Amazon Amandaba—Prevalence, Risk Factors and Self-Care Perception Associated with Diabetic Peripheral Neuropathy in Patients with Type 2 Diabetes: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Setting and Period of Study
2.3. Population
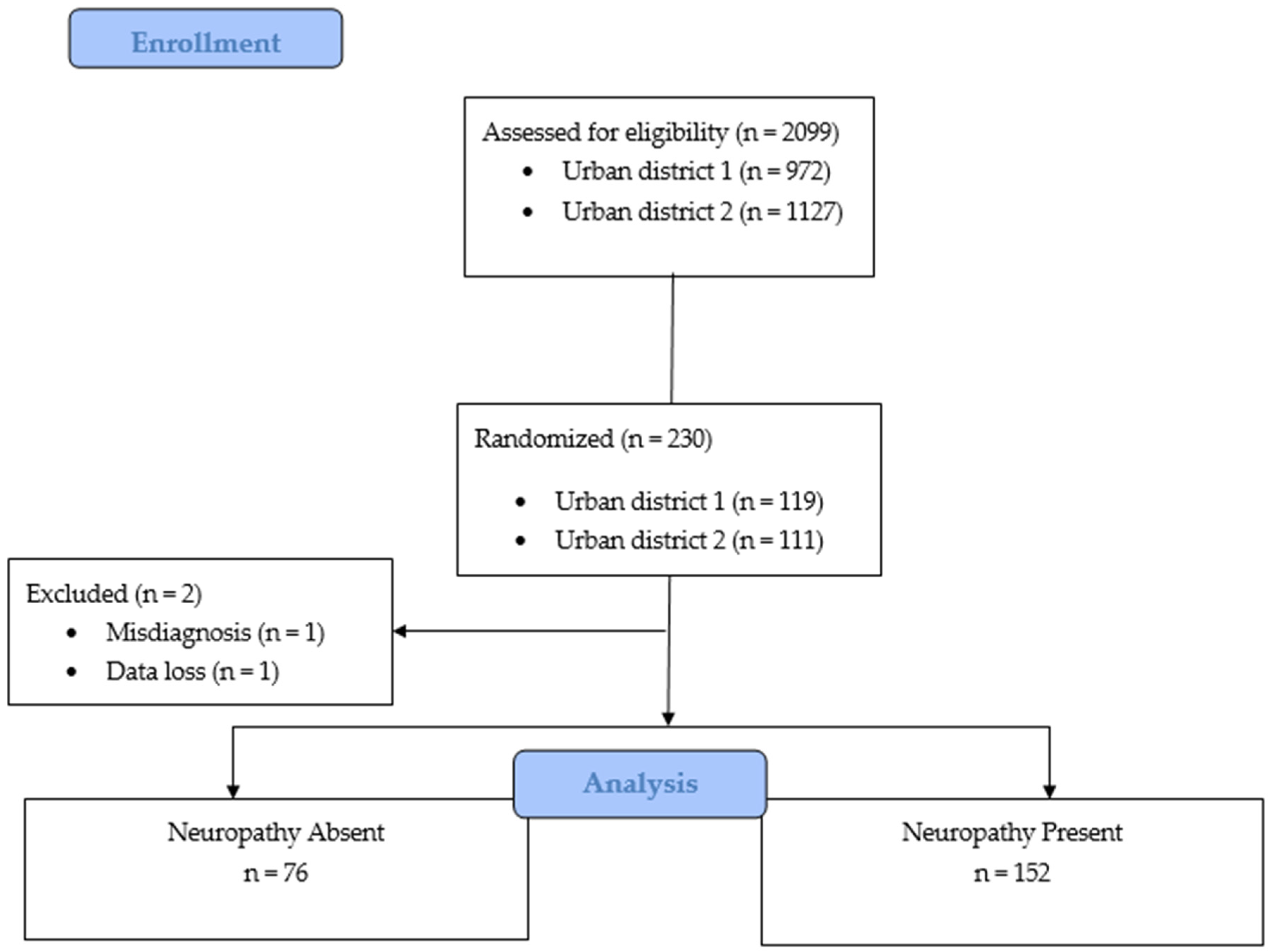
2.4. Sampling
2.5. Sample Size
2.6. Sample
2.7. Eligibility Criteria
2.8. Data Collection and Variables
Michigan Neuropathy Screening Instrument (MSNI)
2.9. Study Outcomes
2.10. Bias
2.11. Statistical Analysis
3. Results
3.1. Prevalence of Diabetic Peripheral Neuropathy
3.2. Social Characteristics
3.3. Health-Related and Clinical Characteristics
3.4. Biochemical Parameters
3.5. Diabetes Self-Care Activities
3.6. Associated Factors with Diabetic Peripheral Neuropathy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Diabetes Federation. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021. [Google Scholar]
- Sociedade Brasileira de Diabetes. Diretrizes da Sociedade Brasileira de Diabetes 2019–2020; Editora Clanad: Sao Paulo, Brasil, 2019. [Google Scholar]
- Bodman, M.A.; Varacallo, M. Peripheral Diabetic Neuropathy; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Ministério da Saúde; Secretaria de Atenção à Saúde; Departamento de Atenção Básica. Estratégias para o Cuidado da Pessoa com Doença Crônica: Diabetes Mellitus; Ministério da Saúde; Secretaria de Atenção à Saúde; Departamento de Atenção Básica: Rio de Janeiro, Brasil, 2013. [Google Scholar]
- Tesfaye, S.; Selvarajah, D. Advances in the epidemiology, pathogenesis and management of diabetic peripheral neuropathy. Diab. Met. Res. Rev. 2012, 28, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Khawaja, N.; Abu-Shennar, J.; Saleh, M.; Dahbour, S.S.; Khader, Y.S.; Ajlouni, K.M. The prevalence and risk factors of peripheral neuropathy among patients with type 2 diabetes mellitus; the case of Jordan. Diabetol. Metab. Syndr. 2018, 10, 8. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.M.; Viana, M.C.A.V.A.; Sousa, N.M.; Penha, A.A.G. Síntese de evidências para políticas de saúde: Prevenção e controle do pé diabético na atenção primária a saúde. Bis 2019, 20, 2–4. [Google Scholar] [CrossRef]
- Brinati, L.M.; Diogo, N.A.S.; Moreira, T.R.; Mendonça, E.T.; Amaro, M.O.F. Prevalência e fatores associados à neuropatia periférica em indivíduos com diabetes mellitus. Rev. Fund Care 2017, 9, 347–355. [Google Scholar] [CrossRef]
- Nascimento, J.W.A.; Silva, E.C.S.; Roque, G.S.L.; Ferreira Júnior, L.; Jesus, S.B. Correlação entre o tipo de calçado com alterações físicas em pés de diabéticos. Rev. Enferm. UFPI 2020, 9, e10189. [Google Scholar] [CrossRef]
