A Simple Nomogram for Predicting Stroke-Associated Pneumonia in Patients with Acute Ischemic Stroke
Abstract
:1. Introduction
2. Materials and Methods
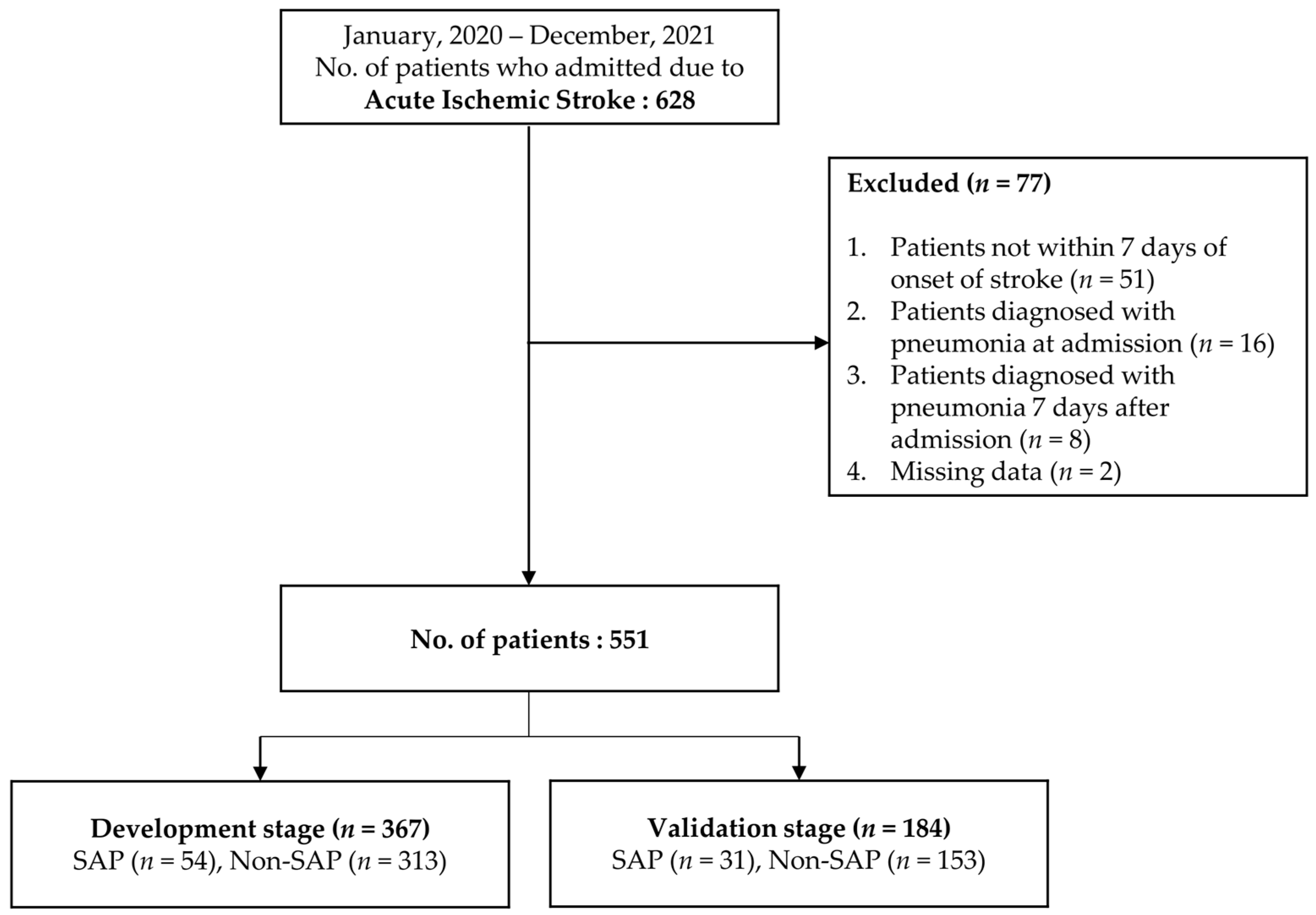
2.1. Study Design and Subjects
2.2. Research Tools
2.3. Data Collection
2.4. Data Analysis
3. Results
3.1. Comparison of the Characteristics of SAP and Non-SAP Groups
3.2. Development and Validation of the SAP Prediction Models
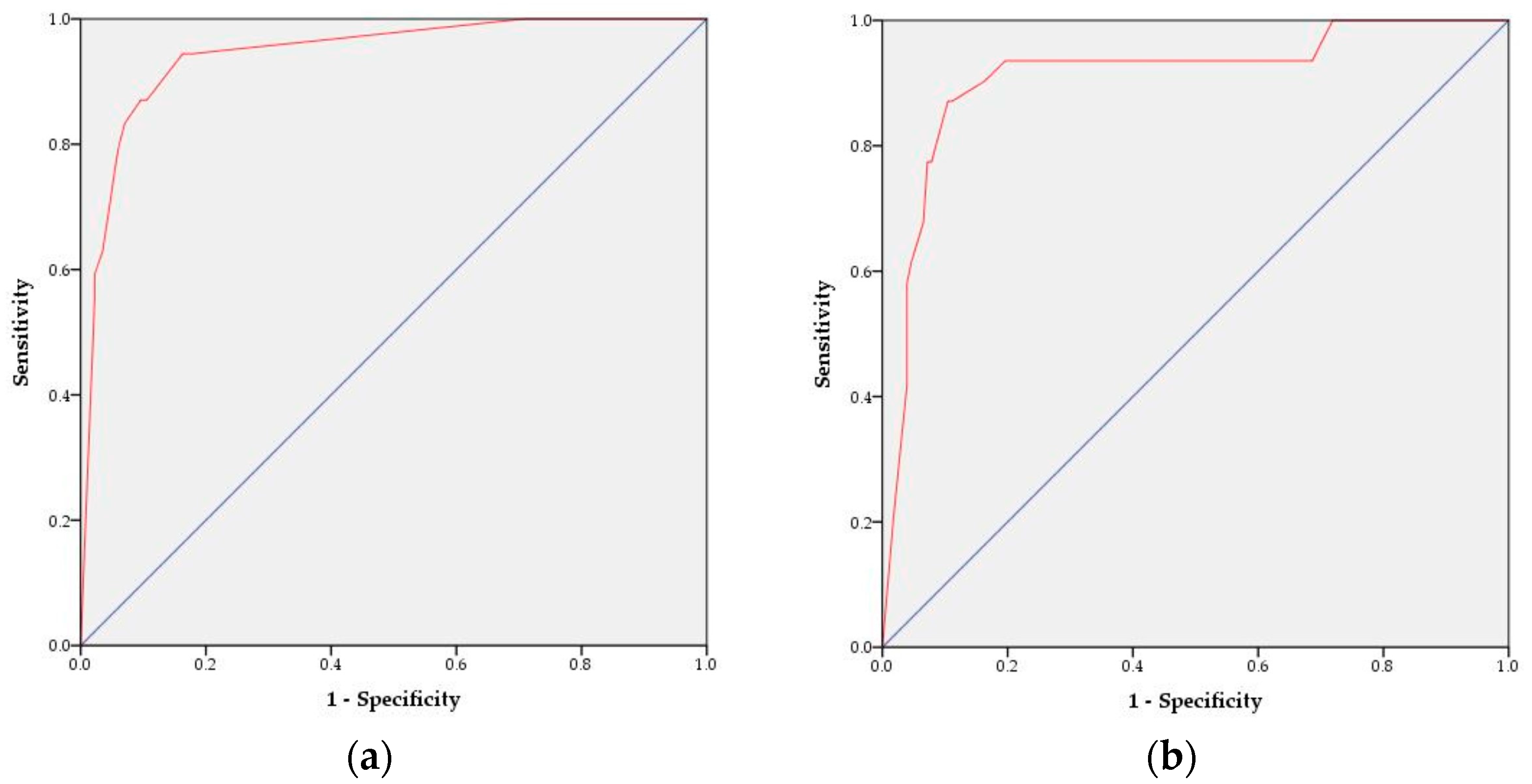
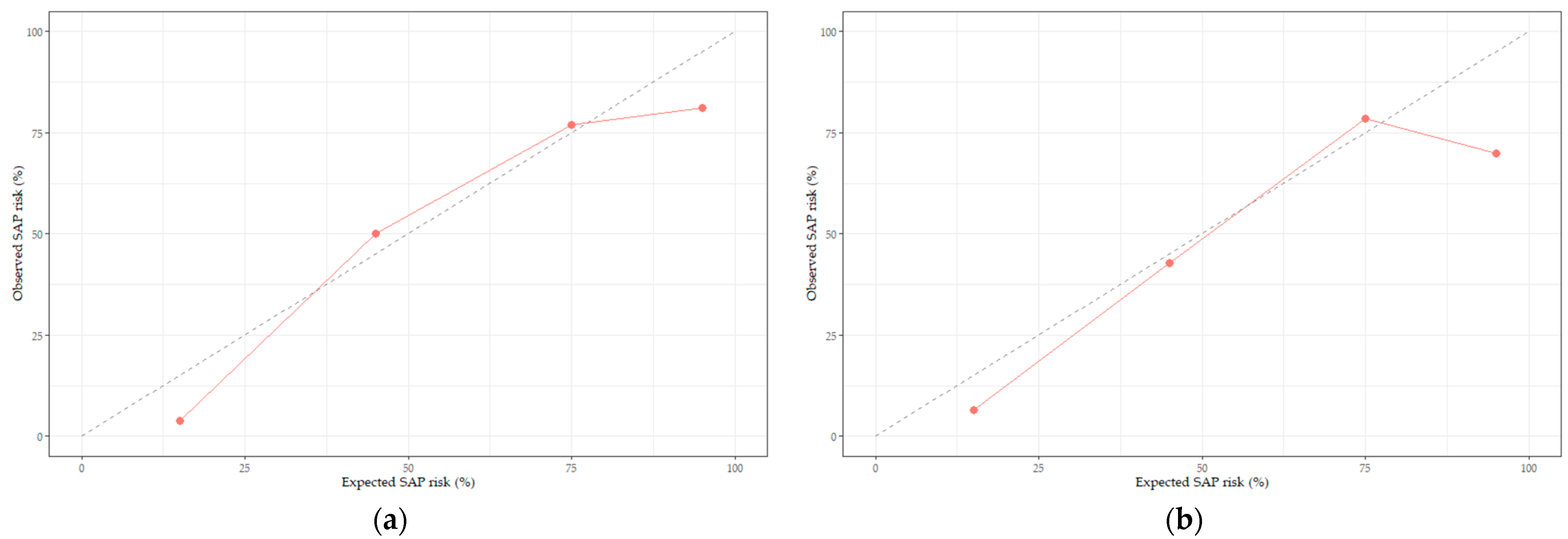
3.3. Development of the SAP Prediction Nomogram and Validity Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hurford, R.; Sekhar, A.; Hughes, T.A.T.; Muir, K.W. Diagnosis and management of acute ischaemic stroke. Pract. Neurol. 2020, 20, 304–316. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. The Top 10 Causes of Death. 2019. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 10 May 2022).
- Yang, S.; Choo, Y.J.; Chang, M.C. The Preventive Effect of Dysphagia Screening on Pneumonia in Acute Stroke Patients: A Systematic Review and Meta-Analysis. Healthcare 2021, 9, 1764. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Howe, J.; Majid, A.; Redgrave, J.; Pownall, S.; Abdelhafiz, A. The economic cost of stroke-associated pneumonia in a UK setting. Top. Stroke Rehabil. 2018, 25, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. 2018 Guidelines for the Early Management of Patients With Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2018, 49, e46–e110. [Google Scholar] [CrossRef]
- Ni, J.; Shou, W.; Wu, X.; Sun, J. Prediction of stroke-associated pneumonia by the A2DS2, AIS-APS, and ISAN scores: A systematic review and meta-analysis. Expert. Rev. Respir. Med. 2021, 15, 1461–1472. [Google Scholar] [CrossRef]
- Arigami, T.; Uenosono, Y.; Ishigami, S.; Okubo, K.; Kijima, T.; Yanagita, S.; Okumura, H.; Uchikado, Y.; Kijima, Y.; Nakajo, A.; et al. A Novel Scoring System Based on Fibrinogen and the Neutrophil-Lymphocyte Ratio as a Predictor of Chemotherapy Response and Prognosis in Patients with Advanced Gastric Cancer. Oncology 2016, 90, 186–192. [Google Scholar] [CrossRef]
