGregor, G.; Tullis, E.; Van Braeckel, E.; Wainwright, C.E.; Watson, D.; Ahluwalia, N.; et al. Efficacy and safety of elexacaftor plus tezacaftor plus ivacaftor versus tezacaftor plus ivacaftor in people with cystic fibrosis homozygous for F508del-CFTR: A 24-week, multicentre, randomised, double-blind, active-controlled, phase 3b trial. Lancet Respir. Med. 2022, 10, 267–277. [Google Scholar] [CrossRef]
- Middleton, P.G.; Mall, M.A.; Dřevínek, P.; Lands, L.C.; McKone, E.F.; Polineni, D.; Ramsey, B.W.; Taylor-Cousar, J.L.; Tullis, E.; Vermeulen, F.; et al. Elexacaftor–Tezacaftor–Ivacaftor for Cystic Fibrosis with a Single Phe508del Allele. N. Engl. J. Med. 2019, 381, 1809–1819. [Google Scholar] [CrossRef] [PubMed]
- Heijerman, H.G.M.; McKone, E.F.; Downey, D.G.; Van Braeckel, E.; Rowe, S.M.; Tullis, E.; Mall, M.A.; Welter, J.J.; Ramsey, B.W.; McKee, C.M.; et al. Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: A double-blind, randomised, phase 3 trial. Lancet 2019, 394, 1940–1948. [Google Scholar] [CrossRef] [PubMed]
- Quittner, A.L. Measurement of quality of life in cystic fibrosis. Curr. Opin. Pulm. Med. 1998, 4, 326–331. [Google Scholar] [CrossRef]
- Quittner, A.L.; Buu, A.; Messer, M.A.; Modi, A.C.; Watrous, M. Development and Validation of the Cystic Fibrosis Questionnaire in the United States. Chest 2005, 128, 2347–2354. [Google Scholar] [CrossRef]
- Dill, E.J.; Dawson, R.; Sellers, D.E.; Robinson, W.M.; Sawicki, G.S. Longitudinal Trends in Health-Related Quality of Life in Adults with Cystic Fibrosis. Chest 2013, 144, 981–989. [Google Scholar] [CrossRef]
- Ancel, J.; Launois, C.; Perotin, J.-M.; Ravoninjatovo, B.; Mulette, P.; Hagenburg, J.; Malet, J.; Griffon, M.; Carré, S.; Lebargy, F.; et al. Health-Related Quality of Life in Adults with Cystic Fibrosis: Familial, Occupational, Social, and Mental Health Predictors. Healthcare 2022, 10, 1351. [Google Scholar] [CrossRef] [PubMed]
- Cronly, J.A.; Duff, A.J.; A Riekert, K.; Fitzgerald, A.P.; Perry, I.J.; A Lehane, E.; Horgan, A.; A Howe, B.; Ni Chroinin, M.; Savage, E. Health-Related Quality of Life in Adolescents and Adults with Cystic Fibrosis: Physical and Mental Health Predictors. Respir. Care 2019, 64, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Aspinall, S.A.; Mackintosh, K.A.; Hill, D.M.; Cope, B.; McNarry, M.A. Evaluating the Effect of Kaftrio on Perspectives of Health and Wellbeing in Individuals with Cystic Fibrosis. Int. J. Environ. Res. Public Health 2022, 19, 6114. [Google Scholar] [CrossRef] [PubMed]
