Effects of Neuromuscular Electrical Stimulation on Spasticity and Walking Performance among Individuals with Chronic Stroke: A Pilot Randomized Clinical Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Site
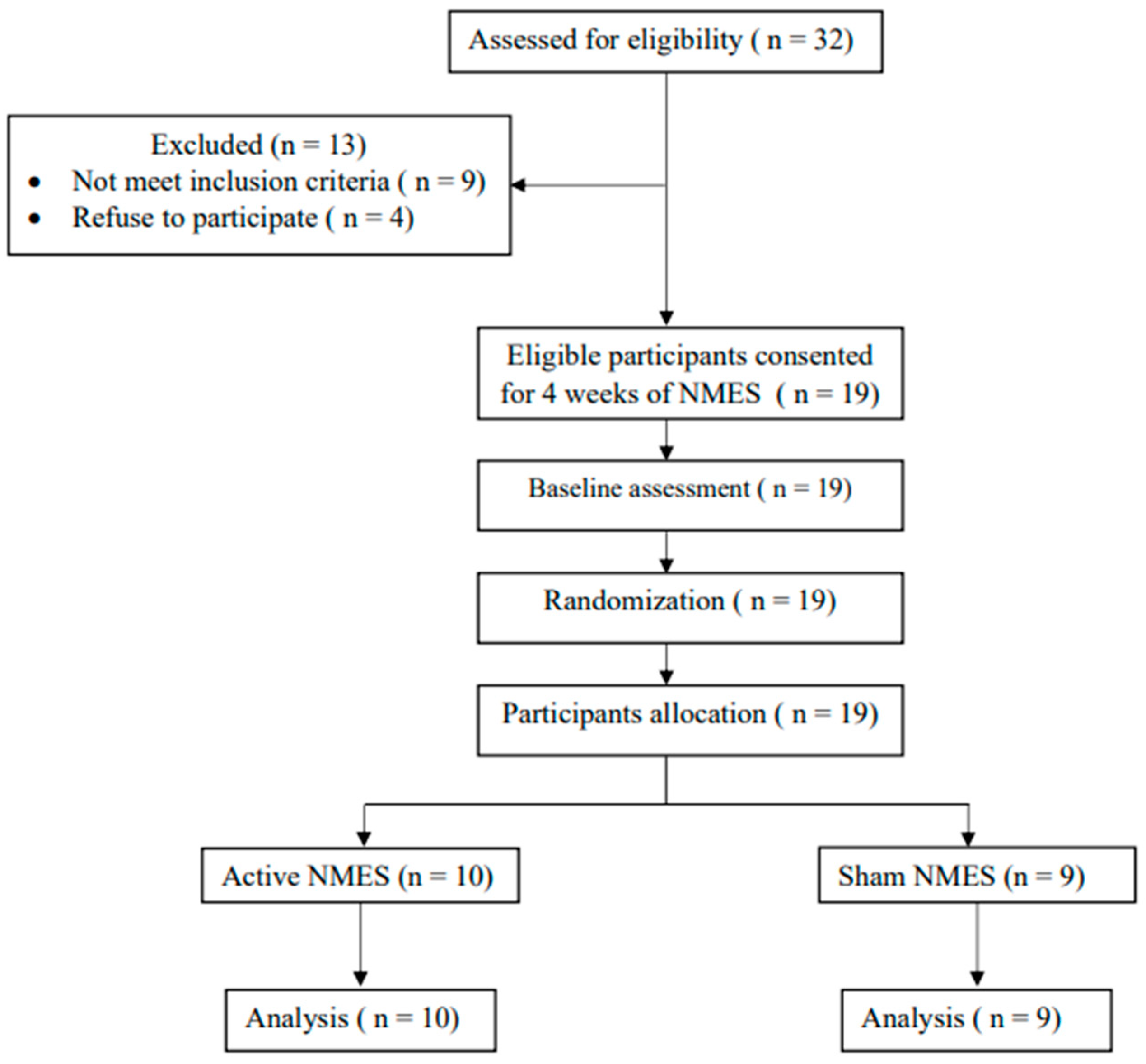
2.3. Participants
2.4. Data Collection and Procedure
2.5. Intervention
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lindsay, M.P.; Norrving, B.; Sacco, R.L.; Brainin, M.; Hacke, W.; Martins, S.; Pandian, J.; Feigin, V. World Stroke Organization (WSO): Global Stroke Fact Sheet. 2019. Available online: https://journals.sagepub.com/doi/10.1177/1747493019881353 (accessed on 2 October 2020).
