Development of Clinical Prediction Score for Chemotherapy Response in Advanced Non-Small Cell Lung Cancer Patients
Abstract
:1. Introduction
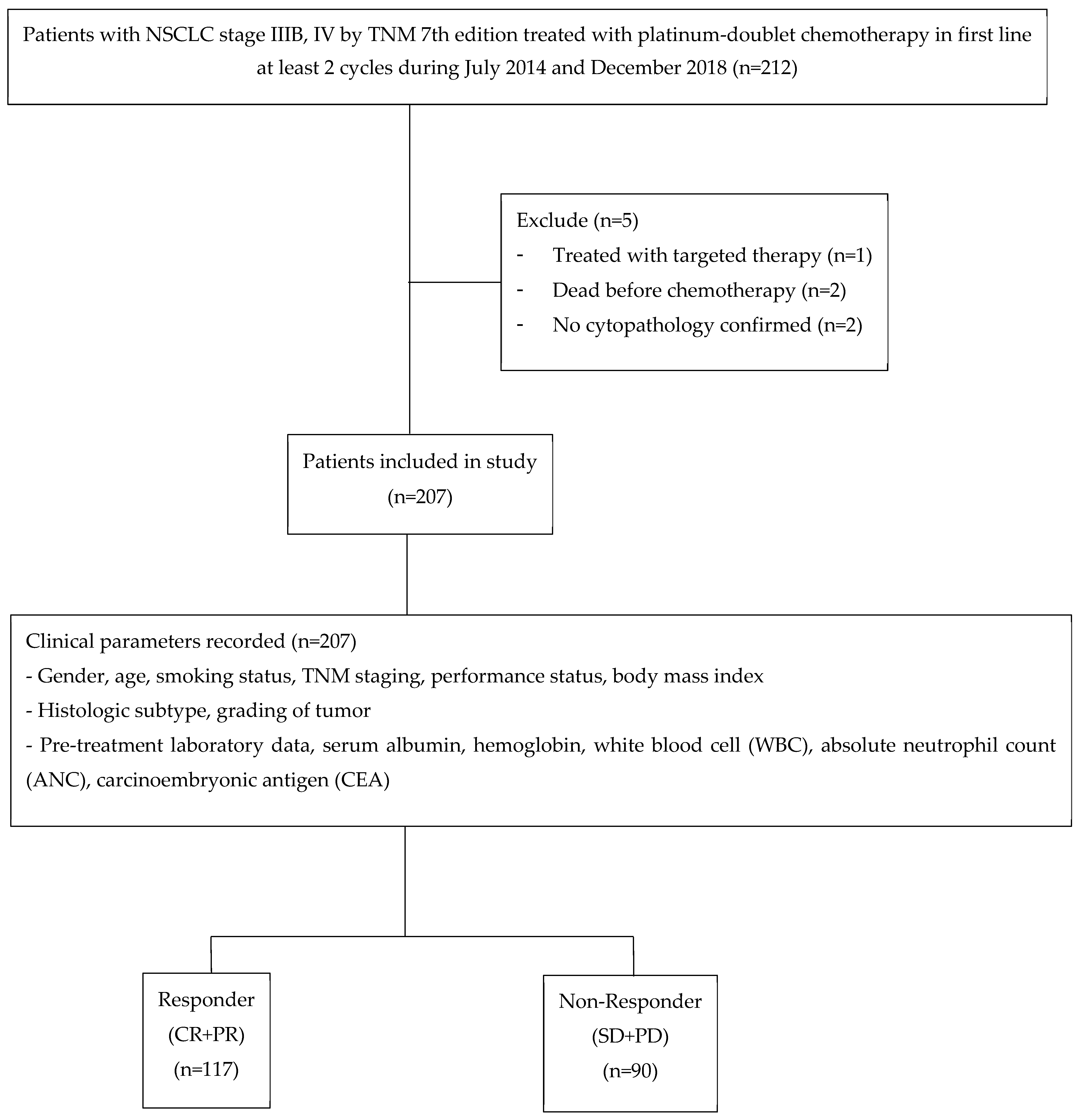
2. Materials and Methods
2.1. Participants
2.2. Outcome
2.3. Predictors
2.4. Statistical Analysis
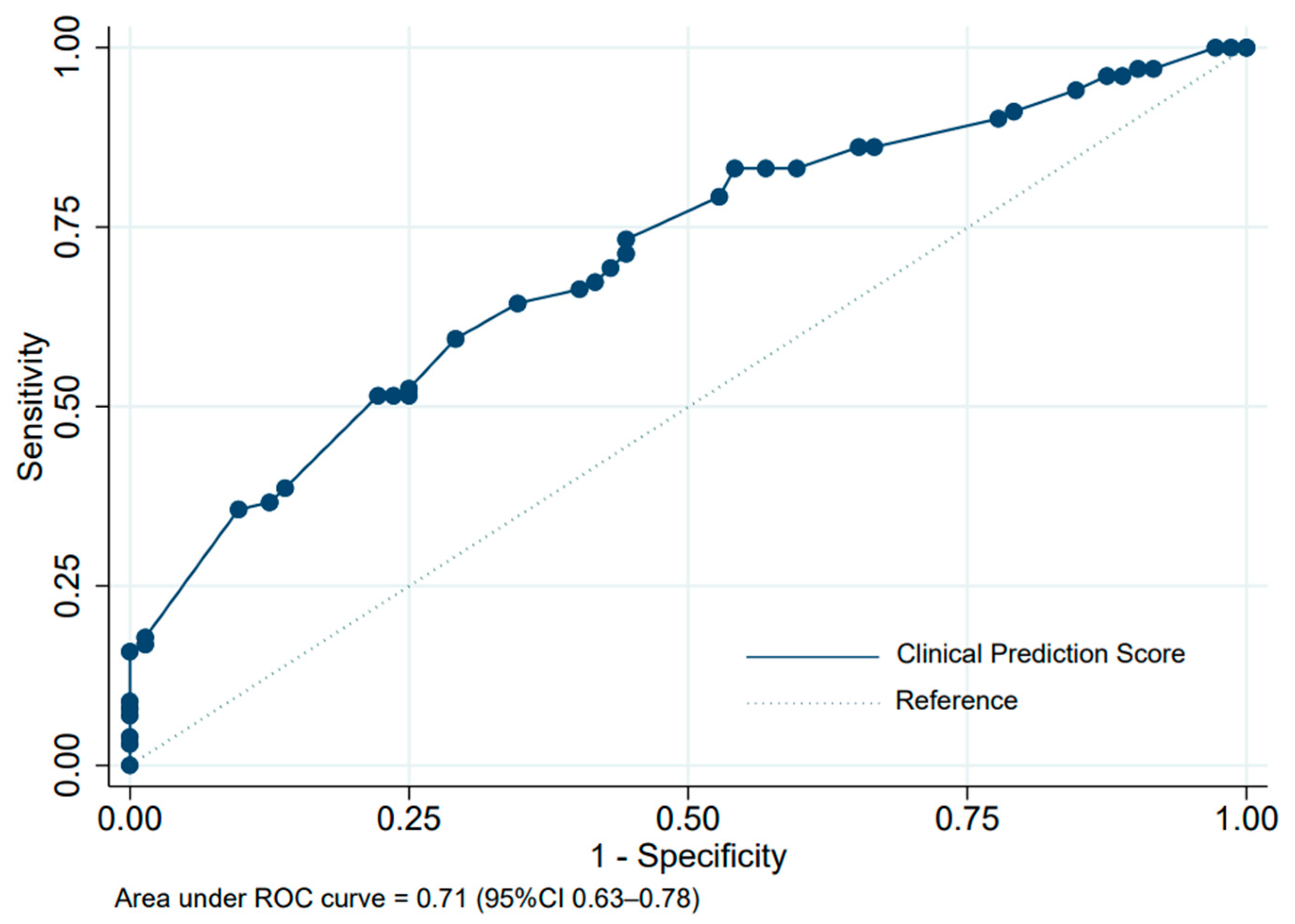
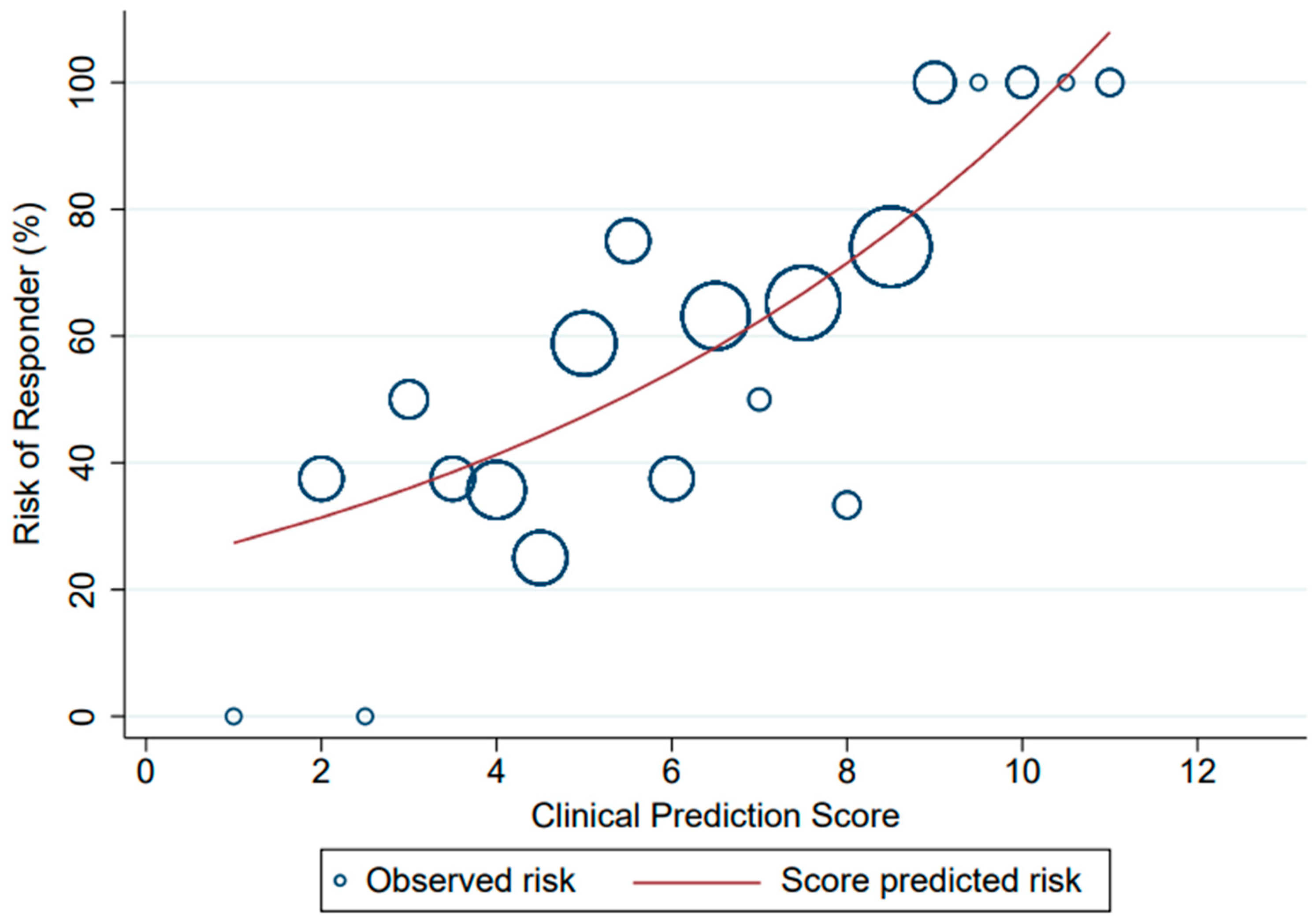
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Mithoowani, H.; Febbraro, M. Non-Small-Cell Lung Cancer in 2022: A Review for General Practitioners in Oncology. Curr. Oncol. 2022, 29, 1828–1839. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Leighl, N.B.; Wu, Y.-L.; Zhong, W.-Z. Emerging Therapies for Non-Small Cell Lung Cancer. J. Hematol. Oncol. 2019, 12, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eaton, K.D.; Jagels, B.; Martins, R.G. Value-Based Care in Lung Cancer. Oncologist 2016, 21, 903–906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Permsuwan, U.; Niamhun, N.; Tanatip, N.; Thongprasert, S. Epidermal Growth Factor Receptor Mutation Testing in Thailand: A Cost-Utility Analysis. Value Health Reg. Issues 2014, 3, 39–43. [Google Scholar] [CrossRef]
- Johnson, S.B.; Park, H.S.; Gross, C.P.; Yu, J.B. Complementary Medicine, Refusal of Conventional Cancer Therapy, and Survival Among Patients with Curable Cancers. JAMA Oncol. 2018, 4, 1375–1381. [Google Scholar] [CrossRef]
- Zhong, J.; Zheng, Q.; Gao, E.; Dong, Z.; Zhao, J.; An, T.; Wu, M.; Zhuo, M.; Wang, Y.; Li, J.; et al. Influence of Body Mass Index on the Therapeutic Efficacy of Gemcitabine plus Cisplatin and Overall Survival in Lung Squamous Cell Carcinoma. Thorac. Cancer 2018, 9, 291–297. [Google Scholar] [CrossRef] [Green Version]