- Nichols, D.P.; Paynter, A.C.; Heltshe, S.L.; Donaldson, S.H.; Frederick, C.A.; Freedman, S.D.; Gelfond, D.; Hoffman, L.R.; Kelly, A.; Narkewicz, M.R.; et al. Clinical Effectiveness of Elexacaftor/Tezacaftor/Ivacaftor in People with Cystic Fibrosis: A Clinical Trial. Am. J. Respir. Crit. Care Med. 2022, 205, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Carnovale, V.; Iacotucci, P.; Terlizzi, V.; Colangelo, C.; Medio, P.; Ferrillo, L.; De Gregorio, F.; Francalanci, M.; Taccetti, G.; Buonaurio, S.; et al. Effectiveness and safety of elexacaftor/tezacaftor/ivacaftor in patients with cystic fibrosis and advanced lung disease with the Phe508del/minimal function genotype. Respir. Med. 2021, 189, 106646. [Google Scholar] [CrossRef] [PubMed]
- DiMango, E.; Spielman, D.B.; Overdevest, J.; Keating, C.; Francis, S.F.; Dansky, D.; Gudis, D.A. Effect of highly effective modulator therapy on quality of life in adults with cystic fibrosis. Int. Forum Allergy Rhinol. 2021, 11, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Ejiofor, L.C.K.; Mathiesen, I.H.M.; Jensen-Fangel, S.; Olesen, H.V.; Skov, M.; Philipsen, L.K.D.; Pedersen, C.L.; Pressler, T. Patients with cystic fibrosis and advanced lung disease benefit from lumacaftor/ivacaftor treatment. Pediatr. Pulmonol. 2020, 55, 3364–3370. [Google Scholar] [CrossRef] [PubMed]
- Fajac, I.; Daines, C.; Durieu, I.; Goralski, J.L.; Heijerman, H.; Knoop, C.; Majoor, C.; Bruinsma, B.G.; Moskowitz, S.; Prieto-Centurion, V.; et al. Non-respiratory health-related quality of life in people with cystic fibrosis receiving elexacaftor/tezacaftor/ivacaftor. J. Cyst. Fibros. 2022, 22, 119–123. [Google Scholar] [CrossRef]
- Welsner, M.; Gruber, W.; Mellies, U.; Olivier, M.; Sutharsan, S.; Taube, C.; Dillenhoefer, S.; Koerner-Rettberg, C.; Stehling, F. Trainability of Health-Related and Motor Performance Fitness in Adults with Cystic Fibrosis within a 12-Month Partially Supervised Exercise Program. Pulm. Med. 2021, 2021, 5581812. [Google Scholar] [CrossRef]
- Gruber, W.; Stehling, F.; Blosch, C.; Dillenhoefer, S.; Olivier, M.; Koerner-Rettberg, C.; Sutharsan, S.; Mellies, U.; Taube, C.; Welsner, M. Effects of a Long-Term Monitored Exercise Program on Aerobic Fitness in a Small Group of Children with Cystic Fibrosis. Int. J. Environ. Res. Public Health 2022, 19, 7923. [Google Scholar] [CrossRef]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; Van Der Grinten, C.P.M.; Gustafsson, P.; et al. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef] [PubMed]
- Quanjer, P.H.; Stanojevic, S.; Cole, T.J.; Baur, X.; Hall, G.L.; Culver, B.H.; Enright, P.L.; Hankinson, J.L.; Ip, M.S.M.; Zheng, J.; et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: The global lung function 2012 equations. Eur. Respir. J. 2012, 40, 1324–1343. [Google Scholar] [CrossRef] [PubMed]
- Castellanos, C.X.; Osterbauer, B.; Hasday, S.; Keens, T.G.; Koempel, J.; Ference, E.H. Improvement in sinonasal quality-of-life indicators for pediatric patients with cystic fibrosis treated with elexacaftor-tezacaftor-ivacaftor. Int. Forum Allergy Rhinol. 2022, 13, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Carrieri-Kohlman, V.; Donesky-Cuenco, D.; Park, S.K.; Mackin, L.; Nguyen, H.Q.; Paul, S.M. Additional evidence for the affective dimension of dyspnea in patients with COPD. Res. Nurs. Health 2010, 33, 4–19. [Google Scholar] [CrossRef] [PubMed]