- Alqahtani, B.A.; Alenazi, A.M.; Hoover, J.C.; Alshehri, M.M.; Alghamdi, M.S.; Osailan, A.M.; Khunti, K. Incidence of stroke among Saudi population: A systematic review and meta-analysis. Neurol. Sci. 2020, 41, 3099–3104. [Google Scholar] [CrossRef] [PubMed]
- Thibaut, A.; Chatelle, C.; Ziegler, E.; Bruno, M.-A.; Laureys, S.; Gosseries, O. Spasticity after stroke: Physiology, assessment and treatment. Brain Inj. 2013, 27, 1093–1105. [Google Scholar] [CrossRef] [PubMed]
- Motta-Oishi, A.A.P.; Magalhães, F.H.; de Azevedo, F.M. Neuromuscular electrical stimulation for stroke rehabilitation: Is spinal plasticity a possible mechanism associated with diminished spasticity? Med. Hypotheses 2013, 81, 784–788. [Google Scholar] [CrossRef] [PubMed]
- Sommerfeld, D.K.; Eek, E.U.-B.; Svensson, A.-K.; Holmqvist, L.W.; Von Arbin, M.H. Spasticity after stroke: Its occurrence and association with motor impairments and activity limitations. Stroke 2004, 35, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Schinwelski, M.; Sławek, J. Prevalence of spasticity following stroke and its impact on quality of life with emphasis on disability in activities of daily living. Systematic review. Neurol. I Neurochir. Pol. 2010, 44, 404–411. [Google Scholar] [CrossRef]
- Demetrios, M.; Khan, F.; Turner-Stokes, L.; Brand, C.; McSweeney, S. Multidisciplinary rehabilitation following botulinum toxin and other focal intramuscular treatment for post-stroke spasticity. Cochrane Database Syst. Rev. 2013, 6, CD009689. [Google Scholar] [CrossRef]
- Lindsay, C.; Kouzouna, A.; Simcox, C.; Pandyan, A.D. Pharmacological interventions other than botulinum toxin for spasticity after stroke. Cochrane Database Syst. Rev. 2016, 10, CD010362. [Google Scholar] [CrossRef]
- Monaghan, K.; Horgan, F.; Blake, C.; Cornall, C.; Hickey, P.P.; Lyons, B.E.; Langhorne, P. Physical treatment interventions for managing spasticity after stroke. Cochrane Database Syst. Rev. 2017, 13, CD009188. [Google Scholar] [CrossRef]
- Cabanas-Valdés, R.; Calvo-Sanz, J.; Urrùtia, G.; Serra-Llobet, P.; Pérez-Bellmunt, A.; Germán-Romero, A. The effectiveness of extracorporeal shock wave therapy to reduce lower limb spasticity in stroke patients: A systematic review and meta-analysis. Top. Stroke Rehabil. 2020, 27, 137–157. [Google Scholar] [CrossRef]
- Jung, K.-S.; In, T.-S.; Cho, H. Effects of sit-to-stand training combined with transcutaneous electrical stimulation on spasticity, muscle strength and balance ability in patients with stroke: A randomized controlled study. Gait Posture 2017, 54, 183–187. [Google Scholar] [CrossRef]
- Stein, C.; Fritsch, C.G.; Robinson, C.; Sbruzzi, G.; Plentz, R.D.M. Effects of electrical stimulation in spastic muscles after stroke: Systematic review and meta-analysis of randomized controlled trials. Stroke 2015, 46, 2197–2205. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Meng, F.; Zhang, Y.; Xu, M.; Yue, S. Full-movement neuromuscular electrical stimulation improves plantar flexor spasticity and ankle active dorsiflexion in stroke patients: A randomized controlled study. Clin. Rehabil. 2016, 30, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.; Mattison, P.; Paul, L.; Wood, L. The effects of transcutaneous electrical nerve stimulation (TENS) on spasticity in multiple sclerosis. Mult. Scler. J. 2007, 13, 527–533. [Google Scholar] [CrossRef]
- Mangold, S.; Schuster, C.; Keller, T.; Zimmermann-Schlatter, A.; Ettlin, T. Motor training of upper extremity with functional electrical stimulation in early stroke rehabilitation. Neurorehabilit. Neural Repair 2009, 23, 184–290. [Google Scholar] [CrossRef] [PubMed]
