Can Gastric Juice Analysis with EndoFaster® Reduce the Environmental Impact of Upper Endoscopy?
Abstract
:1. Introduction
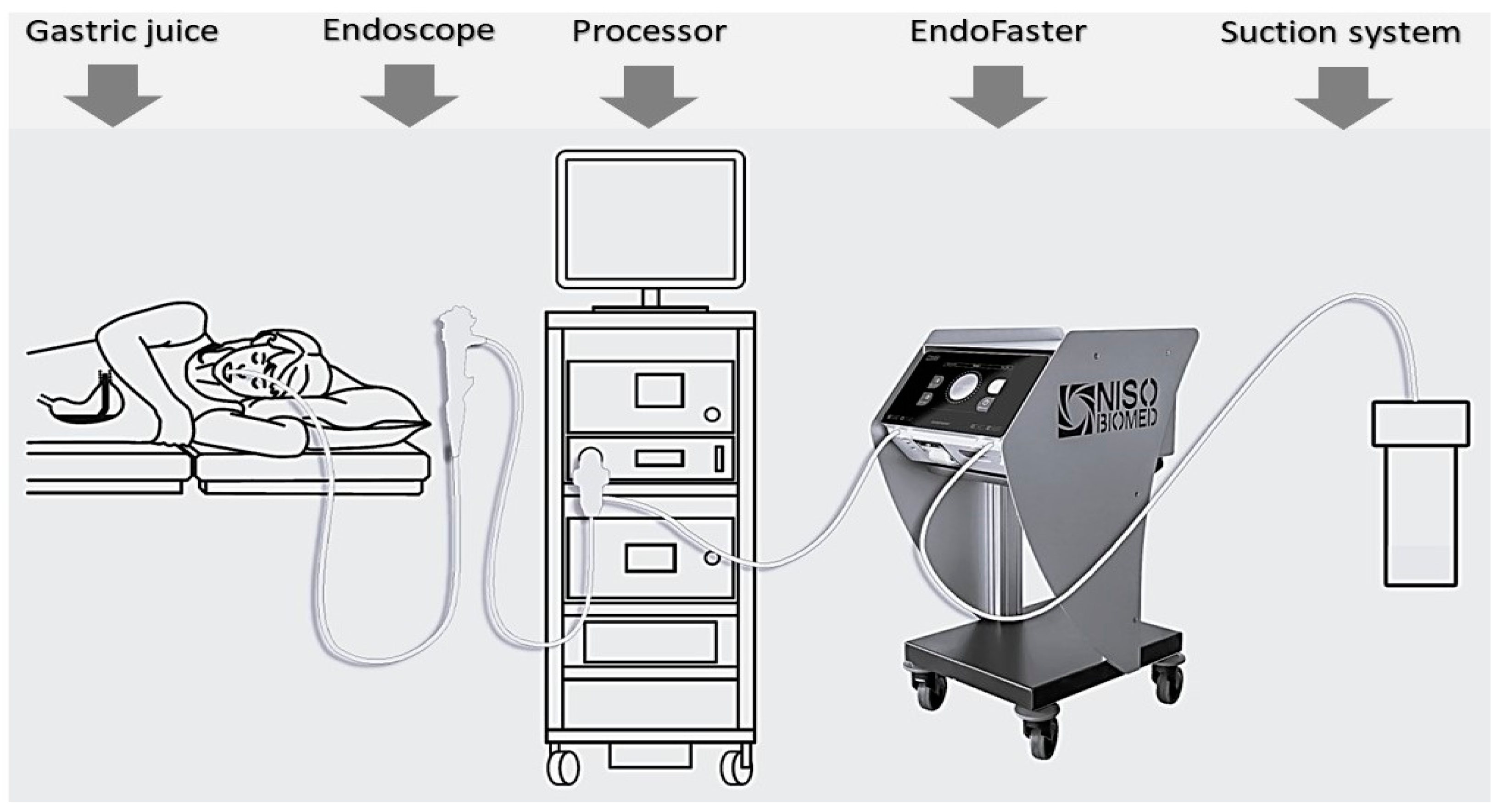
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Santiago, E.R.; Dinis-Ribeiro, M. Reducing the environmental footprint of gastrointestinal endoscopy: European Society of Gastrointestinal Endoscopy (ESGE) and European Society of Gastroenterology and Endoscopy Nurses and Associates (ESGENA) Position Statement. Endoscopy 2022, 54, 797–826. [Google Scholar] [CrossRef] [PubMed]
- Bortoluzzi, F.; Sorge, A. Sustainability in gastroenterology and digestive endoscopy: Position Paper from the Italian Association of Hospital Gastroenterologists and Digestive Endoscopists (AIGO). Dig. Liver Dis. 2022, 54, 1623–1629. [Google Scholar] [CrossRef] [PubMed]
- Lenzen, M.; Malik, A. The environmental footprint of health care: A global assessment. Lancet Planet. Health 2020, 4, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Vaccari, M.; Tudor, T. Costs associated with the management of waste from healthcare facilities: An analysis at national and site level. Waste Manag. Res. 2018, 36, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Malfertheiner, P.; Megraud, F. Management of Helicobacter pylori infection: The Maastricht VI/Florence consensus report. Gut 2022, 71, 1724–1762. [Google Scholar] [CrossRef] [PubMed]
- Pimentel-Nunes, P.; Libanio, D. Management of epithelial precancerous conditions and lesions in the stomach (MAPS II): European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter and Microbiota Study Group (EHMSG), European Society of Pathology (ESP), and Sociedade Portuguesa de Endoscopia Digestiva (SPED) guideline update 2019. Endoscopy 2019, 51, 365–388. [Google Scholar] [CrossRef] [PubMed]
- Pouw, R.E.; Barret, M. Endoscopic tissue sampling—Part 1: Upper gastrointestinal and hepatopancreatobiliary tracts. European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy 2021, 53, 1174–1188. [Google Scholar] [CrossRef] [PubMed]
- Banks, M.; Graham, D. British Society of Gastroenterology guidelines on the diagnosis and management of patients at risk of gastric adenocarcinoma. Gut 2019, 68, 1545–1575. [Google Scholar] [CrossRef] [PubMed]
- De Francesco, V. Alicante. Quality performance measures in upper gastrointestinal endoscopy for lesion detection: Italian AIGO-SIED-SIGE joint position statement. Dig. Liver Dis. 2022, 54, 1479–1485. [Google Scholar] [CrossRef] [PubMed]
