Effect of Lower Limb Venous Dilation on the Autonomic Cardiac Response among Healthy Young Men
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Protocol
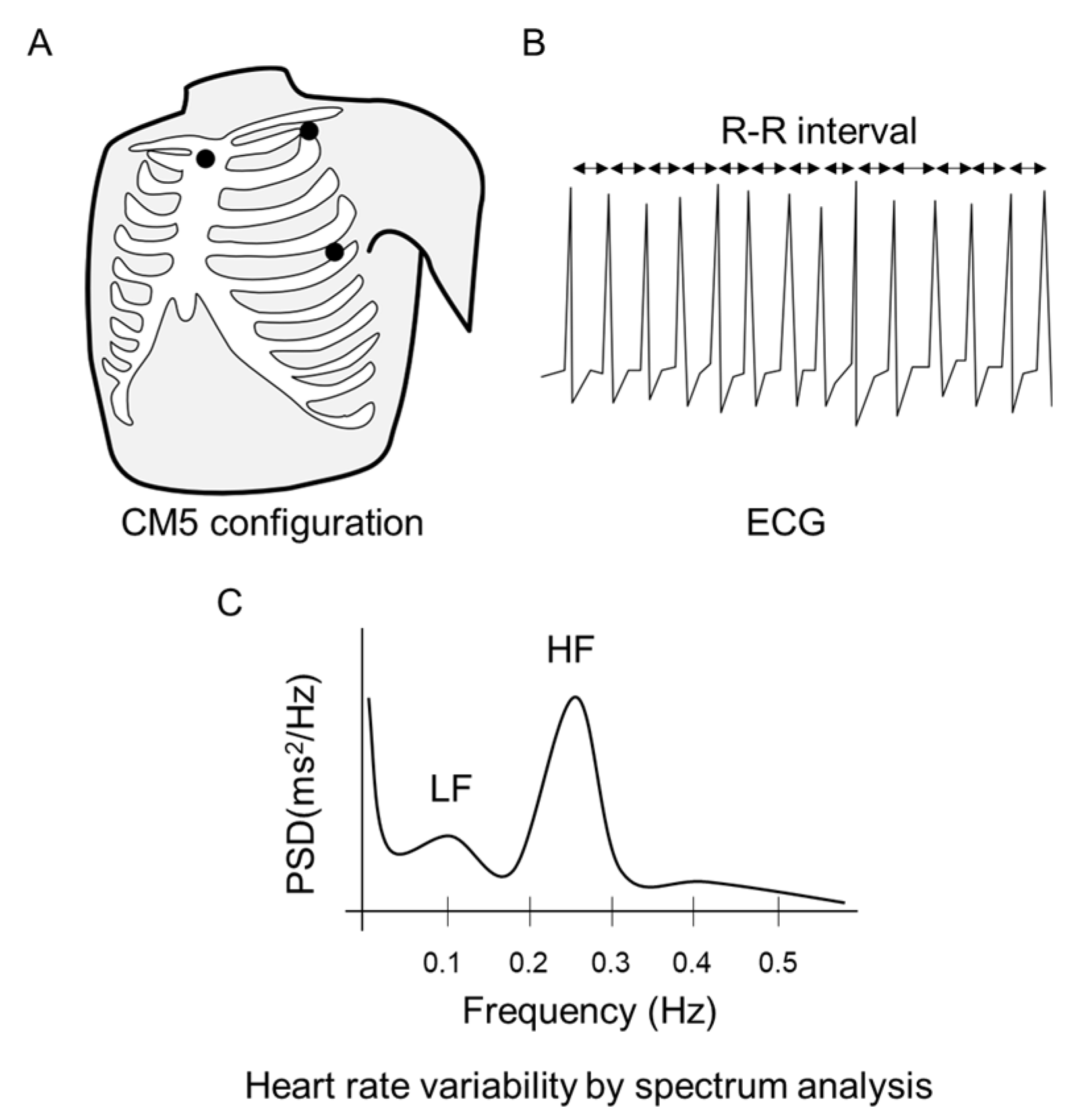
2.3. Physiological Data Collection
2.4. Statistical Analysis
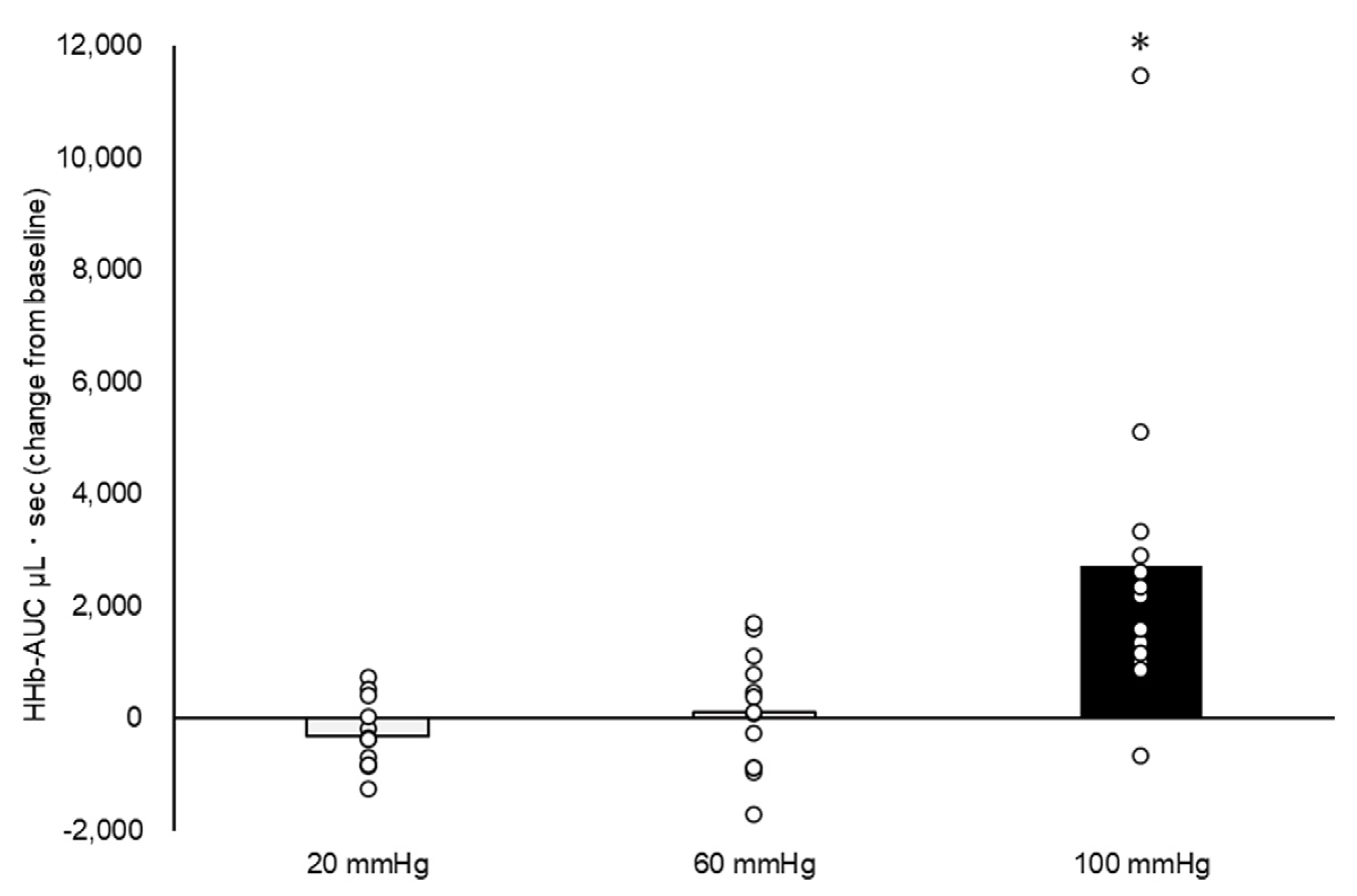
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murphy, M.N.; Mizuno, M.; Mitchell, J.H.; Smith, S.A. Cardiovascular Regulation by Skeletal Muscle Reflexes in Health and Disease. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H1191–H1204. [Google Scholar] [CrossRef]
- Kaufman, M.P.; Hayes, S.G. The Exercise Pressor Reflex. Clin. Auton. Res. 2002, 12, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, M.P.; Longhurst, J.C.; Rybicki, K.J.; Wallach, J.H.; Mitchell, J.H. Effects of Static Muscular Contraction on Impulse Activity of Groups III and IV Afferents in Cats. J. Appl. Physiol. 1983, 55 Pt 1, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Stebbins, C.L.; Brown, B.; Levin, D.; Longhurst, J.C. Reflex Effect of Skeletal Muscle Mechanoreceptor Stimulation on the Cardiovascular System. J. Appl. Physiol. 1988, 65, 1539–1547. [Google Scholar] [CrossRef]
- Angius, L.; Crisafulli, A. Exercise Intolerance and Fatigue in Chronic Heart Failure: Is There a Role for Group III/IV Afferent Feedback? Eur. J. Prev. Cardiol. 2020, 27, 1862–1872. [Google Scholar] [CrossRef]
- Mitchell, J.H. Abnormal Cardiovascular Response to Exercise in Hypertension: Contribution of Neural Factors. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 312, R851–R863. [Google Scholar] [CrossRef]
- Smith, S.A.; Mitchell, J.H.; Naseem, R.H.; Garry, M.G. Mechanoreflex Mediates the Exaggerated Exercise Pressor Reflex in Heart Failure. Circulation 2005, 112, 2293–2300. [Google Scholar] [CrossRef]