- Nam, K.W.; Kim, T.J.; Lee, J.S.; Kwon, H.M.; Lee, Y.S.; Ko, S.B.; Yoon, B.W. High neutrophil-to-lymphocyte ratio predicts stroke-associated pneumonia. Stroke 2018, 49, 1886–1892. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, Y.; Han, L.; He, F.; Cai, N.; Zhang, Q.; Wang, J. Risk factors for acute stroke-associated pneumonia and prediction of neutrophil-to-lymphocyte ratios. Am. J. Emerg. Med. 2021, 41, 55–59. [Google Scholar] [CrossRef]
- Papagianni, M.; Tziomalos, K.; Kostaki, S.; Angelopoulou, S.; Christou, K.; Sofogianni, A.; Alkagiet, S.; Chatzopoulos, G.; Savopoulos, C.; Hatzitolios, A. Obesity is an independent risk factor for pneumonia in patients admitted with acute ischemic stroke. Atherosclerosis 2018, 275, e75. [Google Scholar] [CrossRef]
- Smith, C.J.; Kishore, A.K.; Vail, A.; Chamorro, A.; Garau, J.; Hopkins, S.J.; Di Napoli, M.; Kalra, L.; Langhorne, P.; Montaner, J. Diagnosis of stroke-associated pneumonia: Recommendations from the pneumonia in stroke consensus group. Stroke 2015, 46, 2335–2340. [Google Scholar] [CrossRef]
- Mann, G.; Hankey, G.J.; Cameron, D. Swallowing function after stroke: Prognosis and prognostic factors at 6 months. Stroke 1999, 30, 744–748. [Google Scholar] [CrossRef] [PubMed]
- Horan, T.C.; Andrus, M.; Dudeck, M.A. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am. J. Infect. Control 2008, 36, 309–332. [Google Scholar] [CrossRef] [PubMed]
- Kishore, A.K.; Vail, A.; Bray, B.D.; Chamorro, A.; Napoli, M.D.; Kalra, L.; Langhorne, P.; Montaner, J.; Roffe, C.; Rudd, A.G.; et al. Clinical risk scores for predicting stroke-associated pneumonia: A systematic review. Eur. Stroke J. 2016, 1, 76–84. [Google Scholar] [CrossRef]
- Balachandran, V.P.; Gonen, M.; Smith, J.J.; DeMatteo, R.P. Nomograms in oncology: More than meets the eye. Lancet Oncol. 2015, 16, e173–e180. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, H.; Yan, L.; Gao, J.; Zhu, L. A Novel Clinical Nomogram for Predicting Cancer-Specific Survival in Adult Patients After Primary Surgery for Epithelial Ovarian Cancer: A Real-World Analysis Based on the Surveillance, Epidemiology, and End Results Database and External Validation in a Tertiary Center. Front. Oncol. 2021, 11, 670644. