Early Surfactant Therapy for Respiratory Distress Syndrome in Very Preterm Infants
Abstract
:1. Introduction
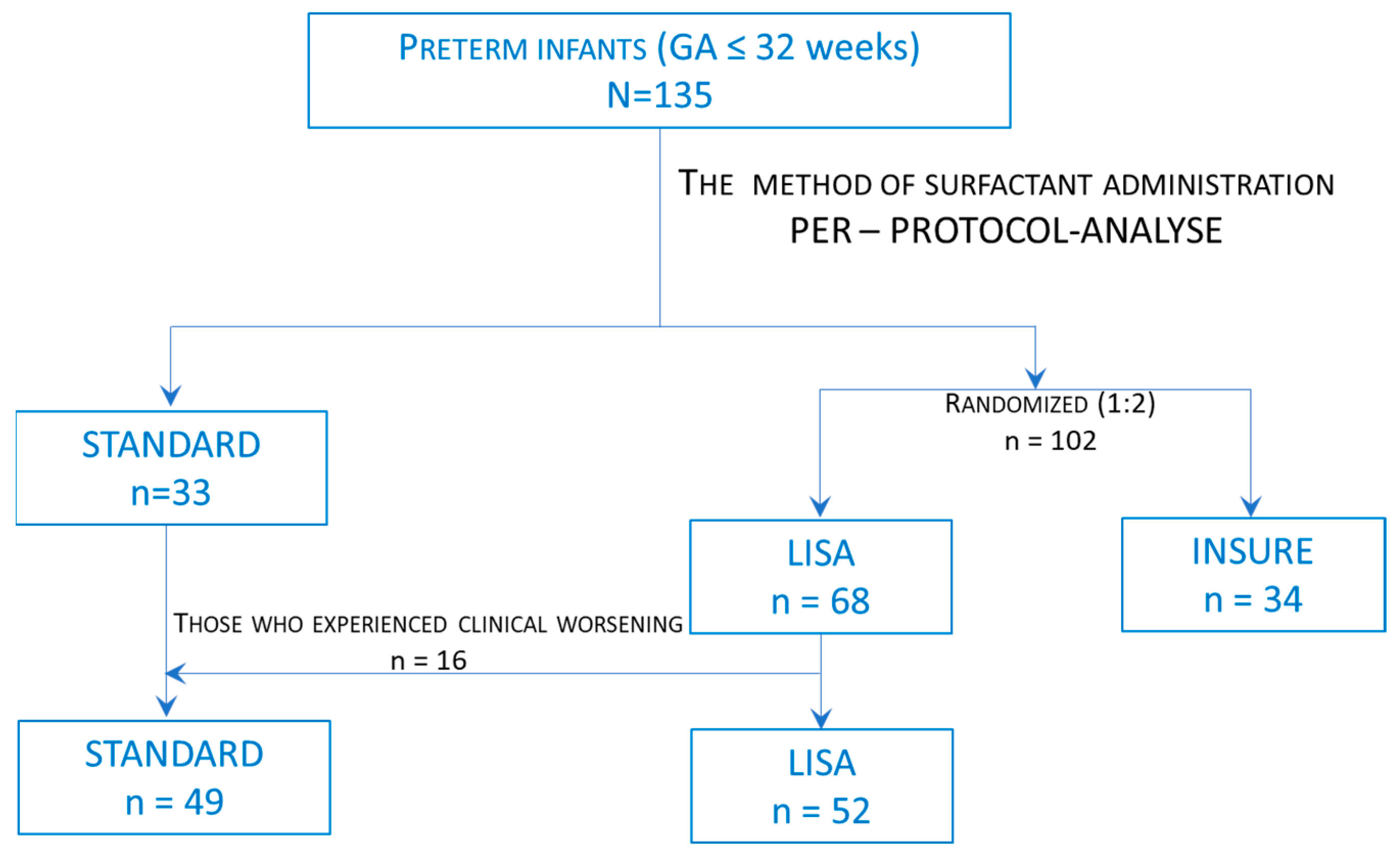
2. Materials and Methods
2.1. Study Design and Patients
2.2. Study Intervention
2.3. Statistical Analysis
3. Results
3.1. Association of Surfactant Administration Methods and Gestational Age with the Subsequent Need for Mechanical Ventilation and the Occurrence of Major Neonatal Morbidities
3.2. Association of the Surfactant Administration Method (LISA and INSURE) and Gestational Age, with the Clinical Evolution of Infants: Need of MV, Need for Subsequent Surfactant Doses, Morbidities, Sepsis/Proven Sepsis and Deaths
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lopez, E.; Gascoin, G.; Flamant, C.; Merhi, M.; Tourneux, P.; Band, O. Exogenous surfactant therapy in 2013: What is next? Who, when and how should we treat newborn infants in the future? BMC Paediatr. 2013, 13, 165. [Google Scholar] [CrossRef]
- Hatch, L.D.; Grubb, P.H.; Lea, A.S.; Walsh, W.F.; Markham, M.H.; Whitney, G.M.; Slaughter, J.C.; Stark, A.R.; Ely, E.W. Endotracheal Intubation in Neonates: A Prospective Study of Adverse Safety Events in 162 Infants. J. Pediatr. 2016, 168, 62–66.e6. [Google Scholar] [CrossRef] [PubMed]
- Gomes Cordeiro, A.M.; Fernandes, J.C.; Troster, E.J. Possible risk factors associated with moderate or severe airway injuries in children who underwent endotracheal intubation. Pediatr. Crit Care Med. 2004, 5, 364–368. [Google Scholar] [CrossRef] [PubMed]
- Lademann, H.; Abshagen, K.; Janning, A.; Däbritz, J.; Olbertz, D. Long-Term Outcome after Asphyxia and Therapeutic Hypothermia in Late Preterm Infants: A Pilot Study. Healthcare 2021, 9, 994. [Google Scholar] [CrossRef] [PubMed]
- Sweet, D.G.; Carnielli, V.; Greisen, G.; Hallman, M.; Ozek, E.; Te Pas, A.; Plavka, R.; Roehr, C.C.; Saugstad, O.D.; Simeoni, U.; et al. European Consensus Guidelines on the Management of Respiratory Distress Syndrome—2019 Update. Neonatology 2019, 115, 432–450. [Google Scholar] [CrossRef]
- Aldana-Aguirre, J.C.; Pinto, M.; Featherstone, R.M.; Kumar, M. Less invasive surfactant administration versus intubation for surfactant delivery in preterm infants with respiratory distress syndrome: A systematic review and meta-analysis. Arch. Dis. Child Fetal Neonatal Ed. 2017, 102, F17–F23. [Google Scholar] [CrossRef]
- Ballard, J.L.; Khoury, J.C.; Wedig, K.; Wang, L.; Eilers-Walsman, B.L.; Lipp, R. New Ballard Score, expanded to include extremely premature infants. J. Pediatr. 1991, 119, 417–423. [Google Scholar] [CrossRef]