- Borges, F.S.C.; Gama, H.S. Avaliação sensório-motora do tornozelo e pé entre idosos diabéticos e não diabéticos. Rev. Brasil Geri. Geront. 2010, 13, 93–102. [Google Scholar] [CrossRef]
- Gonçalves, I.E.S.; Reis, M.S.; Perez, V.C.; Silva, G.G.; Franciulli, P.M. Avaliação sensório motora de tornozelo e pé em pacientes diabéticos. Rev. Iberk-Am. Podol. 2020, 2, 101. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. Retinopathy, neuropathy, and foot care: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022, 45, S185. [Google Scholar] [CrossRef]
- Ministério da Saúde; Secretaria de Atenção à Saúde; Departamento de Atenção Básica. Manual do pé diabético: Estratégias para o cuidado da pessoa com doença crônica; Departamento de Atenção Básica; Secretaria de Atenção à Saúde; Ministério da Saúde: Rio de Janeiro, Brasília, 2016. [Google Scholar]
- Sartor, C.D.; Oliveira, M.D.; Campos, V.; Ferreira, J.S.S.P.; Sacco, I.C.N. Cross-cultural adaptation and measurement properties of the Brazilian Version of the Michigan Neuropathy Screening Instrument. Bra. J. Phys. Ther. 2018, 22, 222–230. [Google Scholar] [CrossRef]
- Selvarajah, D.; Kar, D.; Khunti, K.; Davies, M.J.; Scott, A.R.; Walker, J.; Tesfaye, S. Diabetic peripheral neuropathy: Advances in diagnosis and strategies for screening and early intervention. Lancet Diabetes Endocrinol. 2019, 7, 938–948. [Google Scholar] [CrossRef]
- Bashar, M.D.A.; Verma, M. Prevalence and Determinants of Diabetic Peripheral Neuropathy/Foot Syndrome in the Rural Population of North India. Iberoam J. Med. 2021, 3, 18–25. [Google Scholar] [CrossRef]
- Michels, J.M.; Coral, M.H.C.; Sakae, T.M.; Damas, T.B.; Furlanetto, L.M. Questionário de Atividades de Autocuidado com o Diabetes: Tradução, adaptação e avaliação das propriedades psicométricas. Arq. Bras. De Endocrinol. Metabol. 2010, 54, 644–651. [Google Scholar] [CrossRef]
- Oliveira, F.B.; Botelho, K.K.P.; Bezerra, A.R.; Azevedo, D.I.O.; Paz, C.C.S.C.; Martins, E.F. Cross-cultural adaptation to Brazilian Portuguese of the Michigan Neuropathy Screening Instrument: MNSI-Brazil. Arq. Neuro-Psiquiatr. 2016, 74, 653–661. [Google Scholar] [CrossRef]
- Barbosa, M.; Saavedra, A.; Oliveira, S.; Reis, L.; Rodrigues, F.; Severo, M.; Sittl, R.; Maier, C.; Carvalho, D.M. Prevalence and Determinants of Painful and Painless Neuropathy in Type 1 Diabetes Mellitus. Front. Endocrinol. 2019, 10, 402. [Google Scholar] [CrossRef] [PubMed]
