Evaluation of Midwives’ Practises on Herpetic Infections during Pregnancy: A French Vignette-Based Study
Abstract
1. Introduction
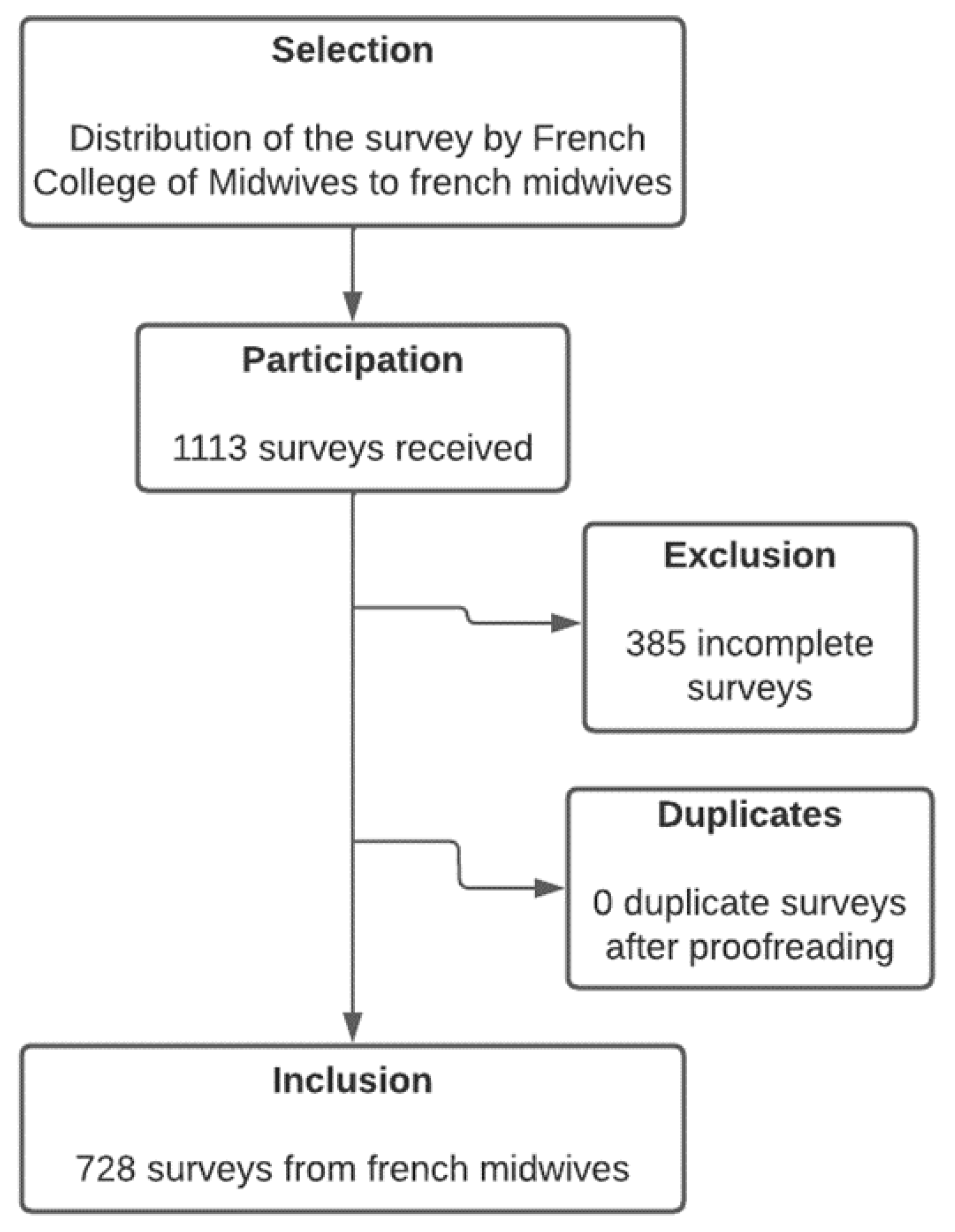
2. Materials and Methods
3. Results
4. Discussion
4.1. Main Findings
4.2. Interpretation
4.3. Strengths and Limitations
4.4. Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- James, C.; Harfouche, M.; Welton, N.J.; Turner, K.M.; Abu-Raddad, L.J.; Gottlieb, S.L.; Looker, K.J. Herpes Simplex Virus: Global Infection Prevalence and Incidence Estimates, 2016. Bull. World Health Organ. 2020, 98, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Parvathy, K.; Reghukumar, A.; Gurudas, A.; Sasidharan, K.V.; Nair, L.J. Herpes Simplex Hepatitis Presenting as Fulminant Hepatic Failure in Pregnancy. Indian J. Clin. Med. 2021, 11, 44–46. [Google Scholar] [CrossRef]
- Melvin, A.J.; Mohan, K.M.; Vora, S.B.; Selke, S.; Sullivan, E.; Wald, A. Neonatal Herpes Simplex Virus Infection: Epidemiology and Outcomes in the Modern Era. J. Pediatr. Infect. Dis. Soc. 2022, 11, 94–101. [Google Scholar] [CrossRef] [PubMed]
- James, S.H.; Sheffield, J.S.; Kimberlin, D.W. Mother-to-Child Transmission of Herpes Simplex Virus. J. Pediatr. Infect. Dis. Soc. 2014, 3, S19–S23. [Google Scholar] [CrossRef]
- Looker, K.J.; Magaret, A.S.; May, M.T.; Turner, K.M.E.; Vickerman, P.; Newman, L.M.; Gottlieb, S.L. First Estimates of the Global and Regional Incidence of Neonatal Herpes Infection. Lancet Glob. Health 2017, 5, e300–e309. [Google Scholar] [CrossRef]
- Sénat, M.-V.; Anselem, O.; Picone, O.; Renesme, L.; Sananès, N.; Vauloup-Fellous, C.; Sellier, Y.; Laplace, J.-P.; Sentilhes, L. Prevention and Management of Genital Herpes Simplex Infection during Pregnancy and Delivery: Guidelines from the French College of Gynaecologists and Obstetricians (CNGOF). Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 224, 93–101. [Google Scholar] [CrossRef]
- Kimberlin, D.W. Neonatal Herpes Simplex Infection. Clin. Microbiol. Rev. 2004, 17, 1–13. [Google Scholar] [CrossRef]
- Whitley, R.J.; Corey, L.; Arvin, A.; Lakeman, F.D.; Sumaya, C.V.; Wright, P.F.; Dunkle, L.M.; Steele, R.W.; Soong, S.J.; Nahmias, A.J. Changing Presentation of Herpes Simplex Virus Infection in Neonates. J. Infect. Dis. 1988, 158, 109–116. [Google Scholar] [CrossRef]
- Samies, N.L.; James, S.H.; Kimberlin, D.W. Neonatal Herpes Simplex Virus Disease: Updates and Continued Challenges. Clin. Perinatol. 2021, 48, 263–274. [Google Scholar] [CrossRef]