- Aimo, A.; Saccaro, L.F.; Borrelli, C.; Fabiani, I.; Gentile, F.; Passino, C.; Emdin, M.; Piepoli, M.F.; Coats, A.J.S.; Giannoni, A. The Ergoreflex: How the Skeletal Muscle Modulates Ventilation and Cardiovascular Function in Health and Disease. Eur. J. Heart Fail. 2021, 23, 1458–1467. [Google Scholar] [CrossRef]
- Keller-Ross, M.L.; Johnson, B.D.; Carter, R.E.; Joyner, M.J.; Eisenach, J.H.; Curry, T.B.; Olson, T.P. Improved Ventilatory Efficiency with Locomotor Muscle Afferent Inhibition Is Strongly Associated with Leg Composition in Heart Failure. Int. J. Cardiol. 2016, 202, 159–166. [Google Scholar] [CrossRef]
- Banks, R.W.; Hulliger, M.; Saed, H.H.; Stacey, M.J. A Comparative Analysis of the Encapsulated End-Organs of Mammalian Skeletal Muscles and of Their Sensory Nerve Endings. J. Anat. 2009, 214, 859–887. [Google Scholar] [CrossRef]
- Yamauchi, K.; Stone, A.J.; Kaufman, M.P. Hindlimb Venous Distention Evokes a Pressor Reflex in Decerebrated Rats. Physiol. Rep. 2014, 2, e12036. [Google Scholar] [CrossRef]
- McClain, J.; Hardy, C.; Enders, B.; Smith, M.; Sinoway, L. Limb Congestion and Sympathoexcitation during Exercise. Implications for Congestive Heart Failure. J. Clin. Investig. 1993, 92, 2353–2359. [Google Scholar] [CrossRef] [PubMed]
- Haouzi, P.; Chenuel, B.; Huszczuk, A. Sensing Vascular Distension in Skeletal Muscle by Slow Conducting Afferent Fibers: Neurophysiological Basis and Implication for Respiratory Control. J. Appl. Physiol. 2004, 96, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Hart, E.C.; Charkoudian, N. Sympathetic Neural Mechanisms in Human Blood Pressure Regulation. Curr. Hypertens. Rep. 2011, 13, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Prochaska, J.H.; Arnold, N.; Falcke, A.; Kopp, S.; Schulz, A.; Buch, G.; Moll, S.; Panova-Noeva, M.; Jünger, C.; Eggebrecht, L.; et al. Chronic Venous Insufficiency, Cardiovascular Disease, and Mortality: A Population Study. Eur. Heart J. 2021, 42, 4157–4165. [Google Scholar] [CrossRef]
