Emerging Therapeutic Strategies for Diffuse Intrinsic Pontine Glioma: A Systematic Review
Abstract
1. Introduction
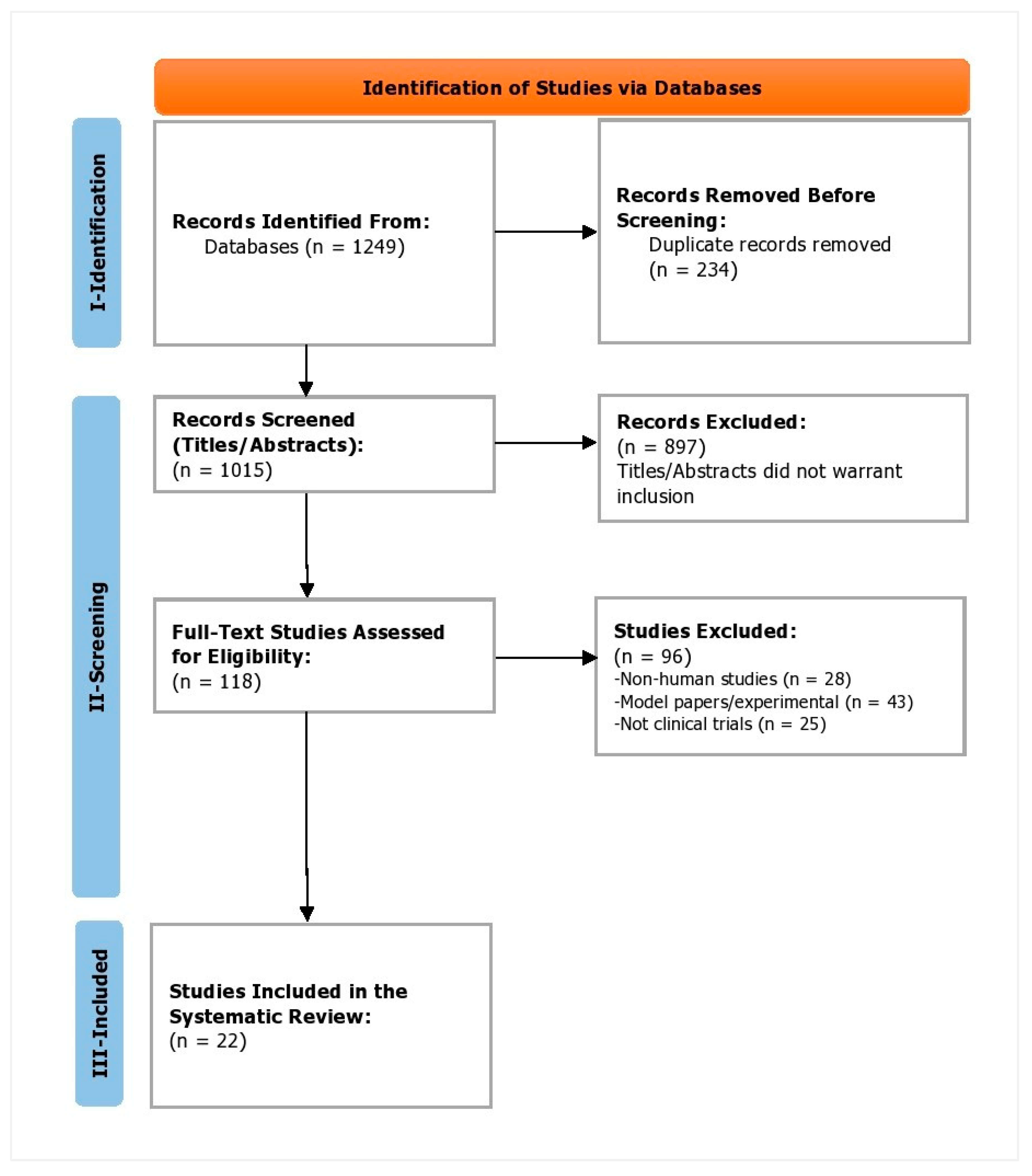
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction (Selection and Coding)
2.4. Risk of Bias (Quality Assessment)
3. Results
3.1. Bypassing the Blood–Brain Barrier
3.1.1. Intra-Arterial Therapy
3.1.2. Convection-Enhanced Delivery
3.2. Different Radiotherapy Regimens
3.2.1. Hypofractionated Radiation Therapy
3.2.2. Re-Irradiation
3.3. Non-Chemotherapeutic Agent Regimens
3.4. Chemotherapeutic Agent Regimens
3.5. Immunotherapy
3.6. Risk of Bias Synthesis
4. Discussion
4.1. Intra-Arterial Delivery
4.2. Convection-Enhanced Delivery
4.3. Radiotherapy
4.4. Other Regimens
4.5. Potential Molecular Targets
4.6. Strengths and Future Directions
4.7. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Linabery, A.M.; Ross, J.A. Trends in childhood cancer incidence in the US (1992–2004). Cancer Interdiscip. Int. J. Am. Cancer Soc. 2008, 112, 416–432. [Google Scholar]
- Ostrom, Q.T.; Cioffi, G.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2014–2018. Neuro Oncol. 2021, 23, iii1–iii105. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; de Blank, P.M.; Kruchko, C.; Petersen, C.M.; Liao, P.; Finlay, J.L.; Stearns, D.S.; Wolff, J.E.; Wolinsky, Y.; Letterio, J.J. Alex’s Lemonade Stand Foundation infant and childhood primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro Oncol. 2015, 16, x1–x36. [Google Scholar] [CrossRef]
- Li, G.; Mitra, S.S.; Monje, M.; Henrich, K.N.; Bangs, C.D.; Nitta, R.T.; Wong, A.J. Expression of epidermal growth factor variant III (EGFRvIII) in pediatric diffuse intrinsic pontine gliomas. J. Neurooncol. 2012, 108, 395–402. [Google Scholar] [CrossRef]
- Rashed, W.M.; Maher, E.; Adel, M.; Saber, O.; Zaghloul, M.S. Pediatric diffuse intrinsic pontine glioma: Where do we stand? Cancer Metastasis Rev. 2019, 38, 759–770. [Google Scholar] [CrossRef] [PubMed]
- Janssens, G.O.; Gandola, L.; Bolle, S.; Mandeville, H.; Ramos-Albiac, M.; van Beek, K.; Benghiat, H.; Hoeben, B.; La Madrid, A.M.; Kortmann, R.-D. Survival benefit for patients with diffuse intrinsic pontine glioma (DIPG) undergoing re-irradiation at first progression: A matched-cohort analysis on behalf of the SIOP-E-HGG/DIPG working group. Eur. J. Cancer 2017, 73, 38–47. [Google Scholar] [CrossRef]
