Induction of Labor in Women with Previous Cesarean Section and Unfavorable Cervix: A Retrospective Cohort Study
Abstract
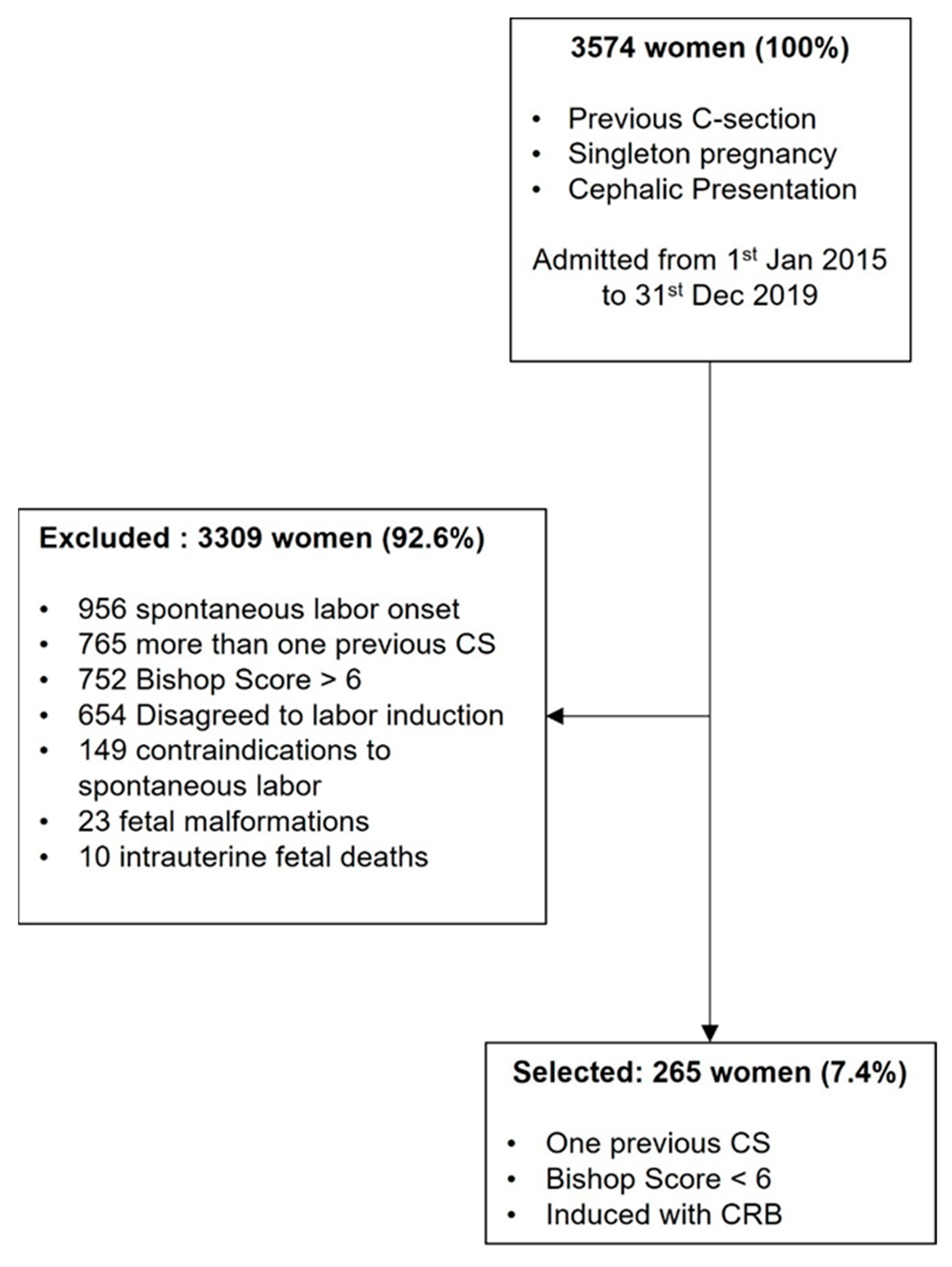
:1. Introduction
2. Materials and Methods
2.1. Outcomes
2.2. Statistical Analysis
3. Results
4. Discussion
4.1. Strengths and Limitations
4.2. Implications for Clinical Practice
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheng, Y.W.; Eden, K.B.; Marshall, N.; Pereira, L.; Caughey, A.B.; Guise, J.M. Delivery after prior cesarean: Maternal morbidity and mortality. Clin. Perinatol. 2011, 38, 297–309. [Google Scholar] [CrossRef]
- Thomas, J.; Paranjothy, S. The National Sentinel Caesarean Section Audit Report. RCOG Press. 2001. Available online: https://orca.cardiff.ac.uk/id/eprint/93112/1/nscs_audit.pdf (accessed on 11 February 2023).