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, M.; Zhang, C.; Chen, H.; Liu, X.; An, Y.; Zhang, L.; Guo, X. Establishment and Clinical Application of the Nomogram Related to Risk or Prognosis of Hepatocellular Carcinoma: A Review. J. Hepatocell. Carcinoma 2023, 10, 1389–1398. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, H.S.; Seo, W.Y.; Nam, C.M.; Kim, K.Y.; Jeung, H.C.; Lai, J.F.; Chung, H.C.; Noh, S.H.; Rha, S.Y. External validation of nomogram for the prediction of recurrence after curative resection in early gastric cancer. Ann. Oncol. 2012, 23, 361–367. [Google Scholar] [CrossRef]
- Huang, G.Q.; Lin, Y.T.; Wu, Y.M.; Cheng, Q.Q.; Cheng, H.R.; Wang, Z. Individualized Prediction Of Stroke-Associated Pneumonia For Patients With Acute Ischemic Stroke. Clin. Interv. Aging 2019, 14, 1951–1962. [Google Scholar] [CrossRef]
- Song, X.; He, Y.; Bai, J.; Zhang, J. A nomogram based on nutritional status and A(2)DS(2) score for predicting stroke-associated pneumonia in acute ischemic stroke patients with type 2 diabetes mellitus: A retrospective study. Front. Nutr. 2022, 9, 1009041. [Google Scholar] [CrossRef]
- Chumbler, N.R.; Williams, L.S.; Wells, C.K.; Lo, A.C.; Nadeau, S.; Peixoto, A.J.; Gorman, M.; Boice, J.L.; Concato, J.; Bravata, D.M. Derivation and validation of a clinical system for predicting pneumonia in acute stroke. Neuroepidemiology 2010, 34, 193–199. [Google Scholar] [CrossRef]
- Hannawi, Y.; Hannawi, B.; Rao, C.P.; Suarez, J.I.; Bershad, E.M. Stroke-associated pneumonia: Major advances and obstacles. Cerebrovasc. Dis. 2013, 35, 430–443. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Marchina, S.; Massaro, J.; Feng, W.; Lahoti, S.; Selim, M.; Herzig, S.J. ACDD4 score: A simple tool for assessing risk of pneumonia after stroke. J. Neurol. Sci. 2017, 372, 399–402. [Google Scholar] [CrossRef] [PubMed]
- Dobbin, K.K.; Simon, R.M. Optimally splitting cases for training and testing high dimensional classifiers. BMC Med. Genom. 2011, 4, 31. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.Z.; Li, F.; Tian, X.; Wang, W.; Jia, M.; Wang, X.F.; Liu, G.W. Risk factors for lung infection in stroke patients: A meta-analysis of observational studies. Expert. Rev. Anti Infect. Ther. 2015, 13, 1289–1298. [Google Scholar] [CrossRef]