- Mehrholz, J.; Wagner, K.; Rutte, K.; Meiβner, D.; Pohl, M. Predictive validity and responsiveness of the functional ambulation category in hemiparetic patients after stroke. Arch. Phys. Med. Rehabil. 2007, 88, 1314–1319. [Google Scholar] [CrossRef] [PubMed]
- Charalambous, C.P. Interrater reliability of a modified Ashworth scale of muscle spasticity. In Classic Papers in Orthopaedics; Springer: London, UK, 2014. [Google Scholar]
- Collen, F.M.; Wade, D.T.; Bradshaw, C.M. Mobility after stroke: Reliability of measures of impairment and disability. Int. Disabil. Stud. 1990, 12, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Perera, S.; Mody, S.H.; Woodman, R.C.; Studenski, S.A. Meaningful change and responsiveness in common physical performance measures in older adults. J. Am. Geriatr. Soc. 2006, 54, 743–749. [Google Scholar] [CrossRef]
- Mesci, N.; Ozdemir, F.; Kabayel, D.D.; Tokuc, B. The effects of neuromuscular electrical stimulation on clinical improvement in hemiplegic lower extremity rehabilitation in chronic stroke: A single-blind, randomised, controlled trial. Disabil. Rehabil. 2009, 31, 2047–2054. [Google Scholar] [CrossRef]
- Yang, Y.-R.; Mi, P.-L.; Huang, S.-F.; Chiu, S.-L.; Liu, Y.-C.; Wang, R.-Y. Effects of neuromuscular electrical stimulation on gait performance in chronic stroke with inadequate ankle control-A randomized controlled trial. PLoS ONE 2018, 13, e0208609. [Google Scholar] [CrossRef]
- Pease, W.S. Therapeutic Electrical Stimulation for Spasticity: Quantitative Gait Analysis: 1. Am. J. Phys. Med. Rehabil. 1998, 77, 351–355. [Google Scholar] [CrossRef]
- Weingarden, H.; Ring, H. Functional electrical stimulation-induced neural changes and recovery after stroke. Eura Medicophys 2006, 42, 87–90. [Google Scholar] [PubMed]
- Kimberley, T.J.; Lewis, S.M.; Auerbach, E.J.; Dorsey, L.L.; Lojovich, J.M.; Carey, J.R. Electrical stimulation driving functional improvements and cortical changes in subjects with stroke. Exp. Brain Res. 2004, 154, 450–460. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.K.; Cho, S.H.; Jeon, H.S.; Lee, Y.H.; Song, J.C.; Jang, S.H.; Lee, C.H.; Kwon, Y.H. Cortical effect and functional recovery by the electromyography-triggered neuromuscular stimulation in chronic stroke patients. Neurosci. Lett. 2008, 442, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Hines, A.E.; Crago, P.E.; Billian, C. Functional electrical stimulation for the reduction of spasticity in the hemiplegic hand. Biomed. Sci. Instrum. 1993, 29, 259–266. [Google Scholar]
- Carda, S.; Molteni, F. Taping versus electrical stimulation after botulinum toxin type A injection for wrist and finger spasticity. A case—Control study. Clin. Rehabil. 2005, 19, 621–626. [Google Scholar] [CrossRef]
- Low, J.; Reed, A. Electrotherapy Explained: Principles and Practice; Butterworth-Heinemann: Oxford, UK, 2000. [Google Scholar]
- Reed, B. The physiology of neuromuscular electrical stimulation. Pediatr. Phys. Ther. 1997, 9, 96–102. [Google Scholar] [CrossRef]
- Allen, K.; Goodman, C. Using Electrical Stimulation: A Guideline for Allied Health Professionals; Sydney Local Health District and Royal Rehabilitation Centre: Sydney, Australia, 2014; Available online: https://www.alliedhealthsupport.com/wp-content/uploads/2019/03/Using-Electrical-Stimulation_A-guideline-for-allied-health-professionals-January-2014.pdf (accessed on 2 October 2020).