- Lahner, E.; Zullo, A. Detection of gastric precancerous conditions in daily clinical practice: A nationwide survey. Helicobacter 2014, 19, 417–424. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, H.J. Forty years of Helicobacter pylori infection and changes in findings at esophagogastroduodenoscopy. Helicobacter 2023, 28, e13026. [Google Scholar] [CrossRef] [PubMed]
- Vasapolli, R.; Ailloud, F. Intraprocedural gastric juice analysis as compared to rapid urease test for real-time detection of Helicobacter pylori. World J. Gastroenterol. 2023, 29, 1638–1647. [Google Scholar] [CrossRef] [PubMed]
- Esposito, G.; Libanio, D. Real-time assessment of H. pylori during the endoscopic assessment of individuals with gastric intestinal metaplasia: A possible way to reduce the burden of care. Eur. J. Gastroenterol. Hepatol. 2023, 35, 1154–1158. [Google Scholar] [CrossRef] [PubMed]
- Caputo, A. Indicatori di Efficienza e Decarbonizzazione del Sistema Energetico Nazionale e del Settore Elettrico; Rapporti 343/2021; ISPRA: Rome, Italy, 2021; ISBN 978-88-448-1049-8. [Google Scholar]
- Gordon, I.O.; Sherman, J.D. Life Cycle Greenhouse Gas Emissions of Gastrointestinal Biopsies in a Surgical Pathology Laboratory. Am. J. Clin. Pathol. 2021, 156, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Material Economics. Industrial Transformation 2050: Pathways to Net-Zero Emissions from EU Heavy Industry; University of Cambridge Institute for Sustainability Leadership (CISL): Cambridge, UK, 2019. [Google Scholar]
- Pipatti, R.; Sharma, C. Waste generation, composition and management data. In 2006 IPCC Guidelines for National Greenhouse Gas Inventories; Eggleston, S., Buendia, L., Eds.; Institute for Global Environmental Strategies: Hayama, Japan, 2006; Volume 5. [Google Scholar]
- Zullo, A.; Brighi, S. Current practice for upper gastrointestinal endoscopy: A multicentre study in Lazio, Italy. J. Gastrointestin Liver Dis. 2023, 32, 261–262. [Google Scholar] [CrossRef] [PubMed]
- Park, S.B.; Cha, J.M. Gastrointestinal endoscopy’s carbon footprint. Clin. Endosc. 2023, 56, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, S.; Dhar, A. Green endoscopy: British Society of Gastroenterology (BSG), Joint Accreditation Group (JAG) and Centre for Sustainable Health (CSH) joint consensus on practical measures for environmental sustainability in endoscopy. Gut 2023, 72, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Yong, K.K.; He, Y. Rationalising the use of specimen pots following colorectal polypectomy: A small step towards greener endoscopy. Frontline Gastroenterol. 2023, 14, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Costamagna, G.; Zullo, A. Real-time diagnosis of H. pylori infection during endoscopy: Accuracy of an innovative tool (EndoFaster). United Eur. Gastroenterol. J. 2015, 4, 339–342. [Google Scholar] [CrossRef] [PubMed]