- Marchina, S.; Doros, G.; Modak, J.; Helenius, J.; Aycock, D.M.; Kumar, S. Acid-suppressive medications and risk of pneumonia in acute stroke patients: A systematic review and meta-analysis. J. Neurol. Sci. 2019, 400, 122–128. [Google Scholar] [CrossRef]
- Perry, L. Screening swallowing function of patients with acute stroke. Part two: Detailed evaluation of the tool used by nurses. J. Clin. Nurs. 2001, 10, 474–481. [Google Scholar] [CrossRef]
- Harms, H.; Grittner, U.; Dröge, H.; Meisel, A. Predicting post-stroke pneumonia: The PANTHERIS score. Acta Neurol. Scand. 2013, 128, 178–184. [Google Scholar] [CrossRef]
- Sellars, C.; Bowie, L.; Bagg, J.; Sweeney, M.P.; Miller, H.; Tilston, J.; Langhorne, P.; Stott, D.J. Risk factors for chest infection in acute stroke: A prospective cohort study. Stroke 2007, 38, 2284–2291. [Google Scholar] [CrossRef]
- Lee, E.J.; Jeong, I.S.; Woo, S.H.; Jung, H.J.; Han, E.J.; Kang, C.W.; Hyun, S. Development of a Diabetic Foot Ulceration Prediction Model and Nomogram. J. Korean Acad. Nurs. 2021, 51, 280–293. [Google Scholar] [CrossRef]
- Ji, R.; Shen, H.; Pan, Y.; Wang, P.; Liu, G.; Wang, Y.; Li, H.; Wang, Y. Novel risk score to predict pneumonia after acute ischemic stroke. Stroke 2013, 44, 1303–1309. [Google Scholar] [CrossRef]
- Kwon, H.M.; Jeong, S.W.; Lee, S.H.; Yoon, B.W. The pneumonia score: A simple grading scale for prediction of pneumonia after acute stroke. Am. J. Infect. Control 2006, 34, 64–68. [Google Scholar] [CrossRef]
- Walter, U.; Knoblich, R.; Steinhagen, V.; Donat, M.; Benecke, R.; Kloth, A. Predictors of pneumonia in acute stroke patients admitted to a neurological intensive care unit. J. Neurol. 2007, 254, 1323–1329. [Google Scholar] [CrossRef] [PubMed]
- Komiya, K.; Ishii, H.; Kadota, J.I. Healthcare-associated pneumonia and aspiration pneumonia. Aging Dis. 2015, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Grief, S.N.; Loza, J.K. Guidelines for the Evaluation and Treatment of Pneumonia. Prim. Care Clin. Off. Pract. 2018, 45, 485–503. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, S.; Malzahn, U.; Harms, H.; Koennecke, H.C.; Berger, K.; Kalic, M.; Walter, G.; Meisel, A.; Heuschmann, P.U. Development of a clinical score (A2DS2) to predict pneumonia in acute ischemic stroke. Stroke 2012, 43, 2617–2623. [Google Scholar] [CrossRef] [PubMed]