- Keyte, R.; Kauser, S.; Mantzios, M.; Egan, H. The psychological implications and health risks of cystic fibrosis pre- and post- CFTR modulator therapy. Chronic Illn. 2022, 19, 539–556. [Google Scholar] [CrossRef] [PubMed]
- Quittner, A.L.; Modi, A.C.; Wainwright, C.; Otto, K.; Kirihara, J.; Montgomery, A.B. Determination of the Minimal Clinically Important Difference Scores for the Cystic Fibrosis Questionnaire-Revised Respiratory Symptom Scale in Two Populations of Patients with Cystic Fibrosis and Chronic Pseudomonas aeruginosa Airway Infection. Chest 2009, 135, 1610–1618. [Google Scholar] [CrossRef] [PubMed]
- Adler, F.R.; Liou, T.G. The Dynamics of Disease Progression in Cystic Fibrosis. PLoS ONE 2016, 11, e0156752. [Google Scholar] [CrossRef]
- Habib, A.-R.R.; Manji, J.; Wilcox, P.G.; Javer, A.R.; Buxton, J.A.; Quon, B.S. A Systematic Review of Factors Associated with Health-Related Quality of Life in Adolescents and Adults with Cystic Fibrosis. Ann. Am. Thorac. Soc. 2015, 12, 420–428. [Google Scholar] [CrossRef]
- Caley, L.; Smith, L.; White, H.; Peckham, D. Average rate of lung function decline in adults with cystic fibrosis in the United Kingdom: Data from the UK CF registry. J. Cyst. Fibros. 2021, 20, 86–90. [Google Scholar] [CrossRef]
- Flume, P.A.; Suthoff, E.D.; Kosinski, M.; Marigowda, G.; Quittner, A.L. Measuring recovery in health-related quality of life during and after pulmonary exacerbations in patients with cystic fibrosis. J. Cyst. Fibros. 2019, 18, 737–742. [Google Scholar] [CrossRef]
- Zaher, A.; ElSaygh, J.; Elsori, D.; ElSaygh, H.; Sanni, A. A Review of Trikafta: Triple Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Modulator Therapy. Cureus 2021, 13, e16144. [Google Scholar] [CrossRef]
- Purkayastha, D.; Agtarap, K.; Wong, K.; Pereira, O.; Co, J.; Pakhale, S.; Kanji, S. Drug-drug interactions with CFTR modulator therapy in cystic fibrosis: Focus on Trikafta®/Kaftrio®. J. Cyst. Fibros. 2023, 22, 478–483. [Google Scholar] [CrossRef]
| ETI Group (n = 21) | Non-ETI Group (n = 6) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | 95% CI Lower/Upper | Mean ± SD | 95% CI Lower/Upper | between Groups p-Value | within Groups p-Value | |||||||
| Variable | T0 | T5 | T0 | T5 | T0 | T5 | T0 | T5 | T0 | T5 | ETI Group | Non-ETI Group |
| Males (%) | 67 | 67 | ||||||||||
| del F508 homozygous (in %) | 60 | 67 | ||||||||||
| del F508 heterozygous (in %) | 28 | 17 | ||||||||||
| Pancreatic insufficient (%) | 82 | 100 | ||||||||||
| Pseudomonas aeruginosa (%) | 54 | 33 | ||||||||||
| time between clinical assessments (yrs) | 5.4 ± 0.8 | 5.0/5.8 | 6.5 ± 0.5 | 6.1/7.0 | 0.001 | |||||||
| duration of intake ETI (weeks) | 33 ± 25 | |||||||||||