- Wiese, M.; Hamdan, F.H.; Kubiak, K.; Diederichs, C.; Gielen, G.H.; Nussbaumer, G.; Carcaboso, A.M.; Hulleman, E.; Johnsen, S.A.; Kramm, C.M. Combined treatment with CBP and BET inhibitors reverses inadvertent activation of detrimental super enhancer programs in DIPG cells. Cell Death Dis. 2020, 11, 673. [Google Scholar] [CrossRef] [PubMed]
- Gallitto, M.; Lazarev, S.; Wasserman, I.; Stafford, J.M.; Wolden, S.L.; Terezakis, S.A.; Bindra, R.S.; Bakst, R.L. Role of radiation therapy in the management of diffuse intrinsic pontine glioma: A systematic review. Adv. Radiat. Oncol. 2019, 4, 520–531. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Reifenberger, G.; Von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef]
- El-Khouly, F.E.; Veldhuijzen van Zanten, S.E.M.; Santa-Maria Lopez, V.; Hendrikse, N.H.; Kaspers, G.J.L.; Loizos, G.; Sumerauer, D.; Nysom, K.; Pruunsild, K.; Pentikainen, V. Diagnostics and treatment of diffuse intrinsic pontine glioma: Where do we stand? J. Neurooncol. 2019, 145, 177–184. [Google Scholar] [CrossRef]
- Long, W.; Yi, Y.; Chen, S.; Cao, Q.; Zhao, W.; Liu, Q. Potential new therapies for pediatric diffuse intrinsic pontine glioma. Front. Pharmacol. 2017, 8, 495. [Google Scholar] [CrossRef]
- Srikanthan, D.; Taccone, M.S.; Van Ommeren, R.; Ishida, J.; Krumholtz, S.L.; Rutka, J.T. Diffuse intrinsic pontine glioma: Current insights and future directions. Chin. Neurosurg. J. 2021, 7, 6. [Google Scholar] [CrossRef]
- McCrea, H.J.; Ivanidze, J.; O’Connor, A.; Hersh, E.H.; Boockvar, J.A.; Gobin, Y.P.; Knopman, J.; Greenfield, J.P. Intraarterial delivery of bevacizumab and cetuximab utilizing blood-brain barrier disruption in children with high-grade glioma and diffuse intrinsic pontine glioma: Results of a phase I trial. J. Neurosurg. Pediatr. 2021, 28, 371–379. [Google Scholar] [CrossRef]
- Heiss, J.D.; Jamshidi, A.; Shah, S.; Martin, S.; Wolters, P.L.; Argersinger, D.P.; Warren, K.E.; Lonser, R.R. Phase I trial of convection-enhanced delivery of IL13-Pseudomonas toxin in children with diffuse intrinsic pontine glioma. J. Neurosurg. Pediatr. 2018, 23, 333–342. [Google Scholar] [CrossRef]
- Gállego Pérez-Larraya, J.; Garcia-Moure, M.; Labiano, S.; Patiño-García, A.; Dobbs, J.; Gonzalez-Huarriz, M.; Zalacain, M.; Marrodan, L.; Martinez-Velez, N.; Puigdelloses, M. Oncolytic DNX-2401 virus for pediatric diffuse intrinsic pontine glioma. N. Engl. J. Med. 2022, 386, 2471–2481. [Google Scholar] [CrossRef]