- Nicklas, J.M.; Bleske, B.E.; Van Harrison, R.; Hogikyan, R.V.; Kwok, Y.; Chavey, W.E. Heart Failure: Clinical Problem and Management Issues. Prim. Care 2013, 40, 17–42. [Google Scholar] [CrossRef]
- Nijst, P.; Verbrugge, F.H.; Grieten, L.; Dupont, M.; Steels, P.; Tang, W.H.W.; Mullens, W. The Pathophysiological Role of Interstitial Sodium in Heart Failure. J. Am. Coll. Cardiol. 2015, 65, 378–388. [Google Scholar] [CrossRef]
- Hirsch, J.A.; Bishop, B. Respiratory Sinus Arrhythmia in Humans: How Breathing Pattern Modulates Heart Rate. Am. J. Physiol. 1981, 241, H620–H629. [Google Scholar] [CrossRef]
- Malik, M.; Bigger, J.T.; Camm, A.J.; Kleiger, R.E.; Malliani, A.; Moss, A.J.; Schwartz, P.J. Heart Rate Variability: Standards of Measurement, Physiological Interpretation, and Clinical Use. Eur. Heart J. 1996, 17, 354–381. [Google Scholar] [CrossRef]
- Jones, S.; Chiesa, S.T.; Chaturvedi, N.; Hughes, A.D. Recent Developments in Near-Infrared Spectroscopy (NIRS) for the Assessment of Local Skeletal Muscle Microvascular Function and Capacity to Utilise Oxygen. Artery Res. 2016, 16, 25–33. [Google Scholar] [CrossRef] [Green Version]
- Amann, M.; Runnels, S.; Morgan, D.E.; Trinity, J.D.; Fjeldstad, A.S.; Wray, D.W.; Reese, V.R.; Richardson, R.S. On the Contribution of Group III and IV Muscle Afferents to the Circulatory Response to Rhythmic Exercise in Humans: Muscle Afferents and Blood Flow. J. Physiol. 2011, 589 Pt 15, 3855–3866. [Google Scholar] [CrossRef]
- Lipsitz, L.A.; Mietus, J.; Moody, G.B.; Goldberger, A.L. Spectral Characteristics of Heart Rate Variability before and during Postural Tilt. Relations to Aging and Risk of Syncope. Circulation 1990, 81, 1803–1810. [Google Scholar] [CrossRef] [PubMed]
- Pomeranz, B.; Macaulay, R.J.; Caudill, M.A.; Kutz, I.; Adam, D.; Gordon, D.; Kilborn, K.M.; Barger, A.C.; Shannon, D.C.; Cohen, R.J. Assessment of Autonomic Function in Humans by Heart Rate Spectral Analysis. Am. J. Physiol. 1985, 248 Pt 2, H151–H153. [Google Scholar] [CrossRef]