| Age (yrs) | 25.9 ± 7.4 | 31.0 ± 7.6 | 22.5/29.2 | 27.5/34.4 | 26.3 ± 9.4 | 32.3 ± 8.7 † | 17.6/35.0 | 24.2/40.4 | 0.640 | 0.796 | <0.001 | 0.016 |
| Heigth (cm) | 171.2 ± 8.8 | 167.0/175.2 | 174.7 ± 11.0 | 164.6/184.9 | ||||||||
| Weight (kg) | 58.7 ± 11.6 | 67.1 ± 11.9 | 53.7/64.0 | 61.7/72.6 | 60.9 ± 7.3 | 62.3 ± 7.3 | 54.1/67.6 | 49.4/75.6 | 0.435 | 0.499 | <0.001 | 0.207 |
| BMI | 19.9 ± 2.8 | 22.8 ± 2.6 | 18.77/21.2 | 21.6/23.9 | 19.9 ± 1.2 | 20.6 ± 1.7 | 18.8/21.0 | 18.9/22.3 | 0.405 | 0.101 | <0.001 | 0.116 |
| ppFEV1 (%pred) | 51.9 ± 16.5 | 53.1 ± 17.9 | 44.4/59.5 | 45.0/61.3 | 78.6 ± 18.3 | 66.0 ± 20.6 | 61.6/95.5 | 46.9/85.1 | 0.003 | 0.249 | 0.578 | 0.028 |
| FEV1 z-Score | −3.92 ± 1.3 | −3.0 ± 1.9 † | −4.5/-3.4 | −3.9/−2.1 | −0.99 ± 1.62 | −2.70 ± 1.68 | −3.49/−0.5 | −4.05/−1.19 | 0.008 | 0.568 | 0.017 | 0.128 |
| ETI Group (n = 21) | Non-ETI Group (n = 6) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | 95% CI Lower/Upper | Mean ± SD | 95% CI Lower/Upper | between Groups p-Value | within Groups p-Value | |||||||
| T0 | T5 | T0 | T5 | T0 | T5 | T0 | T5 | T0 | T5 | ETI Group | Non-ETI Group | |
| Physical domains | ||||||||||||
| Physical Functioning | 71.4 ± 19.5 | 89.2 ± 8.1 | 61.3/79.5 | 85.2/94.0 | 81.9 ± 13.6 | 78.0 ± 15.6 | 67.7/96.2 | 63.6/92.4 | 0.289 | 0.099 | 0.002 | 0.752 |
| Body Image | 56.1 ± 25.2 | 72.6 ± 24.6 | 46.3/69.3 | 66.1/86.7 | 70.4 ± 24.0 | 69.8 ± 25.4 | 45.2/95.6 | 46.3/93.4 | 0.239 | 0.852 | 0.030 | 0.463 |
| Eating | 86.8 ± 19.8 | 100.0 ± 0.0 | 76.7/95.5 | 100.0/100.0 | 87.0 ± 22.7 | 90.5 ± 17.5 | 63.2/110.8 | 74.3/106.6 | 0.712 | 0.408 | 0.007 | 0.180 |
| Health Perceptions | 51.9 ± 21.5 | 69.3 ± 16.0 | 40.5/60.5 | 30.1/77.5 | 68.5 ± 21.6 | 60.3 ± 25.6 | 45.9/91.1 | 36.7/83.9 | 0.175 | 0.383 | 0.011 | 0.345 |
| Vitality | 56.7 ± 18.6 | 69.6 ± 24.3 | 47.1/64.5 | 55.9/82.6 | 66.7 ± 23.0 | 61.9 ± 16.6 | 97.7/69.2 | 46.6/72.2 | 0.376 | 0.288 | 0.073 | 0.892 |
| Digestive | 82.0 ± 15.1 | 87.6 ± 11.7 | 74.5/88.9 | 81.9/94.5 | 87.0 ± 17.8 | 76.2 ± 20.7 | 68.4/105.7 | 57.0/95.4 | 0.408 | 0.234 | 0.096 | 0.684 |
| Respiratory | 62.4 ± 19.37 | 84.9 ± 15.6 | 53.2/71.8 | 76.5/93.6 | 79.6 ± 24.0 | 67.4 ± 21.4 | 54.4/104.8 | 47.7/87.2 | 0.049 | 0.087 | 0.004 | 0.225 |
| Weight | 61.7 ± 36.3 | 94.1 ± 13.1 | 43.4/79.4 | 86.6/100.0 | 83.3 ± 18.3 | 76.2 ± 25.2 | 64.5/102.5 | 52.9/99.5 | 0.242 | 0.114 | 0.005 | 0.498 |
| Psychosocial domains | ||||||||||||
| Treatment Burden | 70.4 ± 15.8 | 77.8 ± 16.7 | 62.4/77.6 | 68.1/86.1 | 63.0 ± 19.5 | 66.7 ± 12.8 | 42.5/83.4 | 54.8/78.6 | 0.408 | 0.147 | 0.068 | 0.463 |
| Emotional Functioning | 75.9 ± 16.8 | 87.1 ± 9.3 | 67.4/83.3 | 83.2/92.6 | 88.9 ± 21.4 | 89.5 ± 13.8 | 66.5/113.3 | 75.8/102.3 | 0.026 | 0.318 | 0.046 | 1.00 |
| Social Functioning | 68.5 ± 14.9 | 76.1 ± 15.4 | 61.2/83.0 | 70.1/85.4 | 74.1 ± 15.2 | 77.0 ± 19.1 | 58.1/90.0 | 59.3/94.6 | 0.512 | 0.852 | 0.246 | 0.463 |