- de Luca, D.; van Kaam, A.H.; Tingay, D.G.; Courtney, S.E.; Danhaive, O.; Carnielli, V.P.; Zimmermann, L.J.; Kneyber, M.C.J.; Tissieres, P.; Brierley, J.; et al. The Montreux definition of neonatal ARDS: Biological and clinical background behind the description of a new entity. Lancet Respir. Med. 2017, 5, 657–666. [Google Scholar] [CrossRef]
- Sola, A.; Golombek, S.G.; Montes Bueno, M.T.; Lemus-Varela, L.; Zuluaga, C.; Domínguez, F.; Baquero, H.; Young Sarmiento, A.E.; Natta, D.; Rodriguez Perez, J.M.; et al. Safe oxygen saturation targeting and monitoring in preterm infants: Can we avoid hypoxia and hyperoxia? Acta Paediatr. 2014, 103, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Jensen, E.A.; Dysart, K.; Gantz, M.G. The diagnosis of bronchopulmonary dysplasia in very preterm infants: An evidence-based approach. Am. J. Respir. Crit Care Med. 2019, 200, 751–759. [Google Scholar] [CrossRef]
- Bell, M.J.; Ternberg, J.L.; Feigin, R.D.; Keating, J.P.; Marshall, R.; Barton, L.; Brotherton, T. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann. Surg. 1978, 187, 1. [Google Scholar] [CrossRef]
- Papile, L.A.; Burstein, J.; Burstein, R.; Koffler, H. Incidence and evolution of subependymal and intraventricular hemorrhage: A study of infants with birth weights less than 1500 gm. J. Pediatr. 1978, 92, 529–534. [Google Scholar] [CrossRef]
- Gillam-Krakauer, M.; Reese, J. Diagnosis and Management of Patent Ductus Arteriosus. Neoreviews 2018, 19, e394–e402. [Google Scholar] [CrossRef] [PubMed]
- Ţarcă, E.; Roșu, S.T.; Cojocaru, E.; Trandafir, L.; Luca, A.C.; Rusu, D.; Ţarcă, V. Socio-Epidemiological Factors with Negative Impact on Infant Morbidity, Mortality Rates, and the Occurrence of Birth Defects. Healthcare 2021, 9, 384. [Google Scholar] [CrossRef]
- Mavroudis, I.; Kazis, D.; Chowdhury, R.; Petridis, F.; Costa, V.; Balmus, I.M.; Ciobica, A.; Luca, A.C.; Radu, I.; Dobrin, R.P.; et al. Post-Concussion Syndrome and Chronic Traumatic Encephalopathy: Narrative Review on the Neuropathology, Neuroimaging and Fluid Biomarkers. Diagnostics 2022, 12, 740. [Google Scholar] [CrossRef]
- International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Arch Ophthalmol. 2005, 123, 991–999. [Google Scholar] [CrossRef]
- Verder, H.; Bohlin, K.; Kamper, J.; Lindwall, R.; Jonsson, B. Nasal CPAP and surfactant for treatment of respiratory distress syndrome and prevention of bronchopulmonary dysplasia. Acta Paediatr. 2009, 98, 1400–1408. [Google Scholar] [CrossRef]
- Cha, J.H.; Choi, N.; Kim, J.; Lee, H.J.; Na, J.Y.; Park, H.K. Cystic Periventricular Leukomalacia Worsens Developmental Outcomes of Very-Low-Birth Weight Infants with Intraventricular Hemorrhage-A Nationwide Cohort Study. J. Clin. Med. 2022, 11, 5886. [Google Scholar] [CrossRef]
- Trembath, A.; Laughon, M.M. Predictors of bronchopulmonary dysplasia. Clin Perinatol. 2012, 39, 585–601. [Google Scholar] [CrossRef] [PubMed]