- Abdissa, D.; Sorsa, R.; Gerbi, A.; Hamba, N.; Banjaw, Z. Magnitude and associated factors of peripheral neuropathy among diabetes patients attending Jimma University Medical Center, Southwest Ethiopia. Heliyon 2021, 7, e08460. [Google Scholar] [CrossRef] [PubMed]
- Arellano-Longinos, S.A.; Godínez-Tamay, E.D.; Hernández-Miranda, M.B. Prevalencia de neuropatía diabética en pacientes con diabetes mellitus tipo 2 en una clínica regional del Estado de México. Aten. Fam. 2018, 25, 7–11. [Google Scholar] [CrossRef]
- Ibarra, C.T.R.; Rocha, J.J.L.; Hernández, R.O.; Nieves, R.E.R.; Leyva, R.J. Prevalencia de neuropatía periférica en diabéticos tipo 2 en el primer nivel de atención. Rev. Méd. Chile 2012, 140, 1126–1131. [Google Scholar] [CrossRef]
- Solís-Villanueva, J.; Michahelles-Barreno, C.; Rodríguez-Lay, E.G.; Farfán-García, J.; Anticona-Sayán, M.; Curo-Carrión, N.; Miranda-Montero, J.J.; Avilez, J.L.; Akehurst, H. Prevalencia y factores de riesgo de neuropatía diabética periférica en pacientes recientemente diagnosticados de diabetes mellitus tipo 2 en un hospital nacional. Rev. Soc. Peru Med. Int. 2019, 32, 4–8. [Google Scholar] [CrossRef]
- Freitas, S.S.; Silva, G.R.F.; Rezende Neta, D.S.; Silva, A.R.V. Analysis of the self-care of diabetics according to by the Summary of Diabetes Self-Care Activities Questionnaire (SDSCA). Acta Sci. Health Sci. 2014, 36, 73–81. [Google Scholar] [CrossRef]
- França, A.A.; Barbosa, J.A.G.; Guimaraes, F.P.; Guimarães, G.L.; Guimarães, J.B. Avaliação da adesão ao autocuidado em diabetes após intervenção educativa realizada com pacientes hospitalizados. Rev. Brasil. Ciênc. Saúde 2020, 24, 47260. [Google Scholar] [CrossRef]
- Imazu, M.F.M.; Faria, B.N.; Arruda, G.O.; Sales, C.A.; Marcon, S.S. Efetividade das intervenções individual e em grupo junto a pessoas com diabetes tipo 2 1. Rev. Lat. Americ. Enferm. 2015, 23, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Meiners, M.M.M.A.; Tavares, N.U.L.; Guimarães, L.S.P.; Bertoldi, A.D. Acesso e adesão a medicamentos entre pessoas com diabetes no Brasil: Evidências da PNAUM. Rev. Brasil. Epidem. 2017, 20, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Wu, T.; Hu, X.; Gao, L. Self-care activities among patients with type 2 diabetes mellitus: A cross-sectional study. Int. J. Nurs. Pract. 2021, 27, e12987. [Google Scholar] [CrossRef] [PubMed]
- Richardson, C.R.; Borgeson, J.R.; Van Harrison, R.; Wyckoff, J.A.; Yoo, A.S. Management of Type 2 Diabetes Mellitus; Michigan Medicine University of Michigan: Ann Arbor, MI, USA, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK579413/ (accessed on 6 December 2022).