- Heiberger, C.J.; Kazi, S.; Mehta, T.I.; Busch, C.; Wolf, J.; Sandhu, D. Effects on stroke metrics and outcomes of a nurse-led stroke triage team in acute stroke management. Cureus 2019, 11, e5590. [Google Scholar] [CrossRef]
- Alsumrain, M.; Melillo, N.; DeBari, V.A.; Kirmani, J.; Moussavi, M.; Doraiswamy, V.; Katapally, R.; Korya, D.; Adelman, M.; Miller, R. Predictors and outcomes of pneumonia in patients with spontaneous intracerebral hemorrhage. J. Intensive Care Med. 2013, 28, 118–123. [Google Scholar] [CrossRef]
- Lee, Y.F.; Hsu, T.W.; Liang, C.S.; Yeh, T.C.; Chen, T.Y.; Chen, N.C.; Chu, C.S. The efficacy and safety of tube feeding in advanced dementia patients: A systemic review and meta-analysis study. J. Am. Med. Dir. Assoc. 2021, 22, 357–363. [Google Scholar] [CrossRef]
- George, B.P.; Kelly, A.G.; Schneider, E.B.; Holloway, R.G. Current practices in feeding tube placement for US acute ischemic stroke inpatients. Neurology 2014, 83, 874–882. [Google Scholar] [CrossRef]
- NanZhu, Y.; Xin, L.; Xianghua, Y.; Jun, C.; Min, L. Risk factors analysis of nosocomial pneumonia in elderly patients with acute cerebral infraction. Medicine 2019, 98, e15045. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, F.; Zhang, Y.; Ge, Z. Risk factors for developing pneumonia in patients with diabetes mellitus following acute ischaemic stroke. J. Int. Med. Res. 2012, 40, 1860–1865. [Google Scholar] [CrossRef] [PubMed]
- Labeit, B.; Michou, E.; Hamdy, S.; Trapl-Grundschober, M.; Suntrup-Krueger, S.; Muhle, P.; Bath, P.M.; Dziewas, R. The assessment of dysphagia after stroke: State of the art and future directions. Lancet Neurol. 2023, 22, 858–870. [Google Scholar] [CrossRef]
- Johnny, J.D.; Drury, Z.; Ly, T.; Scholine, J. Oral care in critically ill patients requiring noninvasive ventilation: An evidence-based review. Crit. Care Nurse 2021, 41, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Modi, A.R.; Kovacs, C.S. Hospital-acquired and ventilator-associated pneumonia: Diagnosis, management, and prevention. Clevel. Clin. J. Med. 2020, 87, 633–639. [Google Scholar] [CrossRef]