- Hunt, K.; Dassios, T.; Ali, K.; Greenough, A. Volume targeting levels and work of breathing in infants with evolving or established bronchopulmonary dysplasia. Arch. Dis. Child Fetal Neonatal Ed. 2019, 104, F46–F49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escobar, V.; Soares, D.S.; Kreling, J.; Ferrari, L.S.; Felcar, J.M.; Camillo, C.A.; Probst, V.S. Influence of time under mechanical ventilation on bronchopulmonary dysplasia severity in extremely preterm infants: A pilot study. BMC Pediatr. 2020, 20, 241. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.B.; Lee, J.; Park, J.; Jun, Y.H. Impact of prolonged mechanical ventilation in very low birth weight infants: Results from a National Cohort Study. J. Pediatr. 2018, 194, 34–39.e3. [Google Scholar] [CrossRef]
- Ho, J.J.; Subramaniam, P.; Henderson-Smart, D.J.; Davis, P.G. Continuous distending pressure for respiratory distress syndrome in preterm infants. Cochrane Database Syst. Rev. 2002, 1, CD002271. [Google Scholar]
- Ho, J.J.; Subramaniam, P.; Davis, P.G. Continuous positive airway pressure (CPAP) for respiratory distress in preterm infants. Cochrane Database Syst. Rev. 2020, 10, CD002271. [Google Scholar]
- Bamat, N.; Fierro, J.; Mukerji, A.; Wright, C.J.; Millar, D.; Kirpalani, H. Nasal continuous positive airway pressure levels for the prevention of morbidity and mortality in preterm infants. Cochrane Database Syst. Rev. 2021, 11, CD012778. [Google Scholar]
- Thomas, A.N.; Hagan, J.L.; Lingappan, K. Noninvasive ventilation strategies: Which to choose? J. Perinatol. 2018, 38, 447–450. [Google Scholar] [CrossRef]
- Kribs, A.; Hummler, H. Ancillary therapies to enhance success of non-invasive modes of respiratory support—Approaches to delivery room use of surfactant and caffeine? Semin. Neonatal Med. 2016, 21, 212–218. [Google Scholar] [CrossRef]
- Cummings, J.J.; Polin, R.A.; Committee on Fetus and Newborn, American Academy of Pediatrics. Noninvasive Respiratory Support. Pediatrics 2016, 137, e20153758. [Google Scholar] [CrossRef] [PubMed]
- Verder, H.; Robertson, B.; Greisen, G.; Ebbesen, F.; Albertsen, P.; Lundstrom, K.; Jacobsen, T. Surfactant therapy and nasal continuous positive airway pressure for newborns with respiratory distress syndrome. Danish-Swedish Multicenter Study Group. N. Engl. J. Med. 1994, 331, 1051–1055. [Google Scholar] [CrossRef]
- Kribs, A.; Pillekamp, F.; Hünseler, C.; Vierzig, A.; Roth, B. Early administration of surfactant in spontaneous breathing with nCPAP: Feasibility and outcome in extremely premature infants (postmenstrual age ≤27 weeks). Paediatr. Anaesth 2007, 17, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Aguar, M.; Cernada, M.; Brugada, M.; Gimeno, A.; Gutierrez, A.; Vento, M. Minimally invasive surfactant therapy with a gastric tube is as effective as the intubation, surfactant, and extubation technique in preterm babies. Acta Paediatr. 2014, 103, e229–e233. [Google Scholar] [CrossRef]