- Villegas-Rivera, G.; Román-Pintos, L.M.; Cardona-Muñoz, E.G.; Arias-Carvajal, O.; Rodríguez-Carrizalez, A.D.; Troyo-Sanromán, R.; Pacheco-Moisés, F.P.; Moreno-Ulloa, A.; Miranda-Díaz, A.G. Effects of Ezetimibe/Simvastatin and Rosuvastatin on Oxidative Stress in Diabetic Neuropathy: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Oxid. Med. Cell Longev. 2015, 2015, 756294. [Google Scholar] [CrossRef]
- Iqbal, Z.; Azmi, S.; Yadav, R.; Ferdousi, M.; Kumar, M.; Cuthbertson, D.J.; Lim, J.; Malik, R.A.; Alam, U. Diabetic Peripheral Neuropathy: Epidemiology, Diagnosis, and Pharmacotherapy. Clin. Ther. 2018, 40, 828–849. [Google Scholar] [CrossRef]
- Bariha, P.K.; Tudu, K.M.; Kujur, S.T. Correlation of microalbuminuria with neuropathy in type-II diabetes mellitus patients. Intern. J. Adv. Med. 2018, 5, 1143–1150. [Google Scholar] [CrossRef]
- Gæde, P.; Oellgaard, J.; Carstensen, B.; Rossing, P.; Lund-Andersen, H.; Parving, H.H.; Pedersen, O. Years of life gained by multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: 21 years follow-up on the Steno-2 randomised trial. Diabetologia 2016, 59, 2298–2307. [Google Scholar] [CrossRef]
- Smith, A.G.; Singleton, J.R. Obesity and hyperlipidemia are risk factors for early diabetic neuropathy. J. Diabetes Complicat. 2013, 27, 436–442. [Google Scholar] [CrossRef]
- Sendi, R.A.; Mahrus, A.M.; Saeed, R.M.; Mohammed, M.A.; Al-Dubai, S.A.R. Diabetic peripheral neuropathy among Saudi diabetic patients: A multicenter cross-sectional study at primary health care setting. J. Family Med. Prim. Care. 2020, 9, 197–201. [Google Scholar] [CrossRef]
- Perrin, B.M.; Allen, P.; Gardner, M.J.; Chappell, A.; Phillips, B.; Massey, C.; Skinner, I.; Skinner, T.C. The foot-health of people with diabetes in regional and rural Australia: Baseline results from an observational cohort study. J. Foot Ankle Res. 2019, 12, 56. [Google Scholar] [CrossRef]
- Ristikj-stomnaroska, D.; Risteska-Nejashmikj, V.; Papazova, M. Role of inflammation in the pathogenesis of diabetic peripheral neuropathy. Open Access Maced. J. Med. Sci. 2019, 7, 2267. [Google Scholar] [CrossRef] [PubMed]
- Gaman, M.A.; Epîngeac, M.E.; Diaconu, C.C.; Găman, A.M. Evaluation of oxidative stress levels in obesity and diabetes by the free oxygen radical test and free oxygen radical defence assays and correlations with anthropometric and laboratory parameters. World J. Diabetes 2020, 11, 193. [Google Scholar] [CrossRef] [PubMed]