- Martin-Loeches, I.; Rodriguez, A.H.; Torres, A. New guidelines for hospital-acquired pneumonia/ventilator-associated pneumonia: USA vs. Europe. Curr. Opin. Crit. Care 2018, 24, 347–352. [Google Scholar] [CrossRef]
- Forget, P.; Machiels, J.P.; Coulie, P.G.; Berliere, M.; Poncelet, A.J.; Tombal, B.; Stainier, A.; Legrand, C.; Canon, J.L.; Kremer, Y. Neutrophil: Lymphocyte ratio and intraoperative use of ketorolac or diclofenac are prognostic factors in different cohorts of patients undergoing breast, lung, and kidney cancer surgery. Ann. Surg. Oncol. 2013, 20, 650–660. [Google Scholar] [CrossRef] [PubMed]
- Nóbrega Lima Rodrigues de Morais, A.; Ribeiro Baylão, V.M.; Martins Silva, T.; Gomes dos Santos, A.; Azevedo, M.; JM de Oliveira, A. Is neutrophil-lymphocyte ratio a useful tool for predicting outcome in subarachnoid hemorrhage? A systematic review. Neurosurg. Rev. 2021, 44, 3023–3028. [Google Scholar] [CrossRef]
- Patel, U.K.; Kodumuri, N.; Dave, M.; Lekshminarayanan, A.; Khan, N.; Kavi, T.; Kothari, R.; Lunagariya, A.; Jani, V. Stroke-associated pneumonia: A retrospective study of risk factors and outcomes. Neurologist 2020, 25, 39–48. [Google Scholar] [CrossRef]
- Huang, Z.; Zhao, Z.; Liu, Y.; Zhou, Z.; Zhang, W.; Kong, Q.; He, Y. A Simple-to-Use Nomogram for Predicting Postoperative Early Death Risk in Elderly Patients with Spinal Tumors: A Population-Based Study. J. Oncol. 2023, 2023, 2805786. [Google Scholar] [CrossRef]
- Zaydfudim, V.; Dossett, L.A.; Starmer, J.M.; Arbogast, P.G.; Feurer, I.D.; Ray, W.A.; May, A.K.; Pinson, C.W. Implementation of a real-time compliance dashboard to help reduce SICU ventilator-associated pneumonia with the ventilator bundle. Arch. Surg. 2009, 144, 656–662. [Google Scholar] [CrossRef]

| Characteristics | Categories | Non-SAP (n = 313) | SAP (n = 54) | χ2/t/Z | p |
|---|---|---|---|---|---|
| n (%)/M ± SD/Median (IQR) | |||||
| Sociodemographic Characteristics | |||||
| Age (years) | <65 | 104 (33.2) | 5 (9.3) | 12.67 | <0.001 |