- Kanmaz, H.G.; Erdeve, O.; Canpolat, F.E.; Mutlu, B.; Dilmen, U. Surfactant administration via thin catheter during spontaneous breathing: Randomized controlled trial. Pediatrics 2013, 131, e502–e509. [Google Scholar] [CrossRef] [PubMed]
- Maiwald, C.A.; Neuberger, P.; Vochem, M.; Poets, C. QuickSF: A New Technique in Surfactant Administration. Neonatology 2017, 111, 211–213. [Google Scholar] [CrossRef] [PubMed]
- Dargaville, P.A.; Aiyappan, A.; Cornelius, A.; Williams, C.; De Paoli, A.G. Preliminary evaluation of a new technique of minimally invasive surfactant therapy. Arch. Dis. Child Fetal Neonatal Ed. 2011, 96, F243–F248. [Google Scholar] [CrossRef]
- Fabbri, L.; Klebermass-Schrehof, K.; Aguar, M.; Harrison, C.; Gulczyńska, E.; Santoro, D.; Di Castri, M.; Rigo, V. Five-country manikin study found that neonatologists preferred using the LISAcath rather than the Angiocath for less invasive surfactant administration. Acta Paediatr. 2018, 107, 780–783. [Google Scholar] [CrossRef] [PubMed]
- Göpel, W.; Kribs, A.; Härtel, C.; Avenarius, S.; Teig, N.; Groneck, P.; Olbertz, D.; Roll, C.; Vochem, M.; Weller, U.; et al. Less invasive surfactant administration is associated with improved pulmonary outcomes in spontaneously breathing preterm infants. Acta Paediatr. 2015, 104, 241–246. [Google Scholar] [CrossRef]
- Krajewski, P.; Chudzik, A.; Strzałko-Głoskowska, B.; Górska, M.; Kmiecik, M.; Więckowska, K.; Mesjasz, A.; Sieroszewski, P. Surfactant administration without intubation in preterm infants with respiratory distress syndrome--our experiences. J. Matern.-Fetal Neonatal Med. Off. J. Eur. Assoc. Perinat. Med. Fed. Asia Ocean. Perinat. Soc. Int. Soc. Perinat. Obstet. 2015, 28, 1161–1164. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.; Chamberlain, R.S.; Sun, S. Less Invasive Surfactant Administration Reduces the Need for Mechanical Ventilation in Preterm Infants: A Meta-Analysis. Glob. Pediatr. Health 2017, 4, 2333794X17696683. [Google Scholar] [CrossRef]
- Kribs, A.; Roll, C.; Göpel, W.; Wieg, C.; Groneck, P.; Laux, R.; Teig, N.; Hoehn, T.; Böhm, W.; Welzing, L.; et al. Nonintubated Surfactant Application vs Conventional Therapy in Extremely Preterm Infants: A Randomized Clinical Trial. JAMA Pediatr. 2015, 169, 723–730. [Google Scholar] [CrossRef]
- Härtel, C.; Paul, P.; Hanke, K.; Humberg, A.; Kribs, A.; Mehler, K.; Vochem, M.; Wieg, C.; Roll, C.; Herting, E.; et al. Less invasive surfactant administration and complications of preterm birth. Sci. Rep. 2018, 8, 8333. [Google Scholar] [CrossRef] [Green Version]