- Mallet, M.L.; Hadjivassiliou, M.; Sarrigiannis, P.G.; Zis, P. The role of oxidative stress in peripheral neuropathy. J. Mol. Neurosci. 2020, 70, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.M.; Wang, B.; An, X.F.; Zhang, J.A.; Ding, L. Testosterone level and risk of type 2 diabetes in men: A systematic review and meta-analysis. Endocr. Connect. 2018, 7, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Velikova, T.V.; Kabakchieva, P.P.; Assyov, Y.S.; Georgiev, T.A. Targeting Inflammatory Cytokines to Improve Type 2 Diabetes Control. Biomed. Res. Int. 2021, 2021, 7297419. [Google Scholar] [CrossRef]
| Characteristics | Total n (%) | Neuropathy Absent N = 76 1 (%) | Neuropathy Present N = 152 1 (%) | χ2 | U | p-Value |
|---|---|---|---|---|---|---|
| SOCIOECONOMIC CHARACTERISTICS | ||||||
| Sex | 5.06 | 0.024 * | ||||
| Male Female | 108 (47.3) 120 (52.6) | 28 (36.8) 48 (63.1) | 80 (52.6) 72 (47.3) | |||
| Race | 4.46 | 0.346 | ||||
| Black Brown White Yellow Indigenous | 60 (26.3) 137 (60.8) 26 (11.4) 4 (1.7) 1 (0.4) | 23 (30.2) 47 (61.8) 6 (7.8) 0 (0) 0 (0) | 37 (24.3) 90 (59.2) 20 (13.1) 4 (2.6) 1 (0.6) | |||
| Age | 0.20 | 0.977 | ||||
| 30–40 41–50 51–60 ≥61 | 6 (2.6) 27 (11.8) 64 (28) 131 (57.4) | 2 (2.6) 8 (10.5) 22 (28.9) 44 (57.8) | 4 (2.6) 19 (12.5) 42 (27.6) 87 (57.2) | |||
| Education | 4.82 | 0.185 | ||||
| ≤4 5–9 10–12 ≥13 | 80 (35) 75 (32.8) 66 (28.9) 7 (3) | 22 (28.9) 25 (32.8) 28 (36.8) 1 (1.3) | 58 (38.1) 50 (32.8) 38 (25) 6 (3.9) | |||
| Marital status | 3.12 | 0.680 | ||||
| Married Stable Union Single Widower Divorced Separated | 110 (48.2) 47 (20.6) 32 (14) 16 (7) 3 (1.3) 20 (8.7) | 34 (44.7) 15 (19.7) 13 (17.1) 6 (7.8) 0 (0) 8 (10.5) | 76 (50) 32 (21) 19 (12.5) 10 (6.5) 3 (1.9) 12 (7.8) | |||
| Income | 396.4 (275; 550.1) | 366.6 (275; 641.8) | 406.2 (275; 550) | 5531.000 | 0.601 | |
| CLINICAL AND HEALTH HABITS CHARACTERISTICS | ||||||
| DM2 diagnosis time [years] | 6.85 | 0.076 | ||||
| ≤4 5–9 10–19 ≥13 | 64 (28) 58 (25.4) 70 (30.7) 36 (15.7) | 29 (38.1) 19 (25) 20 (26.3) 8 (10.5) | 8 (5.2) 39 (25.6) 50 (32.8) 28 (18.4) | |||
| Perception of general health status | 0.08 | 0.960 | ||||
| Good, very good Regular Poor, fair | 89 (39) 108 (47.3) 31 (13.5) | 29 (38.1) 37 (48.6) 10 (13.1) | 60 (39.4) 71 (46.7) 21 (17.7) | |||
| Smoking | 1.02 | 0.599 | ||||
| Non-smoking Ex-smoker Smoker | 110 (48.2) 105 (46) 13 (5.7) | 36 (47.3) 34 (44.7) 6 (7.8) | 74 (30.9) 71 (46.7) 7 (4.6) | |||
| SAH | 0.50 | 0.477 | ||||