- Bander, E.D.; Ramos, A.D.; Wembacher-Schroeder, E.; Ivasyk, I.; Thomson, R.; Morgenstern, P.F.; Souweidane, M.M. Repeat convection-enhanced delivery for diffuse intrinsic pontine glioma. J. Neurosurg. Pediatr. 2020, 26, 661–666. [Google Scholar] [CrossRef]
- Majzner, R.G.; Ramakrishna, S.; Yeom, K.W.; Patel, S.; Chinnasamy, H.; Schultz, L.M.; Richards, R.M.; Jiang, L.; Barsan, V.; Mancusi, R. GD2-CAR T cell therapy for H3K27M-mutated diffuse midline gliomas. Nature 2022, 603, 934–941. [Google Scholar] [CrossRef]
- Zaghloul, M.S.; Nasr, A.; Tolba, M.; Refaat, A.; Youssef, A.; Mosaab, A.; Enayet, A.; Arafa, O.; Maher, E.; Eldebawy, E. Hypofractionated Radiation Therapy For Diffuse Intrinsic Pontine Glioma: A Noninferiority Randomized Study Including 253 Children. Int. J. Radiat. Oncol. Biol. Phys. 2022, 113, 360–368. [Google Scholar] [CrossRef]
- Izzuddeen, Y.; Gupta, S.; Haresh, K.P.; Sharma, D.; Giridhar, P.; Rath, G.K. Hypofractionated radiotherapy with temozolomide in diffuse intrinsic pontine gliomas: A randomized controlled trial. J. Neurooncol. 2020, 146, 91–95. [Google Scholar] [CrossRef]
- Amsbaugh, M.J.; Mahajan, A.; Thall, P.F.; McAleer, M.F.; Paulino, A.C.; Grosshans, D.; Khatua, S.; Ketonen, L.; Fontanilla, H.; McGovern, S.L. A phase 1/2 trial of reirradiation for diffuse intrinsic pontine glioma. Int. J. Radiat. Oncol. Biol. Phys. 2019, 104, 144–148. [Google Scholar] [CrossRef]
- Fleischhack, G.; Massimino, M.; Warmuth-Metz, M.; Khuhlaeva, E.; Janssen, G.; Graf, N.; Rutkowski, S.; Beilken, A.; Schmid, I.; Biassoni, V. Nimotuzumab and radiotherapy for treatment of newly diagnosed diffuse intrinsic pontine glioma (DIPG): A phase III clinical study. J. Neurooncol. 2019, 143, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Su, J.M.; Kilburn, L.B.; Mansur, D.B.; Krailo, M.; Buxton, A.; Adekunle, A.; Gajjar, A.; Adamson, P.C.; Weigel, B.; Fox, E. Phase I/II trial of vorinostat and radiation and maintenance vorinostat in children with diffuse intrinsic pontine glioma: A Children’s Oncology Group report. Neuro Oncol. 2022, 24, 655–664. [Google Scholar] [CrossRef]
- Mueller, S.; Cooney, T.; Yang, X.; Pal, S.; Ermoian, R.; Gajjar, A.; Liu, X.; Prem, K.; Minard, C.G.; Reid, J.M. Wee1 kinase inhibitor Adavosertib with radiation in newly diagnosed diffuse intrinsic pontine glioma: A Children’s Oncology Group phase 1 consortium study. Neuro-Oncol. Adv. 2022, 4, vdac073. [Google Scholar] [CrossRef] [PubMed]