- Vybiral, T.; Bryg, R.J.; Maddens, M.E.; Boden, W.E. Effect of Passive Tilt on Sympathetic and Parasympathetic Components of Heart Rate Variability in Normal Subjects. Am. J. Cardiol. 1989, 63, 1117–1120. [Google Scholar] [CrossRef]
- Hayano, J.; Taylor, J.A.; Yamada, A.; Mukai, S.; Hori, R.; Asakawa, T.; Yokoyama, K.; Watanabe, Y.; Takata, K.; Fujinami, T. Continuous Assessment of Hemodynamic Control by Complex Demodulation of Cardiovascular Variability. Am. J. Physiol. Heart Circ. Physiol. 1993, 264, H1229–H1238. [Google Scholar] [CrossRef] [PubMed]
- Moak, J.P.; Goldstein, D.S.; Eldadah, B.A.; Saleem, A.; Holmes, C.; Pechnik, S.; Sharabi, Y. Supine Low-Frequency Power of Heart Rate Variability Reflects Baroreflex Function, Not Cardiac Sympathetic Innervation. Heart Rhythm 2007, 4, 1523–1529. [Google Scholar] [CrossRef] [PubMed]
- Rahman, F.; Pechnik, S.; Gross, D.; Sewell, L.; Goldstein, D.S. Low Frequency Power of Heart Rate Variability Reflects Baroreflex Function, Not Cardiac Sympathetic Innervation. Clin. Auton. Res. 2011, 21, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Nóbrega, A.C.L.; Williamson, J.W.; Friedman, D.B.; Araújo, C.G.S.; Mitchell, J.H. Cardiovascular Responses to Active and Passive Cycling Movements. Med. Sci. Sports Exerc. 1994, 26, 709–714. [Google Scholar] [CrossRef]
- McDaniel, J.; Hayman, M.A.; Ives, S.; Fjeldstad, A.S.; Trinity, J.D.; Wray, D.W.; Richardson, R.S. Attenuated Exercise Induced Hyperaemia with Age: Mechanistic Insight from Passive Limb Movement: Attenuated Exercise Hyperaemia with Age. J. Physiol. 2010, 588 Pt 22, 4507–4517. [Google Scholar] [CrossRef]
- Vianna, L.C.; Oliveira, R.B.; Ramos, P.S.; Ricardo, D.R.; Araújo, C.G.S. Effect of Muscle Mass on Muscle Mechanoreflex-Mediated Heart Rate Increase at the Onset of Dynamic Exercise. Eur. J. Appl. Physiol. 2010, 108, 429–434. [Google Scholar] [CrossRef]