- Imai, H.; Mori, K.; Ono, A.; Akamatsu, H.; Taira, T.; Kenmotsu, H.; Naito, T.; Kaira, K.; Murakami, H.; Endo, M.; et al. Individual-Level Data on the Relationships of Progression-Free Survival and Post-Progression Survival with Overall Survival in Patients with Advanced Non-Squamous Non-Small Cell Lung Cancer Patients Who Received Second-Line Chemotherapy. Med. Oncol. 2014, 31, 88. [Google Scholar] [CrossRef]
- Sirohi, B.; Ashley, S.; Norton, A.; Popat, S.; Hughes, S.; Papadopoulos, P.; Priest, K.; O’Brien, M. Early Response to Platinum-Based First-Line Chemotherapy in Non-Small Cell Lung Cancer May Predict Survival. J. Thorac. Oncol. 2007, 2, 735–740. [Google Scholar] [CrossRef] [Green Version]
- Tas, F.; Ciftci, R.; Kilic, L.; Karabulut, S. Age Is a Prognostic Factor Affecting Survival in Lung Cancer Patients. Oncol. Lett. 2013, 6, 1507–1513. [Google Scholar] [CrossRef]
- Yu, X.Q.; Yap, M.L.; Cheng, E.S.; Ngo, P.J.; Vaneckova, P.; Karikios, D.; Canfell, K.; Weber, M.F. Evaluating Prognostic Factors for Sex Differences in Lung Cancer Survival: Findings from a Large Australian Cohort. J. Thorac. Oncol. 2022, 17, 688–699. [Google Scholar] [CrossRef]
- Mitchell, P.; Mok, T.; Barraclough, H.; Strizek, A.; Lew, R.; van Kooten, M. Smoking History as a Predictive Factor of Treatment Response in Advanced Non–Small-Cell Lung Cancer: A Systematic Review. Clin. Lung Cancer 2012, 13, 239–251. [Google Scholar] [CrossRef]
- Simmons, C.P.; Koinis, F.; Fallon, M.T.; Fearon, K.C.; Bowden, J.; Solheim, T.S.; Gronberg, B.H.; McMillan, D.C.; Gioulbasanis, I.; Laird, B.J. Prognosis in Advanced Lung Cancer—A Prospective Study Examining Key Clinicopathological Factors. Lung Cancer 2015, 88, 304–309. [Google Scholar] [CrossRef] [Green Version]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and Classification of Cancer Cachexia: An International Consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Kasymjanova, G.; MacDonald, N.; Agulnik, J.S.; Cohen, V.; Pepe, C.; Kreisman, H.; Sharma, R.; Small, D. The Predictive Value of Pre-Treatment Inflammatory Markers in Advanced Non-Small-Cell Lung Cancer. Curr. Oncol. 