| Material | Type | Quantity (g) | Waster Disposal | Quantity/ Test | kg CO2/ kg Waste | kg CO2/ Test |
|---|---|---|---|---|---|---|
| Bottle 1 for calibration + liquid-draining system | Polyethylene (PE) | 78.6 | Plastic recycling | 1.31 | 3.0 | 0.004 |
| Bottle 2 for calibration + liquid-draining system | PE | 51.6 | Plastic recycling | 0.86 | 3.0 | 0.003 |
| Bottle 3 for calibration + liquid-draining system | PE | 51.6 | Plastic recycling | 0.86 | 3.0 | 0.003 |
| Cardboard box for the 3 bottles | Carton | 137 | Cardboard recycling | 2.28 | 0.95 | 0.002 |
| Washing solution tank | High-density polyethylene (HDPE) | 146 | Plastic recycling | 2.43 | 3.0 | 0.007 |
| Gastric juice suction tube | Plastic (mixed) | 25 | Infected waste | 2.08 | 3.0 | 0.006 |
| Biopsy forceps | Plastic (mixed) | 20 | Infected waste | 1 | 3.0 | 0.060 |
| Biopsy jar | Plastic | - | Infected waste | 2 | 3.0 | 0.081 |
| Total procedures/year | 2000 |
| Standard biopsy sampling | 90% |
| Procedures with biopsy sampling | 1800 |
| Reduction in biopsies through the use of EndoFaster® | 50% |
| Biopsy sampling avoided | 900 |
| kg CO2 produced by EndoFaster® materials | 50 |
| kg CO2 produced by EndoFaster® due to energy consumption and liquid residue disposal | 22 |
| kg CO2 total normally produced (without biopsy reduction) | 1262 |
| kg CO2 total saved yearly through the use of EndoFaster® | 630 |
| kg CO2 reduction | 558 |
| Percentage of CO2 reduction | 44% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zullo, A.; Chiovelli, F.; Esposito, E.; Hassan, C.; Casini, B. Can Gastric Juice Analysis with EndoFaster® Reduce the Environmental Impact of Upper Endoscopy? Healthcare 2023, 11, 3186. https://doi.org/10.3390/healthcare11243186
Zullo A, Chiovelli F, Esposito E, Hassan C, Casini B. Can Gastric Juice Analysis with EndoFaster® Reduce the Environmental Impact of Upper Endoscopy? Healthcare. 2023; 11(24):3186. https://doi.org/10.3390/healthcare11243186
Chicago/Turabian StyleZullo, Angelo, Federica Chiovelli, Enrica Esposito, Cesare Hassan, and Beatrice Casini. 2023. "Can Gastric Juice Analysis with EndoFaster® Reduce the Environmental Impact of Upper Endoscopy?" Healthcare 11, no. 24: 3186. https://doi.org/10.3390/healthcare11243186
APA StyleZullo, A., Chiovelli, F., Esposito, E., Hassan, C., & Casini, B. (2023). Can Gastric Juice Analysis with EndoFaster® Reduce the Environmental Impact of Upper Endoscopy? Healthcare, 11(24), 3186. https://doi.org/10.3390/healthcare11243186