- Landon, M.B.; Hauth, J.C.; Leveno, K.J.; Spong, C.Y.; Leindecker, S.; Varner, M.W.; Moawad, A.H.; Caritis, S.N.; Harper, M.; Wapner, R.J.; et al. Maternal and perinatal outcomes associated with a trial of labor after prior cesarean delivery. N. Engl. J. Med. 2004, 351, 2581–2589. [Google Scholar] [CrossRef] [PubMed]
- Curtin, S.C.; Gregory, K.D.; Korst, L.M.; Uddin, S.F. Maternal morbidity for vaginal and cesarean deliveries, according to previous cesarean history: New data from the birth certificate, 2013. Natl. Vital Stat. Rep. 2015, 64, 1–13. [Google Scholar]
- Grobman, W.A.; Lai, Y.; Landon, M.B.; Spong, C.Y.; Leveno, K.J.; Rouse, D.J.; Varner, M.W.; Moawad, A.H.; Caritis, S.N.; Harper, M.; et al. Development of a nomogram for prediction of vaginal birth after cesarean delivery. Obstet. Gynecol. 2007, 109, 806–812. [Google Scholar] [CrossRef] [PubMed]
- Royal College of Obstetricians and Gynaecologists. Birth after Previous Caesarean Birth; Green-Top Guideline No.45; RCOG: London, UK, 2015. [Google Scholar]
- Atia, O.; Rotem, R.; Reichman, O.; Jaffe, A.; Grisaru-Granovsky, S.; Sela, H.Y.; Rottenstreich, M. Number of prior vaginal deliveries and trial of labor after cesarean success. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 256, 189–193. [Google Scholar] [CrossRef]
- Landon, M.B.; Leindecker, S.; Spong, C.Y.; Hauth, J.C.; Bloom, S.; Varner, M.W.; Moawad, A.H.; Caritis, S.N.; Harper, M.; Wapner, R.J.; et al. The MFMU Cesarean Registry: Factors affecting the success of trial of labor after previous cesarean delivery. Am. J. Obstet. Gynecol. 2005, 193 Pt 2, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Sentilhes, L.; Vayssière, C.; Beucher, G.; Deneux-Tharaux, C.; Deruelle, P.; Diemunsch, P.; Gallot, D.; Haumonté, J.B.; Heimann, S.; Kayem, G.; et al. Delivery for women with a previous cesarean: Guidelines for clinical practice from the French College of Gynecologists and Obstetricians (CNGOF). Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 170, 25–32. [Google Scholar] [CrossRef] [PubMed]
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 205: Vaginal Birth After Cesarean Delivery. Obstet. Gynecol. 2019, 133, e110–e127. [Google Scholar] [CrossRef] [PubMed]
- Martel, M.J.; MacKinnon, C.J. RETIRED: No. 155—Guidelines for Vaginal Birth After Previous Caesarean Birth. J. Obstet. Gynaecol. Can. 2018, 40, e195–e207. [Google Scholar] [CrossRef]
- Fondazione Confalonieri Ragonese on behalf of SIGO, AOGOI, AGUI. Linea Guida 15. Induzione al Travaglio di Parto; SIGO Rome, Italy. 2022. Available online: https://www.sigo.it/wp-content/uploads/2022/02/LG15_Induzione_Travaglio_Parto.pdf (accessed on 11 February 2023).