- Takeda, K.; Tanino, G.; Miyasaka, H. Review of devices used in neuromuscular electrical stimulation for stroke rehabilitation. Med. Devices Evid. Res. 2017, 10, 207–213. [Google Scholar] [CrossRef]
- Dantas, M.T.A.P.; Fernani, D.C.G.L.; Silva, T.D.; da Assis, I.S.A.; de Carvalho, A.C.; de Silva, S.B.; Abreu, L.C.D.; Barbieri, F.A.; Monteiro, C.B.D.M. Gait Training with Functional Electrical Stimulation Improves Mobility in People Post-Stroke. Int. J. Environ. Res. Public Health 2023, 20, 5728. [Google Scholar] [CrossRef]
| Components | Time | Description |
|---|---|---|
| Warming up | 5 min | Bicycling using a stationary bicycle or ergometer. |
| Stretching exercise | 5 min | Unilateral for the following muscle groups: wrist flexors, biceps, pectoral major, shoulder extensors, quadriceps, hamstrings, gastrocnemius, and thigh adductors. |
| Strengthening exercise | 5 min | Upper extremity strengthening exercise using small pulley weight. Lower extremity strengthening exercise using quadriceps chair. |
| Postural control and balance | 3 min | Sit to stand transition with symmetrical weight bearing and trunk rotation. |
| 3 min | Dynamic balance activity includes low frequency sway and increase weight shifting on the affected side. | |
| 4 min | Gentle perturbations to displace center of gravity (COG) using a gymnastic ball or equilibrium. | |
| Upper extremity control | 5 min | Moving the upper extremity with emphasis on scapular motion. For example, hand to mouth, hand to opposite side, and hand functions. |
| Grasping and releasing objective. | ||
| Lower extremity control | 3 min | Pre-gait mat activity includes bridging, hook lying, and lower trunk rotation. |
| Gait training | 12 min | Gait training using parallel bar; gait training includes forward, backward, sideward step, and in crossed pattern. |
| Carrier Wave | 2.5 kHz |
|---|---|
| Burst | 80 Hz |
| On time | 5 s |
| Off time | 15 s |
| Treatment time | 30 min |
| Current type | Constant current |
| Waveform | Sinusoidal |
| Active NMES (n = 10) | Sham NMES (n = 9) | p-Value | |
|---|---|---|---|
| Age (years), mean ± SD | 57.9 ± 9.9 | 54.7 ± 12.3 | 0.55 |
| Sex, males, n (%) | 10 (100) | 7 (77) | 0.21 |
| Time since stroke (months), mean ± SD | 32.5 ± 34 | 26.44 ± 30 | 0.69 |
| Side of paralysis, left, n (%) | 6 (60.0) | 7 (77.8) | 0.62 |
| MAS, mean ± SD | 2.8 ± 0.9 | 3.4 ± 1 | 0.16 |
| 10-MWT (s), mean ± SD | 24.22 ± 21 | 16.97 ± 7.8 | 0.84 |
| FAC, mean ± SD | 3.80 ± 0.63 | 3.33 ± 0.86 | 0.35 |
| Active NMES (n = 10) | p-Value | Sham NMES (n = 9) | p-Value | |
|---|---|---|---|---|
| MAS, mean difference ± SD | 1.00 ± 0.66 | 0.008 | 0.22 ± 1.1 | 0.57 |
| 10-MWT (s), mean difference ± SD | 4.27 ± 6.7 | 0.028 | 1.76 ± 1.42 | 0.011 |
| FAC, mean difference ± SD | −0.40 ± 0.51 | 0.046 | −0.22 ± 0.66 | 0.32 |
| Active NMES (n = 10) | Sham NMES (n = 9) | p-Value | |
|---|---|---|---|
| MAS, mean ± SD | 1.80 ± 0.91 | 3.22 ± 0.83 | 0.006 |
| 10-MWT (s), mean ± SD | 19.95 ± 16.22 | 15.20 ± 7.15 | 0.96 |
| FAC, mean ± SD | 4.20 ± 0.42 | 3.55 ± 0.72 | 0.053 |
| Active NMES (n = 10) | Sham NMES (n = 9) | p-Value | |
|---|---|---|---|
| MAS, percent change | 35.00 | −10.74 | 0.035 |
| 10-MWT (s), percent change | 15.59 | 9.82 | 0.35 |
| FAC, percent change | −11.11 | −12.50 | 0.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almutairi, S.M.; Khalil, M.E.; Almutairi, N.; Alsaadoon, S.M.; Alharbi, D.S.; Al Assadi, S.D.; Alghamdi, S.F.; Albattah, S.N.; Alenazi, A.M. Effects of Neuromuscular Electrical Stimulation on Spasticity and Walking Performance among Individuals with Chronic Stroke: A Pilot Randomized Clinical Trial. Healthcare 2023, 11, 3137. https://doi.org/10.3390/healthcare11243137
Almutairi SM, Khalil ME, Almutairi N, Alsaadoon SM, Alharbi DS, Al Assadi SD, Alghamdi SF, Albattah SN, Alenazi AM. Effects of Neuromuscular Electrical Stimulation on Spasticity and Walking Performance among Individuals with Chronic Stroke: A Pilot Randomized Clinical Trial. Healthcare. 2023; 11(24):3137. https://doi.org/10.3390/healthcare11243137
Chicago/Turabian StyleAlmutairi, Sattam M., Mohamed E. Khalil, Nadiah Almutairi, Saud M. Alsaadoon, Dalal S. Alharbi, Sultan D. Al Assadi, Salem F. Alghamdi, Sahar N. Albattah, and Aqeel M. Alenazi. 2023. "Effects of Neuromuscular Electrical Stimulation on Spasticity and Walking Performance among Individuals with Chronic Stroke: A Pilot Randomized Clinical Trial" Healthcare 11, no. 24: 3137. https://doi.org/10.3390/healthcare11243137