| No Yes | 70 (30.7) 158 (69.2) | 21 (35.5) 55 (72.3) | 49 (32.2) 103 (67.7) | |||
| BMI | 28.8 (25.7; 32.9) | 28 (25.1; 31.5) | 29.3 (26; 33.7) | 4948.000 | 0.077 | |
| BIOCHEMICAL PARAMETERS | ||||||
| Blood glucose | 0.25 | 0.615 | ||||
| 55–130 mg/dL ≥130 mg/dL | 73 (32) 155 (67.9) | 26 (34.2) 50 (65.7) | 47 (30.9) 105 (69) | |||
| Triglycerides | 3.73 | 0.053* | ||||
| 36–149 mg/dL ≥150 mg/dL | 86 (37.7) 142 (62.2) | 22 (28.9) 54 (71) ⧫ | 64 (42.1) ⧫ 88 (57.8) | |||
| Total cholesterol | 1.97 | 0.159 | ||||
| 87–189 mg/dL ≥190 mg/dL | 111 (48.6) 117 (51.3) | 32 (42.1) 44 (57.8) | 79 (51.9) 73 (48) | |||
| HDL | 4.64 | 0.031 * | ||||
| 20–39 mg/dL ≥40 mg/dL | 110 (48.2) 118 (51.7) | 29 (38.1) 47 (61.8) | 81 (53.2) 71 (46.7) | |||
| LDL | 0.45 | 0.497 | ||||
| 15–99 mg/dL ≥100 mg/dL | 85 (37.2) 143 (62.7) | 26 (34.2) 50 (65.7) | 59 (38.8) 93 (61.1) | |||
| Non-HDL | 3.85 | 0.049 * | ||||
| 59–129 mg/dL ≥130 mg/dL | 80 (35) 148 (64.9) | 20 (26.3) 56 (73.6) | 60 (39.4) 92 (60.5) | |||
| HbA1c | 0.13 | 0.709 | ||||
| 4.8–6.9% ≥7% | 39 (17.1) 189 (82.8) | 14 (18.4) 62 (81.5) | 25 (16.4) 127 (83.5) | |||
| Microalbuminuria | 6.79 | 0.033 * | ||||
| <30 mg/g 30–300 mg/g >300 mg/g | 162 (71) 46 (20.1) 20 (8.7) | 61 (80.2) 13 (17.1) 2 (2.6) | 101 (66.4) 33 (21.7) 18 (11.8) | |||
| PERCEPTION OF SELF-CARE IN DIABETES | ||||||
| General Diet | 2.5 (0; 4.8) | 3 (0; 5) | 0.8 (0; 4.5) | 5711.500 | 0.888 | |
| Specific diet | 2.6 (1.3; 3.3) | 2.5 (1.4; 3.3) | 2.6 (1.3; 3.6) | 5504.000 | 0.561 | |
| Exercise | 0 (0; 3.5) | 0 (0; 3) | 0 (0; 3.5) | 5140.500 | 0.139 | |
| Blood Glucose testing | 0 (0; 1) | 0 (0; 1) | 0 (0; 1) | 5623.000 | 0.699 | |
| Foot Care | 4 (2.3; 5) | 4 (2.3; 5) | 4 (2.3; 4.9) | 5642.500 | 0.774 | |
| Medication | 4.6 (4; 4.6) | 4.66 (3.4; 4.6) | 4.6 (4; 4.6) | 5346.000 | 0.315 |
| Univariate Analysis (n = 228) | Multivariate Analysis (n = 228) | |||||
|---|---|---|---|---|---|---|
| Variables | OR 1 | 95% CI | p-value | OR 2 | 95% CI | p-value |
| Sex—reference male | 1.90 | 1.08, 3.34 | 0.025 * | 0.50 | 0.26, 0.96 | 0.039 ** |
| Education—reference ≥13 | 0.199 * | 0.356 | ||||
| ≤4 5–9 10–12 | 0.43 0.33 0.22 | 0.05, 3.86 0.03, 2.92 0.02, 1.98 | 0.458 0.321 0.179 * | 0.46 0.34 0.25 | 0.35, 1.61 0.25, 1.16 0.21, 21.82 | 0.469 0.118 0.510 |
| DM2 diagnosis time [years]—reference ≥13 | 0.082 * | 0.345 | ||||
| ≤4 5–9 10–19 | 0.34 0.58 0.71 | 0.13, 0.87 0.22, 1.52 0.27, 1.83 | 0.024 * 0.275 0.484 | 0.50 0.79 1.03 | 0.17, 1.51 0.26, 2.33 0.35, 3.01 | 0.223 0.671 0.947 |