- American College of Obstetricians and Gynecologists. Obstetric care consensus no. 1: Safe prevention of the primary cesarean delivery. Obstet. Gynecol. 2014, 123, 693–711. [Google Scholar] [CrossRef] [PubMed]
- Ayres-de-Campos, D.; Spong, C.Y.; Chandraharan, E. FIGO consensus guidelines on intrapartum fetal monitoring: Cardiotocography. Int. J. Gynaecol. Obstet. 2015, 131, 13–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kehl, S.; Weiss, C.; Rath, W. Balloon catheters for induction of labor at term after previous cesarean section: A systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 204, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Grobman, W.A.; Rice, M.M.; Reddy, U.M.; Tita, A.T.N.; Silver, R.M.; Mallett, G.; Hill, K.; Thom, E.A.; El-Sayed, Y.Y.; Perez-Delboy, A.; et al. Labor Induction versus Expectant Management in Low-Risk Nulliparous Women. N. Engl. J. Med. 2018, 379, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Durnwald, C.P.; Ehrenberg, H.M.; Mercer, B.M. The impact of maternal obesity and weight gain on vaginal birth after cesarean sectionsuccess. Am. J. Obstet. Gynecol. 2004, 191, 954–957. [Google Scholar] [CrossRef]
- Jozwiak, M.; Bloemenkamp, K.W.; Kelly, A.J.; Mol, B.W.; Irion, O.; Boulvain, M. Mechanical methods for induction of labor. Cochrane Database Syst. Rev. 2012, 3, CD001233. [Google Scholar]
- Cromi, A.; Ghezzi, F.; Agosti, M.; Serati, M.; Uccella, S.; Arlant, V.; Bolis, P. Is transcervical Foley catheter actually slower than prostaglandins in ripening the cervix? A randomized study. Am. J. Obstet. Gynecol. 2011, 204, 338.e1–338.e7. [Google Scholar] [CrossRef]
- Ande, A.B.; Ezeanochie, C.M.; Olagbuji, N.B. Induction of labor in prolonged pregnancy with unfavorable cervix: Comparison of sequential intracervical Foley catheter-intravaginal misoprostol and intravaginal misoprostol alone. Arch. Gynecol. Obstet. 2012, 285, 967–971. [Google Scholar] [CrossRef] [PubMed]
- Shah, U.; Bellows, P.; Drexler, K.; Hawley, L.; Davidson, C.; Sangi-Haghpeykar, H.; Gandhi, M. Comparison of induction of labor methods for unfavorable cervices in trial of labor after cesarean delivery. J. Matern. Fetal Neonatal Med. 2017, 30, 1010–1015. [Google Scholar] [CrossRef]
- Ralph, J.A.; Leftwich, H.K.; Leung, K.; Zaki, M.N.; Della Torre, M.; Hibbard, J.U. Morbidity associated with the use of Foley balloon for cervical ripening in women with prior cesarean delivery. J. Matern. Fetal Neonatal Med. 2020, 35, 3937–3942. [Google Scholar] [CrossRef]
- Fitzpatrick, K.E.; Kurinczuk, J.J.; Alfirevic, Z.; Spark, P.; Brocklehurst, P.; Knight, M. Uterine rupture by intended mode of delivery in the UK: A national case-control study. PLoS Med. 2012, 9, e1001184. [Google Scholar] [CrossRef] [PubMed]
- Stock, S.J.; Ferguson, E.; Duffy, A.; Ford, I.; Chalmers, J.; Norman, J.E. Outcomes of induction of labor in women with previous caesarean delivery: A retrospective cohort study using a population database. PLoS ONE 2013, 8, e60404. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, H.; Luo, S.; Gu, W. Oxytocin use in trial of labor after cesarean and its relationship with risk of uterine rupture in women with one previous cesarean section: A meta-analysis of observational studies. BMC Pregnancy Childbirth 2021, 21, 11. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, L.T.; Gardner, B.; Rahman, M.; Schoen, C.; Connolly, K.A.; Hankins, G.D.; Saade, G.R.; Saad, A.F. Cervical Ripening Using Foley Balloon with or without Oxytocin: A Systematic Review and Meta-Analysis. Am. J. Perinatol. 2019, 36, 406–421. [Google Scholar] [CrossRef] [PubMed]
| Age (Years) | 34 (29–37) |
|---|---|
| Ethnicity | |
| Caucasian | 208 (78.6%) |
| Black | 24 (9%) |
| Asian | 21 (7.9%) |
| Hispanic | 12 (4.5%) |
| Previous vaginal birth | |
| Yes | 47 (17.7%) |
| No | 218 (82.3%) |
| Gestational Age at Induction (weeks) | 40 (38–41) |
| BMI | |