2010, 17, 52–58. [Google Scholar]
- Grunnet, M.; Sorensen, J.B. Carcinoembryonic Antigen (CEA) as Tumor Marker in Lung Cancer. Lung Cancer 2012, 76, 138–143. [Google Scholar] [CrossRef]
- Yuan, M.; Huang, L.-L.; Chen, J.-H.; Wu, J.; Xu, Q. The Emerging Treatment Landscape of Targeted Therapy in Non-Small-Cell Lung Cancer. Signal Transduct Target Ther. 2019, 4, 61. [Google Scholar] [CrossRef] [Green Version]
- Majeed, U.; Manochakian, R.; Zhao, Y.; Lou, Y. Targeted Therapy in Advanced Non-Small Cell Lung Cancer: Current Advances and Future Trends. J. Hematol. Oncol. 2021, 14, 108. [Google Scholar] [CrossRef]
- Olaussen, K.A.; Dunant, A.; Fouret, P.; Brambilla, E.; André, F.; Haddad, V.; Taranchon, E.; Filipits, M.; Pirker, R.; Popper, H.H.; et al. DNA Repair by ERCC1 in Non-Small-Cell Lung Cancer and Cisplatin-Based Adjuvant Chemotherapy. N. Engl. J. Med. 2006, 355, 983–991. [Google Scholar] [CrossRef]
- Olaussen, K.A.; Fouret, P.; Kroemer, G. ERCC1-Specific Immunostaining in Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2007, 357, 1559–1561. [Google Scholar] [CrossRef]
- Rosell, R.; Danenberg, K.D.; Alberola, V.; Bepler, G.; Sanchez, J.J.; Camps, C.; Provencio, M.; Isla, D.; Taron, M.; Diz, P.; et al. Ribonucleotide Reductase Messenger RNA Expression and Survival in Gemcitabine/cisplatin-Treated Advanced Non-Small Cell Lung Cancer Patients. Clin. Cancer Res. 2004, 10, 1318–1325. [Google Scholar] [CrossRef] [PubMed]