- Heggarty, E.; Sibiude, J.; Mandelbrot, L.; Vauloup-Fellous, C.; Picone, O. Genital Herpes and Pregnancy: Evaluating Practices and Knowledge of French Health Care Providers. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 249, 84–91. [Google Scholar] [CrossRef]
- Hollier, L.M.; Wendel, G.D. Third Trimester Antiviral Prophylaxis for Preventing Maternal Genital Herpes Simplex Virus (HSV) Recurrences and Neonatal Infection. Cochrane Database Syst. Rev. 2008, CD004946. [Google Scholar] [CrossRef] [PubMed]
- Eysenbach, G. Improving the Quality of Web Surveys: The Checklist for Reporting Results of Internet E-Surveys (CHERRIES). J. Med. Internet Res. 2004, 6, e34. [Google Scholar] [CrossRef] [PubMed]
- Bailie, R.S.; Robinson, G.; Kondalsamy-Chennakesavan, S.N.; Halpin, S.; Wang, Z. Investigating the Sustainability of Outcomes in a Chronic Disease Treatment Programme. Soc. Sci. Med. 2006, 63, 1661–1670. [Google Scholar] [CrossRef] [PubMed]
- Brand, C.; Landgren, F.; Hutchinson, A.; Jones, C.; Macgregor, L.; Campbell, D. Clinical Practice Guidelines: Barriers to Durability after Effective Early Implementation. Intern. Med. J. 2005, 35, 162–169. [Google Scholar] [CrossRef]
- Morris, Z.S.; Wooding, S.; Grant, J. The Answer Is 17 Years, What Is the Question: Understanding Time Lags in Translational Research. J. R. Soc. Med. 2011, 104, 510–520. [Google Scholar] [CrossRef]
- Flodgren, G.; Parmelli, E.; Doumit, G.; Gattellari, M.; O’Brien, M.A.; Grimshaw, J.; Eccles, M.P. Local Opinion Leaders: Effects on Professional Practice and Health Care Outcomes. Cochrane Database Syst. Rev. 2011, CD000125. [Google Scholar] [CrossRef]
- Carpenter, C.R.; Sherbino, J. How Does an “Opinion Leader” Influence My Practice? Can. J. Emerg. Med. 2010, 12, 431–434. [Google Scholar] [CrossRef]
- Code de La Santé Publique—Article R4127-318. Volume R4127-318. Available online: https://www.legifrance.gouv.fr/codes/article_lc/LEGIARTI000019412438/2012-04-05 (accessed on 26 January 2023).
- Lee, A.I.; Wong, C.A.; Healy, L.; Toledo, P. Impact of a Third Stage of Labor Oxytocin Protocol on Cesarean Delivery Outcomes. Int. J. Obstet. Anesth. 2014, 23, 18–22. [Google Scholar] [CrossRef]
- Dagraca, J.; Malladi, V.; Nunes, K.; Scavone, B. Outcomes after Institution of a New Oxytocin Infusion Protocol during the Third Stage of Labor and Immediate Postpartum Period. Int. J. Obstet. Anesth. 2013, 22, 194–199. [Google Scholar] [CrossRef]