| Role Functioning | 70.8 ± 24.5 | 91.2 ± 9.5 | 46.3/69.3 | 86.5/96.8 | 84.7 ± 13.4 | 89.3 ± 11.5 | 70.7/98.7 | 78.6/99.9 | 0.251 | 0.757 | 0.012 | 0.416 |
| ETI Group (n = 21) | Non-ETI Group (n = 6) | |||||||
|---|---|---|---|---|---|---|---|---|
| p-Value | ||||||||
| Weight | BMI | ppFEV1 | FEV z-Score | Weight | BMI | ppFEV1 | FEV z-Score | |
| Physical domains | ||||||||
| Physical Functioning | 0.951 | 0.817 | 0.746 | 0.204 | 0.979 | 0.923 | 0.990 | 0.980 |
| Body Image | 0.974 | 0.746 | 0.988 | 0.326 | 0.666 | 0.535 | 0.541 | 0.382 |
| Eating | 0.166 | 0.087 | 0.870 | 0.951 | 0.012 | 0.018 | 0.960 | 0.839 |
| Health Perceptions | 0.528 | 0.504 | 0.689 | 0.635 | 0.668 | 0.849 | 0.380 | 0.542 |
| Vitality | 0.998 | 0.969 | 0.844 | 0.488 | 0.239 | 0.200 | 0.838 | 0.858 |
| Digestive | 0.282 | 0.165 | 0.111 | 0.222 | 0.578 | 0.509 | 0.878 | 0.566 |
| Respiratory | 0.931 | 0.846 | 0.442 | 0.631 | 0.791 | 0.721 | 0.721 | 0.802 |
| Weight | 0.960 | 0.681 | 0.479 | 0.593 | 0.736 | 0.695 | 0.574 | 0.366 |
| Psychosocial domains | ||||||||
| Treatment Burden | 0.414 | 0.287 | 0.636 | 0.434 | 0.880 | 0.882 | 0.778 | 0.651 |
| Emotional Functioning | 0.784 | 0.691 | 0.761 | 0.903 | 0.348 | 0.337 | 0.381 | 0.310 |
| Social Functioning | 0.185 | 0.108 | 0.345 | 0.883 | 0.959 | 0.913 | 0.391 | 0.358 |
| Role Functioning | 0.988 | 0.785 | 0.887 | 0.134 | 0.836 | 0.912 | 0.970 | 0.864 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gruber, W.; Welsner, M.; Blosch, C.; Dillenhoefer, S.; Olivier, M.; Brinkmann, F.; Koerner-Rettberg, C.; Sutharsan, S.; Mellies, U.; Taube, C.; et al. Long-Term Follow-Up of Health-Related Quality of Life and Short-Term Intervention with CFTR Modulator Therapy in Adults with Cystic Fibrosis: Evaluation of Changes over Several Years with or without 33 Weeks of CFTR Modulator Therapy. Healthcare 2023, 11, 2873. https://doi.org/10.3390/healthcare11212873
Gruber W, Welsner M, Blosch C, Dillenhoefer S, Olivier M, Brinkmann F, Koerner-Rettberg C, Sutharsan S, Mellies U, Taube C, et al. Long-Term Follow-Up of Health-Related Quality of Life and Short-Term Intervention with CFTR Modulator Therapy in Adults with Cystic Fibrosis: Evaluation of Changes over Several Years with or without 33 Weeks of CFTR Modulator Therapy. Healthcare. 2023; 11(21):2873. https://doi.org/10.3390/healthcare11212873
Chicago/Turabian StyleGruber, Wolfgang, Matthias Welsner, Christopher Blosch, Stefanie Dillenhoefer, Margarete Olivier, Folke Brinkmann, Cordula Koerner-Rettberg, Sivagurunathan Sutharsan, Uwe Mellies, Christian Taube, and et al. 2023. "Long-Term Follow-Up of Health-Related Quality of Life and Short-Term Intervention with CFTR Modulator Therapy in Adults with Cystic Fibrosis: Evaluation of Changes over Several Years with or without 33 Weeks of CFTR Modulator Therapy" Healthcare 11, no. 21: 2873. https://doi.org/10.3390/healthcare11212873