- Grassi, B.; Quaresima, V. Near-Infrared Spectroscopy and Skeletal Muscle Oxidative Function in Vivo in Health and Disease: A Review from an Exercise Physiology Perspective. J. Biomed. Opt. 2016, 21, 091313. [Google Scholar] [CrossRef]
- Manfredini, F.; Lamberti, N.; Rossi, T.; Mascoli, F.; Basaglia, N.; Zamboni, P. A Toe Flexion NIRS Assisted Test for Rapid Assessment of Foot Perfusion in Peripheral Arterial Disease: Feasibility, Validity, and Diagnostic Accuracy. Eur. J. Vasc. Endovasc. Surg. 2017, 54, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Hosoi, Y.; Yasuhara, H.; Shigematsu, H.; Aramoto, H.; Komiyama, T.; Muto, T. A New Method for the Assessment of Venous Insufficiency in Primary Varicose Veins Using Near-Infrared Spectroscopy. J. Vasc. Surg. 1997, 26, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Hamdan, A. Management of Varicose Veins and Venous Insufficiency. JAMA 2012, 308, 2612. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, R.T.; Raffetto, J.D. Chronic Venous Insufficiency. Circulation 2014, 130, 333–346. [Google Scholar] [CrossRef]
- Raju, S.; Walker, W.; May, C. Measurement of Ambulatory Venous Pressure and Column Interruption Duration in Normal Volunteers. J. Vasc. Surg. Venous Lymphat. Disord. 2020, 8, 127–136. [Google Scholar] [CrossRef]
- Agu, O.; Baker, D.; Seifalian, A.M. Effect of graduated compression stockings on limb oxygenation and venous function during exercise in patients with venous insufficiency. Vascular 2004, 12, 69–76. [Google Scholar] [CrossRef]
- Hiatt, W.R.; Huang, S.Y.; Regensteiner, J.G.; Micco, A.J.; Ishimoto, G.; Manco-Johnson, M.; Drose, J.U.L.I.A.; Reeves, J.T. Venous occlusion plethysmography reduces arterial diameter and flow velocity. J. Appl. Physiol. 1989, 66, 2239–2244. [Google Scholar] [CrossRef] [PubMed]