| Pre-pregnancy | 24.2 (21.6–27.8) |
| Term pregnancy | 28.9 (26.2–32.4) |
| Mode of conception | |
| Spontaneous | 261 (98.5%) |
| ART | 4 (1.5%) |
| VBAC n = 152 | CS n = 113 | p | |
|---|---|---|---|
| Maternal age (years) | 34 (29–36) | 35 (30–38) | 0.479 |
| Maternal age ≥40 | 7.2% | 15.9% | 0.025 |
| Gestational age at induction (weeks) | 40 (38–41) | 40 (39–41) | 0.101 |
| Gestational age ≥39 weeks | 55.9% | 61.1% | 0.402 |
| Mode of conception | 0.764 | ||
| Spontaneous | 150 (98.7%) | 111 (98.2%) | |
| ART | 2 (1.3%) | 2 (1.8%) | |
| Previous vaginal birth | 25% | 8% | <0.001 |
| Ethnicity | 0.395 | ||
| Caucasian | 121 (79.6%) | 87 (77%) | |
| Black | 13 (8.6%) | 11 (9.7%) | |
| Asian | 12 (7.9%) | 9 (8%) | |
| Hispanic | 6 (3.9%) | 6 (5.3%) | |
| Pre-pregnancy BMI | 23.3 (21–26) | 25.6 (22.9–30.6) | <0.001 |
| ≥30 | 11.8% | 28.3% | 0.001 |
| Term-pregnancy BMI | 27.7 (25.5–30.5) | 30.6 (27.5–34.2) | 0.054 |
| Indication of labor induction | 0.386 | ||
| Post-term pregnancy | 49 (32.2%) | 45 (39.8%) | |
| Diabetes | 30 (19.7%) | 17 (15%) | |
| Hypertensive disorders | 21 (13.8%) | 18 (15.9%) | |
| IUGR/oligohydramnios | 10 (6.6%) | 10 (8.9%) | |
| Intrahepatic cholestasis | 13 (8.6%) | 10 (8.9%) | |
| Others | 29 (19.1%) | 13 (11.5%) | |
| Indication for previous CS | 0.210 | ||
| Breech presentation | 30 (19.7%) | 18 (37.5%) | |
| Dystocia | 18 (11.8%) | 21 (53.8%) | |
| Pathological FHR tracing | 25 (16.4%) | 21 (45.7%) | |
| Failed labor induction | 10 (6.6%) | 15 (60%) | |
| Maternal will | 1 (0.7%) | 0 (0%) | |
| Others | 47 (30.9%) | 28 (37.3%) | |
| Unknown | 21 (13.8%) | 10 (32.3%) | |
| Duration of CRB application (h) | 12 (9–15) | 13 (12–16) | 0.285 |
| Time of CRB application—labor onset (h) | 16 (12–22) | 19 (16–25) | 0.018 |
| Time of CRB application—delivery (h) | 21 (15–27) | 22 (17–31) | 0.207 |
| CRB plus oxytocin induction | 44.7% | 45.1% | 0.949 |
| Oxytocin augmentation | 32.2% | 21.2% | 0.047 |
| Use of intrapartum analgesia | 58.6% | 34.5% | <0.001 |
| Incidence of CAMO | 12.5% | 8% | 0.235 |
| Incidence of CAFO | 3.3% | 12.4% | 0.005 |
| CRB n = 146 | CRB Plus Oxytocin n = 119 | p | |
|---|---|---|---|
| Incidence of CAMO | 7 (4.8%) | 21 (17.6%) | 0.001 |
| Uterine rupture | 0 | 1 (0.8%) | |
| Post-partum hemorrhage | 4 (2.7%) | 6 (5%) | |
| Endometritis | 0 | 1 (0.8%) | |
| Wound re-opening | 1 (0.68%) | 1 (0.8%) | |
| Thromboembolism | 2 (1.36%) | 0 | |
| Surgical lesions | 0 | 1 (0.8%) | |
| Others (laparotomy, hysterectomy) | 0 | 3 (2.5%) | |
| Blood transfusion | 0 | 5 (4.2%) | |
| Intensive care unit admission | 0 | 3 (2.5%) | |
| Incidence of CAFO | 7 (4.8%) | 12 (10.1%) | 0.097 |
| Neonatal intensive care unit admission | 1 (0.68%) | 4 (3.3%) | |
| Apgar score (5′) < 7 | 2 (1.36%) | 3 (2.5%) | |
| Umbilical pH < 7 | 1 (0.68%) | 2 (1.68%) | |
| Pathological FHR tracing | 3 (2%) | 3 (2.5%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Germano, C.; Mappa, I.; Cromi, A.; Busato, E.; Incerti, M.; Lojacono, A.; Rizzo, G.; Attini, R.; Patrizi, L.; Revelli, A.; et al. Induction of Labor in Women with Previous Cesarean Section and Unfavorable Cervix: A Retrospective Cohort Study. Healthcare 2023, 11, 543. https://doi.org/10.3390/healthcare11040543
Germano C, Mappa I, Cromi A, Busato E, Incerti M, Lojacono A, Rizzo G, Attini R, Patrizi L, Revelli A, et al. Induction of Labor in Women with Previous Cesarean Section and Unfavorable Cervix: A Retrospective Cohort Study. Healthcare. 2023; 11(4):543. https://doi.org/10.3390/healthcare11040543
Chicago/Turabian StyleGermano, Chiara, Ilenia Mappa, Antonella Cromi, Enrico Busato, Maddalena Incerti, Andrea Lojacono, Giuseppe Rizzo, Rossella Attini, Lodovico Patrizi, Alberto Revelli, and et al. 2023. "Induction of Labor in Women with Previous Cesarean Section and Unfavorable Cervix: A Retrospective Cohort Study" Healthcare 11, no. 4: 543. https://doi.org/10.3390/healthcare11040543