| Baseline Characteristics | Study Group (n = 135) | p-Value | ||
|---|---|---|---|---|
| LISA (n = 52) | INSURE (n = 34) | STANDARD (n = 49) | ||
| Mother’s age: years (mean ± SD) † | 30.2 ± 5.8 | 29.7 ± 7.1 | 29.7 ± 6 | 0.920 |
| Gender (F/M), n (%) ‡ | 27/25 | 15/19 | 23/26 | 0.6112 |
| (51.9/48.1) | (44.1/55.9) | (46.9/53.1) | ||
| GA, weeks † | 27.6 ± 2 | 28.1 ± 1.9 | 25.8 ± 2.5 | < 0.001 * |
| 22–25 weeks | 5 (9.6) | 5 (14.7) | 27 (55.1) | |
| 26–29 weeks | 36 (69.2) | 19 (55.9) | 17 (34.7) | |
| 30–32 weeks | 11 (21.2) | 10 (24.4) | 5 (10.2) | |
| BW, g, (mean ± SD) † | 1043.8 ± 335.9 | 1095.3 ± 272.4 | 828.4 ± 325.5 | 0.0002 * |
| LGA/AGA/SGA, n (%) ‡ | 1/44/7 | 0/32/2 | 1/47/1 | 0.1693 |
| (1.9/84.6/13.5) | (0/94.1/5.9) | (2.1/959/2.1) | ||
| Cesarian section/vaginal delivery, n (%) | 32/20 | 21/13 | 31/18 | 0.9821 |
| (61.5/38.5) | (61.8/38.2) | (63.3/36.7) | ||
| Apgar score, median (IQR) | ||||
| 1 min | 7 (6–7) | 6 (5–7) | 3 (2–5) | <0.001 * |
| 5 min | 8 (8–8) | 8 (7–8) | 5 (5–7) | 0.0124 * |
| Clinical Characteristics | ||||
| Duration of MV, hours (mean ± SD) # | 89.2 ± 33.9 | 143.8 ± 54.5 | 0.0103 * | |
| Antenatal corticosteroids, n (%) ‡ | 36 (69.2) | 17 (50) | 0.2214 | |
| Need for subsequent surfactant doses, n (%) ‡ | 4 (7.7) | 6 (17.7) | 0.0219 * | |
| Morbidity, n (%) ‡ | 32 (61.5) | 26 (76.5) | 0.0168 * | |
| Pneumothorax, n (%) ‡ | 2 (3.9) | 3 (8.8) | 0.0351 * | |
| BPD, n (%) ‡ | 13 (25) | 13 (38.2) | 0.2541 | |
| NEC, n (%) ‡ | 6 (11.5) | 5 (14.7) | 0.7218 | |
| IVH, n (%) ‡ | 9 (17.3) | 8 (23.5) | 0.0054 * | |
| IVH 3–4, n (%) ‡ | 2 (3.9) | 2 (5.9) | 0.0038 * | |
| PVL, n (%) ‡ | 0 (0) | 4 (11.8) | 0.0269 * | |
| PDA, n (%) ‡ | 14 (26.9) | 11 (32.4) | 0.8254 | |
| Ventriculomegaly, n (%) ‡ | 0 (0) | 3 (8.8) | 0.0051 * | |
| Sepsis/probable sepsis, n (%) ‡ | 6 (11.5) | 6 (17.7) | 0.0356 * | |
| ROP, n (%) ‡ | 5 (16.7) | 8 (26.7) | 0.0397 * | |
| NICU, days, median (IQR) # | 19 (14–25) | 20 (14–27) | 0.0242 * | |
| Deaths, n (%) ‡ | 2 (3.9) | 2 (5.9) | 0.0059 * | |
| STANDARD, (n = 49) | |
|---|---|
| Duration of MV, hours (mean ± SD) | 217.6 ± 173 |
| Antenatal corticosteroids, n (%) | 26 (53.1) |
| Need for subsequent surfactant doses, n (%) | 12 (24.5) |
| Morbidity, n (%) | 43 (87.8) |
| Pneumothorax, n (%) | 9 (18.4) |
| BPD, n (%) | 22 (44.9) |
| NEC, n (%) | 9 (18.4) |
| IVH, n (%) | 27 (55.1) |
| IVH 3–4, n (%) | 13 (26.5) |
| PVL, n (%) | 3 (6.1) |
| PDA, n (%) | 12 (24.5) |
| Ventriculomegaly, n (%) | 12 (24.5) |
| Sepsis/probable sepsis, n (%) | 11 (23.4) |
| ROP, n (%) | 17 (56.7) |
| NICU, days, median (IQR) | 26 (7–43) |