- Chawla, K.; Furlong, R.; Kamo, N.; Gerbino, I.; Smith, D.; Blackmore, C. Clinical Spotlight Intervention to Accelerate Translation of Evidence-Based Practices in Primary Care. BMJ Open Qual. 2022, 11, e002032. [Google Scholar] [CrossRef]
- Simpson, S.H.; Eurich, D.T.; Majumdar, S.R.; Padwal, R.S.; Tsuyuki, R.T.; Varney, J.; Johnson, J.A. A Meta-Analysis of the Association between Adherence to Drug Therapy and Mortality. BMJ 2006, 333, 15. [Google Scholar] [CrossRef] [PubMed]
- Althabe, F.; Buekens, P.; Bergel, E.; Belizán, J.M.; Campbell, M.K.; Moss, N.; Hartwell, T.; Wright, L.L. Guidelines Trial Group A Behavioral Intervention to Improve Obstetrical Care. N. Engl. J. Med. 2008, 358, 1929–1940. [Google Scholar] [CrossRef]
- Baldvinsdóttir, T.; Blomberg, M.; Lilliecreutz, C. Improved Clinical Management but Not Patient Outcome in Women with Postpartum Haemorrhage—An Observational Study of Practical Obstetric Team Training. PLoS ONE 2018, 13, e0203806. [Google Scholar] [CrossRef]
- Gaucher, L.; Occelli, P.; Deneux-Tharaux, C.; Colin, C.; Gaucherand, P.; Touzet, S.; Dupont, C. Non-Clinical Interventions to Prevent Postpartum Haemorrhage and Improve Its Management: A Systematic Review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 240, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Pezaro, S.; Maher, K.; Fissell, M. Midwives Need a Useable Past to Shape Their Future. Lancet 2022, 399, 1046–1047. [Google Scholar] [CrossRef] [PubMed]
- Renfrew, M.J.; Malata, A.M. Scaling up Care by Midwives Must Now Be a Global Priority. Lancet Glob. Health 2021, 9, e2–e3. [Google Scholar] [CrossRef]
- Naidu, S.K.; Nabi, R.; Cheemarla, N.R.; Stanfield, B.A.; Rider, P.J.; Jambunathan, N.; Chouljenko, V.N.; Carter, R.; Del Piero, F.; Langohr, I.; et al. Intramuscular Vaccination of Mice with the Human Herpes Simplex Virus Type-1(HSV-1) VC2 Vaccine, but Not Its Parental Strain HSV-1(F) Confers Full Protection against Lethal Ocular HSV-1 (McKrae) Pathogenesis. PLoS ONE 2020, 15, e0228252. [Google Scholar] [CrossRef]
- Awasthi, S.; Friedman, H.M. An MRNA Vaccine to Prevent Genital Herpes. Transl. Res. J. Lab. Clin. Med. 2022, 242, 56–65. [Google Scholar] [CrossRef]
| Characteristics | n | (%) |
|---|---|---|
| Age, median | 34 | |
| Gender | ||
| Women | 705 | 96.8 |