- Olaussen, K.A.; Postel-Vinay, S. Predictors of Chemotherapy Efficacy in Non-Small-Cell Lung Cancer: A Challenging Landscape. Ann. Oncol. 2016, 27, 2004–2016. [Google Scholar] [CrossRef] [PubMed]
- Ravanelli, M.; Farina, D.; Morassi, M.; Roca, E.; Cavalleri, G.; Tassi, G.; Maroldi, R. Texture Analysis of Advanced Non-Small Cell Lung Cancer (NSCLC) on Contrast-Enhanced Computed Tomography: Prediction of the Response to the First-Line Chemotherapy. Eur. Radiol. 2013, 23, 3450–3455. [Google Scholar] [CrossRef]
- Yu, J.; Li, W.; Zhang, Z.; Yu, T.; Li, D. Prediction of Early Response to Chemotherapy in Lung Cancer by Using Diffusion-Weighted MR Imaging. Sci. World J. 2014, 2014, 135841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yabuuchi, H.; Kawanami, S.; Iwama, E.; Okamoto, I.; Kamitani, T.; Sagiyama, K.; Yamasaki, Y.; Honda, H. Prediction of Therapeutic Effect of Chemotherapy for NSCLC Using Dual-Input Perfusion CT Analysis: Comparison among Bevacizumab Treatment, Two-Agent Platinum-Based Therapy without Bevacizumab, and Other Non-Bevacizumab Treatment Groups. Radiology 2018, 286, 685–695. [Google Scholar] [CrossRef] [Green Version]
- Badawy, A.; Khedr, G.; Arafat, W.; Bae, S.; Omar, A.; Grant, S. P2.03a-033 Prediction of Response to First Line Treatment for Metastatic Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2017, 12, S908. [Google Scholar] [CrossRef]
| Characteristics | Responder | Non-Responder | p-Value | ||
|---|---|---|---|---|---|
| CR + PR (n = 117) | SD + PD (n = 90) | ||||
| n | % | n | % | ||
| I. Patient factors | |||||
| Gender | |||||
| Male | 50 | 42.7 | 55 | 61.1 | 0.011 |
| Female | 67 | 57.3 | 35 | 38.9 | |
| Age (year) * | 62.8 | (±9.6) | 61.4 | (±10.4) | 0.298 |
| Smoking status | |||||
| Current/ex-smoking | 40 | 34.2 | 33 | 38.8 | 0.554 |
| Never | 77 | 65.8 | 52 | 61.2 | |
| TNM staging | |||||