| Deaths, n (%) | 18 (36.7) |
| Study Group (n = 135) | |||
|---|---|---|---|
| LISA (n = 52) | INSURE (n = 34) | STANDARD (n = 49) | |
| RDS, n (%) | 8/39/5 | 0/27/7 | 1/31/17 |
| mild/moderate/severe | (15.4/75/9.6) | (0/79.4/20.6) | (2.1/63.3/34.6) |
| Need of MV (<72 h), n (%) | 13 (25) | 13 (38.2) | 49 (100) |
| GA, Weeks | p-Value ‡ | |||
|---|---|---|---|---|
| 22–25 Weeks | 26–29 Weeks | 30–32 Weeks | ||
| Need of MV (<72 h), n (%) | ||||
| LISA, n MV/n total | 4/5 (80) | 8/36 (22.2) | 1/11 (9.1) | 0.0133 * |
| INSURE, n MV/n total | 2/5 (40) | 7/19 (36.8) | 4/10 (40) | 0.9824 |
| Need for subsequent surfactant doses | ||||
| LISA, n subsequent doses/n total | 0/5 (0) | 4/36 (11.1) | 0/11 (0) | 0.0025 * |
| INSURE, n subsequent doses/n total | 0/5 (0) | 4/19 (21.1) | 2/10 (20) | 0.0017 * |
| STANDARD, n subsequent doses/n total | 5/27 (18.5) | 4/17 (23.5) | 3/5 (60) | 0.0108 * |
| Morbidity, n (%) | ||||
| LISA, n morbidity/n total | 4/5 (80) | 22/36 (61.1) | 6/11 (54.6) | 0.0174 * |
| INSURE, n morbidity/n total | 5/5 (100) | 14/19 (73.7) | 7/10 (70) | 0.0351 * |
| STANDARD, n morbidity/n total | 23/27 (85.2) | 15/17 (88.2) | 5/5 (100) | 0.4802 |
| Pneumothorax, n (%) | ||||
| LISA, n Pneumothorax/n total | 0/5 (0) | 2/36 (5.6) | 0/11 (0) | 0.4709 |
| INSURE, n Pneumothorax/n total | 0/5 (0) | 2/19 (10.5) | 1/10 (10) | 0.6049 |
| STANDARD, n Pneumothorax/n total | 4/27 (14.8) | 3/17 (17.7) | 2/5 (40) | 0.0364 * |
| BPD, n (%) | ||||
| LISA, n BPD/n total | 2/5 (40) | 10/36 (27.8) | 1/11 (9.1) | 0.0031 * |
| INSURE, n BPD/n total | 4/5 (80) | 8/19 (42.1) | 1/10 (10) | 0.0196 * |
| STANDARD, n BPD/n total | 11/27 (40.7) | 8/17 (47.1) | 3/5 (60) | 0.7116 |
| IVH grade 3–4, n (%) | ||||
| LISA, n IVH/n total | 2/5 (40) | 0/36 (0) | 0/11 (0) | 0.0060 * |
| INSURE, n IVH/n total | 0/5 (0) | 1/19 (5.3) | 1/10 (10) | 0.6453 |
| STANDARD, n IVH/n total | 9/27 (33.3) | 4/17 (23.5) | 0/5 (0) | 0.0284 * |
| PVL, n (%) | ||||
| LISA, n PVL/n total | - | - | - | - |
| INSURE, n PVL/n total | 0/5 (0) | 3/19 (15.8) | 1/10 (10) | 0.4596 |
| STANDARD, n PVL/n total | 1/27 (3.7) | 1/17 (5.9) | 1/5 (20) | 0.4948 |
| PDA, n (%) | ||||
| LISA, n PDA/n total | 2/5 (40) | 9/36 (25) | 3/11 (27.3) | 0.7905 |
| INSURE, n PDA/n total | 2/5 (40) | 4/19 (21.1) | 5/10 (50) | 0.2649 |
| STANDARD, n PDA/n total | 3/27 (11.1) | 8/17 (47.1) | 1/5 (20) | 0.0272 * |
| Sepsis/probable sepsis, n (%) | ||||
| LISA, nsepsis/probable sepsis/n total | 1/5 (10) | 4/36 (11.1) | 1/11 (9.1) | 0.8305 |
| INSURE, nsepsis/probable sepsis/n total | 3/5 (60) | 3/19 (15.8) | 0/10 (0) | 0.0151 * |
| STANDARD, nsepsis/probable sepsis/n total | 5/27 (18.5) | 6/16 (37.5) | 0/4 (0) | 0.0028 * |
| Deaths, n (%) | ||||