| Men | 23 | 3.2 |
| Practise mode | ||
| Nonhospital midwife | 43 | 5.9 |
| Midwife in hospital <1000 births | 68 | 9.3 |
| Midwife in hospital 1000–3000 births | 371 | 51.0 |
| Midwife in hospital >3000 births | 246 | 33.8 |
| Years of practise, median | 10 years | |
| Region | ||
| Ile de France | 166 | 22.8 |
| Auvergne Rhône Alpes | 195 | 26.8 |
| Hauts de France | 25 | 3.4 |
| PACA | 27 | 3.7 |
| Normandie | 29 | 4.0 |
| Grand Est | 54 | 7.4 |
| Occitanie | 36 | 4.9 |
| Nouvelle Aquitaine | 72 | 9.9 |
| Centre Val de Loire | 19 | 2.6 |
| Bourgogne-Franche-Comté | 20 | 2.7 |
| Bretagne | 36 | 4.9 |
| Corse | 7 | 1.0 |
| Pays de la Loire | 22 | 3.0 |
| DOM TOM | 17 | 2.3 |
| Unknown | 3 | 0.4 |
| Knowledge of guidelines | 190 | 26.1 |
| Context of the Clinical Vignette | Number (Percentage) of Answers According to the Term of the Pregnancy | |
|---|---|---|
| Primary Initial Infection | 25 Weeks of Gestation | 38 Weeks of Gestation |
| Diagnosis | n (%) | n (%) |
| Primary initial | 693 (95.2) * | 708 (97.2) * |
| Nonprimary initial | 20 (2.7) | 8 (1.2) |
| Recurrence | 2 (0.3) | 3 (0.4) |
| Don’t know | 13 (1.8) | 9 (1.2) |
| Treatment | ||
| Prophylactic | 425 (58.4) * | 278 (38.2) |
| Curative | 692 (95.1) * | 555 (76.2) * |
| No treatment | 3 (0.4) | 5 (0.6) |
| Mode of birth | ||
| Caesarean section at term | 19 (2.6) | 364 (50.0) * |
| Vaginal birth at term | 622 (85.4) * | 9 (1.2) |
| Don’t know | 87 (11.9) | 355 (48.8) |
| Neonatal risk | ||
| High risk | 19 (2.6) | 374 (51.4) * |
| Low risk | 319 (43.8) * | 207 (28.4) |
| No risk | 295 (40.5) * | 39 (5.4) |
| Don’t know | 95 (13.1) | 108 (14.8) |
| Breastfeeding | ||
| Yes | 727 (99.9) * | 704 (96.7) * |
| No | 1 (0.1) | 24 (3.3) |
| Overall conformity | (41.5) | (18.4) |
| Context of the Clinical Vignette | Number (Percentage) of Answers According to the Term of the Pregnancy | |
|---|---|---|
| Nonprimary Initial Infection | 25 Weeks of Gestation | 38 Weeks of Gestation |
| Diagnosis | ||
| Primary initial | 84 (11.5) | 110 (15.1) |
| Nonprimary initial | 415 (57.0) * | 345 (47.4) * |
| Recurrence | 192 (26.4) | 246 (33.8) |
| Don’t know | 37 (5.1) | 27 (3.7) |
| Treatment | ||
| Prophylactic | 510 (70.1) * | 363 (49.9) |
| Curative | 661 (90.8) * | 500 (68.8) * |
| No treatment | 2 (0.2) | 2 (0.3) |
| Mode of birth | ||
| Caesarean section at term | 13 (1.8) | 257 (35.3) * |