- Hansteen, V.; Sivertssen, E.; Lorentsen, E. The Influence of Venous Collecting Pressure on Measurements of Calf Blood Flow by Venous Occlusion Plethysmography. Angiology 1970, 21, 661–677. [Google Scholar] [CrossRef]




| Variable | Baseline | 20 mmHg | 60 mmHg | 100 mmHg | F Value | p Value |
|---|---|---|---|---|---|---|
| HR, beats/min | 69.0 ± 2.2 | 68.6 ± 2.2 | 67.4 ± 2.2 | 68.9 ± 2.2 | 1.58 | 0.21 |
| LF, ms2 | 912.0 ± 455.5 | 910.6 ± 455.5 | 2237.3 ± 455.5 | 1732.1 ± 455.5 | 3.04 | 0.04 |
| LF, normalized unit | 46.3 ± 3.3 | 50.7 ± 3.3 | 57.2 ± 3.3 * | 58.7 ± 3.3 * | 5.35 | 0.00 |
| HF, ms2 | 1143.7 ± 289.0 | 923.7 ± 289.0 | 1555.0 ± 289.0 | 1123.2 ± 289.0 | 1.47 | 0.24 |
| HF, normalized unit | 53.2 ± 3.2 | 48.6 ± 3.2 | 42.4 ± 3.2 * | 41.1 ± 3.2 * | 5.55 | 0.00 |
| LF/HF | 1.1 ± 0.2 | 1.1 ± 0.2 | 1.5 ± 0.2 | 1.8 ± 0.2 * | 3.88 | 0.02 |
| SBP, mmHg | 114.3 ± 3.0 | 109.7 ± 3.0 | 111.5 ± 3.0 | 110.6 ± 3.0 | 2.84 | 0.05 |
| DBP, mmHg | 64.3 ± 2.6 | 60.7 ± 2.6 * | 60.6 ± 2.6 * | 60.5 ± 2.6 * | 4.70 | 0.01 |
| MAP, mmHg | 81.0 ± 2.7 | 77.1 ± 2.7 * | 77.5 ± 2.7 * | 77.2 ± 2.7 * | 5.35 | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujita, D.; Kubo, Y.; Tagawa, T. Effect of Lower Limb Venous Dilation on the Autonomic Cardiac Response among Healthy Young Men. Healthcare 2023, 11, 548. https://doi.org/10.3390/healthcare11040548
Fujita D, Kubo Y, Tagawa T. Effect of Lower Limb Venous Dilation on the Autonomic Cardiac Response among Healthy Young Men. Healthcare. 2023; 11(4):548. https://doi.org/10.3390/healthcare11040548
Chicago/Turabian StyleFujita, Daisuke, Yusuke Kubo, and Tatsuya Tagawa. 2023. "Effect of Lower Limb Venous Dilation on the Autonomic Cardiac Response among Healthy Young Men" Healthcare 11, no. 4: 548. https://doi.org/10.3390/healthcare11040548
APA StyleFujita, D., Kubo, Y., & Tagawa, T. (2023). Effect of Lower Limb Venous Dilation on the Autonomic Cardiac Response among Healthy Young Men. Healthcare, 11(4), 548. https://doi.org/10.3390/healthcare11040548