| ≥65 | 209 (66.8) | 49 (90.7) | |||
| M ± SD | 69.83 ± 12.18 | 77.80 ± 9.29 | −4.58 | <0.001 | |
| Sex | Male | 179 (57.2) | 29 (53.7) | 0.23 | 0.633 |
| Female | 134 (42.8) | 25 (46.3) | |||
| Alcohol | 89 (28.4) | 9 (16.7) | 3.26 | 0.071 | |
| Smoking | 62 (19.8) | 10 (18.5) | 0.05 | 0.826 | |
| Clinical Parameters at admission | |||||
| NIHSS | <7 | 287 (91.7) | 18 (33.3) | 111.72 | <0.001 |
| ≥7 | 26 (8.3) | 36 (66.7) | |||
| Median (IQR) | 3.00 (1–5) | 13 (5–16) | −8.11 | <0.001 | |
| mRS | <3 | 292 (93.3) | 47 (87.0) | 2.56 | 0.110 |
| ≥3 | 21 (6.7) | 7 (13.0) | |||
| Median (IQR) | 0 (0–0) | 0 (0–1) | −2.24 | 0.025 | |
| Dysarthria | 134 (42.8) | 34 (63.0) | 7.54 | 0.006 | |
| Dysphagia | 25 (8.0) | 44 (81.5) | 162.95 | <0.001 | |
| OCSP | LACS | 119 (38.0) | 3 (5.6) | 128.24 | <0.001 |
| TACS | 21 (6.7) | 36 (66.7) | |||
| PACS | 125 (39.9) | 12 (22.2) | |||
| POCS | 48 (15.3) | 3 (5.6) | |||
| BMI | <18.5 | 17 (5.4) | 7 (13.0) | −2.07 | 0.038 |
| 18.5–22.99 | 114 (36.4) | 26 (48.1) | |||
| 23–24.99 | 73 (23.3) | 8 (14.9) | |||
| ≥25 | 109 (34.8) | 13 (24.1) | |||
| M ± SD | 23.64 ± 4.03 | 22.64 ± 4.10 | 1.67 | 0.096 | |
| Nasogastric tube | 21 (6.7) | 44 (81.5) | 176.67 | <0.001 | |
| Ventilator | 2 (0.6) | 10 (18.5) | 46.55 | <0.001 | |
| PPI (during hosplitalization) | 287 (91.7) | 53 (98.1) | 2.81 | 0.093 | |
| Pre-existing Comorbidities | |||||
| Previous stroke | 80 (25.6) | 15 (27.8) | 0.19 | 0.731 | |
| Hypertension | 185 (59.1) | 35 (64.8) | 0.63 | 0.429 | |
| Diabetes mellitus | 99 (31.6) | 19 (35.2) | 0.27 | 0.605 | |
| Chronic obstructive pulmonary disease | 5 (1.6) | 2 (3.7) | 1.09 | 0.296 | |
| Atrial fibrillation | 44 (14.1) | 24 (44.4) | 28.17 | <0.001 | |
| Congestive heart failure | 7 (2.2) | 5 (9.3) | 7.18 | 0.007 | |
| Ischemic heart disease | 19 (6.1) | 4 (7.4) | 0.14 | 0.708 | |
| Chronic kidney disease | 13 (4.2) | 1 (1.9) | 0.66 | 0.415 | |
| Laboratory Parameters at admission | |||||
| Albumin (g/dL) | ≤3.5 | 12 (3.8) | 5 (9.3) | 3.07 | 0.008 |
| >3.5 | 301 (96.2) | 49 (90.7) | |||
| M ± SD | 4.28 ± 0.38 | 4.09 ± 0.51 | 3.24 | 0.001 | |
| WBC (×103/uL) | <12.0 | 298 (95.2) | 42 (77.8) | 20.53 | <0.001 |