| Vaginal birth at term | 580 (79.7) * | 5 (0.7) |
| Don’t know | 135 (18.5) | 466 (64.0) |
| Neonatal risk | ||
| High risk | 14 (1.9) | 290 (39.8) * |
| Low risk | 354 (48.6) * | 268 (36.8) |
| No risk | 233 (32.0) * | 45 (6.2) |
| Don’t know | 127 (17.5) | 125 (17.2) |
| Breastfeeding | ||
| Yes | 726 (99.7) * | 714 (98.1) * |
| No | 2 (0.3) | 14 (1.9) |
| Overall conformity | (29.1) | (5.4) |
| Context of the Clinical Vignette | Number (Percentage) of Answers According to the Term of the Pregnancy | |
|---|---|---|
| Recurrence | 25 Weeks of Gestation | 38 Weeks of Gestation |
| Diagnosis | ||
| Primary initial | 18 (2.5) | 4 (0.5) |
| Nonprimary initial | 47 (6.5) | 52 (7.1) |
| Recurrence | 647 (88.8) * | 659 (90.5) * |
| Don’t know | 16 (2.2) | 13 (1.8) |
| Treatment | ||
| Prophylactic | 531 (72.9) * | 442 (60.7) |
| Curative | 547 (75.1) * | 409 (56.1) * |
| No treatment | 33 (4.5) | 16 (2.2) |
| Mode of birth | ||
| Caesarean section at term | 12 (1.7) | 161 (22.1) * |
| Vaginal birth at term | 581 (79.8) * | 15 (2.1) * |
| Don’t know | 135 (18.5) | 552 (75.8) |
| Neonatal risk | ||
| High risk | 10 (1.4) | 129 (17.7) |
| Low risk | 253 (34.8) * | 368 (50.6) * |
| No risk | 364 (50.0) * | 97 (13.3) |
| Don’t know | 101 (13.8) | 134 (18.4) |
| Breastfeeding | ||
| Yes | 723 (99.3) * | 71 (98.2) * |
| No | 5 (0.7) | 13 (1.8) |
| Overall conformity | (49.7) | (8.7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mrozik, A.; Sellier, Y.; Lemaitre, D.; Gaucher, L. Evaluation of Midwives’ Practises on Herpetic Infections during Pregnancy: A French Vignette-Based Study. Healthcare 2023, 11, 364. https://doi.org/10.3390/healthcare11030364
Mrozik A, Sellier Y, Lemaitre D, Gaucher L. Evaluation of Midwives’ Practises on Herpetic Infections during Pregnancy: A French Vignette-Based Study. Healthcare. 2023; 11(3):364. https://doi.org/10.3390/healthcare11030364
Chicago/Turabian StyleMrozik, Adrian, Yann Sellier, Déborah Lemaitre, and Laurent Gaucher. 2023. "Evaluation of Midwives’ Practises on Herpetic Infections during Pregnancy: A French Vignette-Based Study" Healthcare 11, no. 3: 364. https://doi.org/10.3390/healthcare11030364
APA StyleMrozik, A., Sellier, Y., Lemaitre, D., & Gaucher, L. (2023). Evaluation of Midwives’ Practises on Herpetic Infections during Pregnancy: A French Vignette-Based Study. Healthcare, 11(3), 364. https://doi.org/10.3390/healthcare11030364