| II | 1 | 0.9 | 0 | 0.0 | 0.503 |
| IIIA | 3 | 2.6 | 2 | 2.2 | |
| IIIB | 8 | 6.8 | 4 | 4.4 | |
| IV | 105 | 89.7 | 84 | 93.4 | |
| PS | |||||
| ECOG 0–1 | 102 | 90.3 | 70 | 85.4 | 0.369 |
| ECOG ≥ 2 | 11 | 9.7 | 12 | 14.6 | |
| Significant weight loss ** | |||||
| Yes | 35 | 30.4 | 28 | 31.5 | 0.880 |
| No | 80 | 69.6 | 61 | 68.5 | |
| BMI (kg/m2) | |||||
| <18.5 | 35 | 29.9 | 35 | 38.9 | 0.251 |
| 18.5–22.9 | 48 | 41.0 | 37 | 41.1 | |
| ≥23 | 34 | 29.1 | 18 | 20.0 | |
| II. Pathological factors | |||||
| Histology | |||||
| Adenocarcinoma | 72 | 64.3 | 62 | 69.7 | 0.013 |
| Squamous cell carcinoma | 37 | 33.0 | 17 | 19.1 | |
| Large cell carcinoma | 0 | 0.0 | 1 | 1.1 | |
| NOS | 3 | 2.7 | 9 | 10.1 | |
| Tumor grading | |||||
| Well-differentiated | 5 | 4.3 | 7 | 7.8 | 0.209 |
| Moderately differentiated | 5 | 4.3 | 9 | 10.0 | |
| Poorly differentiated | 21 | 18.0 | 13 | 14.4 | |
| Undifferentiated | 0 | 0.0 | 1 | 1.1 | |
| NA | 86 | 73.4 | 60 | 66.7 | |
| III. Pre-treatment laboratory results | |||||
| Albumin (g/dL) * | 3.5 | (±0.5) | 3.4 | (±0.4) | 0.069 |
| Hemoglobin (g/dL) * | 11.4 | (±1.6) | 11.5 | (±2.3) | 0.627 |
| WBCs (cells/mm3) * | 9737.3 | (±4549.8) | 9389.8 | (±3112.6) | 0.536 |
| ANC (cells/mm3) * | 6617.6 | (±3249.3) | 6613.9 | (±2742.1) | 0.993 |
| CEA (ng/mL) *** | 47.9 | (10.9, 258.4) | 42.4 | (10.5, 116.2) | 0.411 |
| Parameters | Coefficient | RR | 95% CI of RR | p-Value |
|---|---|---|---|---|
| Female | 0.56 | 1.75 | 1.20–2.55 | 0.004 |
| Age ≥ 60 years | 0.15 | 1.16 | 0.90–1.49 | 0.259 |
| Current/ex-smoking | 0.32 | 1.38 | 0.94–2.02 | 0.100 |
| ECOG 0–1 | 0.18 | 1.20 | 0.75–1.90 | 0.447 |
| Squamous cell carcinoma | 0.35 | 1.41 | 1.10–1.81 | 0.006 |
| Albumin ≥ 3.5 mg/dL | 0.33 | 1.40 | 1.06–1.85 | 0.019 |