| LISA, n Deaths/n total | 2/5 (40) | 0/36 (0) | 0/11 (0) | 0.0047 * |
| INSURE, n Deaths/n total | 1/5 (20) | 0/19 (0) | 1/10 (10) | 0.1566 |
| STANDARD, n Deaths/n total | 17/27 (62.9) | 1/17 (5.9) | 0/5 (0) | 0.00002 * |
| ROP, n (%) | ||||
| LISA, n ROP/n total | 2/5 (40) | 6/36 (16.7) | 0/11 (0) | 0.0409 * |
| INSURE, n ROP/n total | 1/5 (20) | 2/19 (105) | 2/10 (20) | 0.7422 |
| STANDARD, n ROP/n total | 8/27 (29.6) | 8/17 (47.1) | 1/5 (20) | 0.3811 |
| NICU, days | ||||
| LISA, median (IQR) | 27 (23–27) | 21 (15–27) | 12 (10–20) | 0.0329 * |
| INSURE, median (IQR) | 25 (22–27) | 21 (14–26) | 17 (13–19) | 0.0414 * |
| STANDARD, median (IQR) | 11 (2–28) | 40 (26–44) | 29 (28–60) | 0.0103 * |
| Univariate Analysis (n LISA = 52; n INSURE = 34) | Odds Ratio (95% Confidence Interval) | SE | p-Value |
|---|---|---|---|
| Need of MV (<72 h) | |||
| LISA (ref. INSURE) | 0.538 (0.212–0.730) | 0.047 | 0.019 * |
| GA, weeks (ref. GA > 26 weeks) | 2.735 (1.608–5.971) | 0.183 | <0.001 * |
| Need for subsequent surfactant doses | |||
| LISA (ref. INSURE) | 0.389 (0.101–0.894) | 0.088 | 0.017 * |
| GA, weeks (ref. GA < 26 weeks) | 2.134 (1.391–5.380) | 0.068 | 0.021 * |
| Morbidity, n (%) | |||
| LISA (ref. INSURE) | 0.492 (0.187–0.892) | 0.049 | 0.015 * |
| GA, weeks (ref. GA > 26 weeks) | 2.821 (1.691–4.976) | 0.065 | 0.026 * |
| Sepsis/probable sepsis, n (%) | |||
| LISA (ref. INSURE) | 0.609 (0.179–0.988) | 0.025 | 0.042 * |
| GA, weeks (ref. GA > 26 weeks) | 3.065 (1.874–6.057) | 0.034 | 0.003 * |
| Deaths, n (%) | |||
| LISA (ref. INSURE) | 0.640 (0.386–0.774) | 0.025 | 0.036 * |
| GA, weeks (ref. GA > 26 weeks) | 5.367 (3.245–7.551) | 0.014 | <0.001 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cucerea, M.; Moscalu, M.; Moldovan, E.; Santa, R.; Gall, Z.; Suciu, L.M.; Simon, M. Early Surfactant Therapy for Respiratory Distress Syndrome in Very Preterm Infants. Healthcare 2023, 11, 439. https://doi.org/10.3390/healthcare11030439
Cucerea M, Moscalu M, Moldovan E, Santa R, Gall Z, Suciu LM, Simon M. Early Surfactant Therapy for Respiratory Distress Syndrome in Very Preterm Infants. Healthcare. 2023; 11(3):439. https://doi.org/10.3390/healthcare11030439
Chicago/Turabian StyleCucerea, Manuela, Mihaela Moscalu, Elena Moldovan, Reka Santa, Zsuzsanna Gall, Laura Mihaela Suciu, and Marta Simon. 2023. "Early Surfactant Therapy for Respiratory Distress Syndrome in Very Preterm Infants" Healthcare 11, no. 3: 439. https://doi.org/10.3390/healthcare11030439
APA StyleCucerea, M., Moscalu, M., Moldovan, E., Santa, R., Gall, Z., Suciu, L. M., & Simon, M. (2023). Early Surfactant Therapy for Respiratory Distress Syndrome in Very Preterm Infants. Healthcare, 11(3), 439. https://doi.org/10.3390/healthcare11030439