| ≥12.0 | 15 (4.8) | 12 (22.2) | |||
| M ± SD | 7.52 ± 2.45 | 9.80 ± 5.17 | −5.16 | <0.001 | |
| NLR | <3.53 | 228 (72.8) | 21 (38.9) | 24.34 | <0.001 |
| ≥3.53 | 85 (27.2) | 33 (61.1) | |||
| M ± SD | 3.03 ± 2.26 | 6.02 ± 5.53 | −6.85 | <0.001 | |
| CRP (mg/dL) | <5.0 | 280 (89.5) | 33 (61.1) | 29.49 | <0.001 |
| ≥5.0 | 33 (10.5) | 21 (38.9) | |||
| M ± SD | 6.42 ± 12.58 | 26.75 ± 63.57 | −5.13 | <0.001 | |
| Glucose (mg/dL) | <200 | 266 (85.0) | 45 (83.3) | 0.98 | 0.755 |
| ≥200 | 47 (15.0) | 9 (16.7) | |||
| M ± SD | 146.94 ± 63.61 | 156.50 ± 87.61 | −0.96 | 0.338 | |
| BUN (mg/dL) | <23.0 | 274 (87.5) | 35 (64.8) | 17.87 | <0.001 |
| ≥23.0 | 39 (12.5) | 19 (35.2) | |||
| M ± SD | 16.23 ± 7.52 | 19.76 ± 7.96 | −3.15 | 0.002 | |
| Cr (mg/dL) | <1.3 | 280 (89.5) | 40 (74.1) | 9.76 | 0.002 |
| ≥1.3 | 33 (10.5) | 14 (25.9) | |||
| M ± SD | 1.00 ± 1.07 | 1.01 ± 0.45 | −0.06 | 0.953 | |
| Characteristics | B Coefficient | S.E. | OR | 95% CI | p |
|---|---|---|---|---|---|
| Constant | −4.83 | 0.66 | 0.00 | <0.001 | |
| Age (years) ≥ 65 | 1.38 | 0.62 | 3.99 | 1.18–13.49 | 0.026 |
| NIHSS score ≥ 7 | 1.40 | 0.50 | 4.06 | 1.54–10.72 | 0.005 |
| Nasogastric tube | 3.32 | 0.48 | 27.75 | 10.85–70.93 | <0.001 |
| CRP ≥ 5.0 (mg/dL) | 1.06 | 0.53 | 2.90 | 1.02–8.20 | 0.045 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.-J.; Jang, H.J. A Simple Nomogram for Predicting Stroke-Associated Pneumonia in Patients with Acute Ischemic Stroke. Healthcare 2023, 11, 3015. https://doi.org/10.3390/healthcare11233015
Lee Y-J, Jang HJ. A Simple Nomogram for Predicting Stroke-Associated Pneumonia in Patients with Acute Ischemic Stroke. Healthcare. 2023; 11(23):3015. https://doi.org/10.3390/healthcare11233015
Chicago/Turabian StyleLee, Youn-Jung, and Hee Jung Jang. 2023. "A Simple Nomogram for Predicting Stroke-Associated Pneumonia in Patients with Acute Ischemic Stroke" Healthcare 11, no. 23: 3015. https://doi.org/10.3390/healthcare11233015
APA StyleLee, Y.-J., & Jang, H. J. (2023). A Simple Nomogram for Predicting Stroke-Associated Pneumonia in Patients with Acute Ischemic Stroke. Healthcare, 11(23), 3015. https://doi.org/10.3390/healthcare11233015