| Clinical Parameters | Coefficient | Transformed Coefficient | Assigned Score |
|---|---|---|---|
| Gender | |||
| Female | 0.56 | 3.83 | 4 |
| Male | - | - | 0 |
| Age | |||
| ≥60 years | 0.15 | 1.00 | 1 |
| <60 years | - | - | 0 |
| Smoking status | |||
| Current/ex-smoking | 0.32 | 2.21 | 2 |
| Never | - | - | 0 |
| ECOG | |||
| 0–1 | 0.18 | 1.24 | 1 |
| 2 | - | - | 0 |
| Albumin | |||
| ≥3.5 mg/dL | 0.33 | 2.29 | 2.5 |
| <3.5 mg/dL | - | - | 0 |
| Histologic subtype | |||
| Squamous cell carcinoma | 0.35 | 2.37 | 2.5 |
| Non-squamous cell carcinoma | - | - | 0 |
| Risk Level | Responder | Non-Responder | PPV (%) | LHR+ | 95% CI of LHR+ | p-Value |
|---|---|---|---|---|---|---|
| n (%) | n (%) | |||||
| Low (0–8) | 65 (50.0) | 65 (50.0) | 50.0 | 0.34 | 0.18–0.66 | <0.001 |
| High (8.5–13) | 36 (83.7) | 7 (16.3) | 83.7 | 3.67 | 1.73–7.77 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chayangsu, C.; Khorana, J.; Charoentum, C.; Sriuranpong, V.; Patumanond, J.; Tantraworasin, A. Development of Clinical Prediction Score for Chemotherapy Response in Advanced Non-Small Cell Lung Cancer Patients. Healthcare 2023, 11, 293. https://doi.org/10.3390/healthcare11030293
Chayangsu C, Khorana J, Charoentum C, Sriuranpong V, Patumanond J, Tantraworasin A. Development of Clinical Prediction Score for Chemotherapy Response in Advanced Non-Small Cell Lung Cancer Patients. Healthcare. 2023; 11(3):293. https://doi.org/10.3390/healthcare11030293
Chicago/Turabian StyleChayangsu, Chawalit, Jiraporn Khorana, Chaiyut Charoentum, Virote Sriuranpong, Jayanton Patumanond, and Apichat Tantraworasin. 2023. "Development of Clinical Prediction Score for Chemotherapy Response in Advanced Non-Small Cell Lung Cancer Patients" Healthcare 11, no. 3: 293. https://doi.org/10.3390/healthcare11030293