| BMI | 1.03 | 0.98, 1.07 | 0.153 * | 1.05 | 1.00, 1.10 | 0.051 ** |
| Triglycerides | 1.78 | 0.98, 3.22 | 0.054 * | 1.68 | 0.85, 3.31 | 0.134 |
| Total cholesterol | 1.48 | 0.85, 2.59 | 0.160 * | 0.81 | 0.32, 2.05 | 0.659 |
| HDL | 1.84 | 1.05, 3.24 | 0.032 * | 2.06 | 1.07, 3.97 | 0.031 ** |
| Non-HDL | 1.82 | 0.99, 3.34 | 0.051 * | 1.84 | 0.69, 4.91 | 0.223 |
| Microalbuminuria—reference >300 mg/g | 0.054 * | 0.236 | ||||
| <30 mg/g 30–300 mg/g | 0.18 0.28 | 0.04, 0.82 0.05, 1.39 | 0.026 * 0.120 | 0.24 0.26 | 0.05, 1.24 0.04, 1.44 | 0.090 0.124 |
| Exercise (Summary of Diabetes Self-Care Activities Questionnaire) | 1.11 | 0.97, 1.26 | 0.118 * | 1.12 | 0.96, 1.30 | 0.131 |
| Medication (Summary of Diabetes Self-Care Activities Questionnaire) | 1.10 | 0.95, 1.28 | 0.188 * | 1.18 | 0.99, 1.40 | 0.061 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farias, A.L.d.; Silva, A.S.A.d.; Tavares, V.B.; Souza, J.d.S.e.; Silva, H.P.d.; Oliveira Bastos, M.d.S.C.-B.d.; Melo-Neto, J.S.d. Amazon Amandaba—Prevalence, Risk Factors and Self-Care Perception Associated with Diabetic Peripheral Neuropathy in Patients with Type 2 Diabetes: A Cross-Sectional Study. Healthcare 2023, 11, 518. https://doi.org/10.3390/healthcare11040518
Farias ALd, Silva ASAd, Tavares VB, Souza JdSe, Silva HPd, Oliveira Bastos MdSC-Bd, Melo-Neto JSd. Amazon Amandaba—Prevalence, Risk Factors and Self-Care Perception Associated with Diabetic Peripheral Neuropathy in Patients with Type 2 Diabetes: A Cross-Sectional Study. Healthcare. 2023; 11(4):518. https://doi.org/10.3390/healthcare11040518
Chicago/Turabian StyleFarias, Aline Lobato de, Amanda Suzane Alves da Silva, Victória Brioso Tavares, Josiel de Souza e Souza, Hilton Pereira da Silva, Maria do Socorro Castelo-Branco de Oliveira Bastos, and João Simão de Melo-Neto. 2023. "Amazon Amandaba—Prevalence, Risk Factors and Self-Care Perception Associated with Diabetic Peripheral Neuropathy in Patients with Type 2 Diabetes: A Cross-Sectional Study" Healthcare 11, no. 4: 518. https://doi.org/10.3390/healthcare11040518
APA StyleFarias, A. L. d., Silva, A. S. A. d., Tavares, V. B., Souza, J. d. S. e., Silva, H. P. d., Oliveira Bastos, M. d. S. C.-B. d., & Melo-Neto, J. S. d. (2023). Amazon Amandaba—Prevalence, Risk Factors and Self-Care Perception Associated with Diabetic Peripheral Neuropathy in Patients with Type 2 Diabetes: A Cross-Sectional Study. Healthcare, 11(4), 518. https://doi.org/10.3390/healthcare11040518