- Su, J.M.; Murray, J.C.; McNall-Knapp, R.Y.; Bowers, D.C.; Shah, S.; Adesina, A.M.; Paulino, A.C.; Jo, E.; Mo, Q.; Baxter, P.A. A phase 2 study of valproic acid and radiation, followed by maintenance valproic acid and bevacizumab in children with newly diagnosed diffuse intrinsic pontine glioma or high-grade glioma. Pediatr. Blood Cancer 2020, 67, e28283. [Google Scholar] [CrossRef]
- Macy, M.E.; Kieran, M.W.; Chi, S.N.; Cohen, K.J.; MacDonald, T.J.; Smith, A.A.; Etzl, M.M.; Kuei, M.C.; Donson, A.M.; Gore, L. A pediatric trial of radiation/cetuximab followed by irinotecan/cetuximab in newly diagnosed diffuse pontine gliomas and high-grade astrocytomas: A Pediatric Oncology Experimental Therapeutics Investigators’ Consortium study. Pediatr. Blood Cancer 2017, 64, e26621. [Google Scholar] [CrossRef] [PubMed]
- El-Khouly, F.E.; Veldhuijzen van Zanten, S.E.M.; Jansen, M.H.A.; Bakker, D.P.; Sanchez Aliaga, E.; Hendrikse, N.H.; Vandertop, W.P.; van Vuurden, D.G.; Kaspers, G.J.L. A phase I/II study of bevacizumab, irinotecan and erlotinib in children with progressive diffuse intrinsic pontine glioma. J. Neurooncol. 2021, 153, 263–271. [Google Scholar] [CrossRef]
- DeWire, M.; Lazow, M.; Campagne, O.; Leach, J.; Fuller, C.; Senthil Kumar, S.; Stanek, J.; de Blank, P.; Hummel, T.R.; Pillay-Smiley, N. Phase I study of ribociclib and everolimus in children with newly diagnosed DIPG and high-grade glioma: A CONNECT pediatric neuro-oncology consortium report. Neuro-Oncol. Adv. 2022, 4, vdac055. [Google Scholar] [CrossRef] [PubMed]
- DeWire, M.; Fuller, C.; Hummel, T.R.; Chow, L.M.L.; Salloum, R.; de Blank, P.; Pater, L.; Lawson, S.; Zhu, X.; Dexheimer, P. A phase I/II study of ribociclib following radiation therapy in children with newly diagnosed diffuse intrinsic pontine glioma (DIPG). J. Neurooncol. 2020, 149, 511–522. [Google Scholar] [CrossRef]
- Baxter, P.A.; Su, J.M.; Onar-Thomas, A.; Billups, C.A.; Li, X.-N.; Poussaint, T.Y.; Smith, E.R.; Thompson, P.; Adesina, A.; Ansell, P. A phase I/II study of veliparib (ABT-888) with radiation and temozolomide in newly diagnosed diffuse pontine glioma: A Pediatric Brain Tumor Consortium study. Neuro Oncol. 2020, 22, 875–885. [Google Scholar] [CrossRef] [PubMed]
- Kilburn, L.B.; Kocak, M.; Baxter, P.; Poussaint, T.Y.; Paulino, A.C.; McIntyre, C.; Lemenuel-Diot, A.; Lopez-Diaz, C.; Kun, L.; Chintagumpala, M. A pediatric brain tumor consortium phase II trial of capecitabine rapidly disintegrating tablets with concomitant radiation therapy in children with newly diagnosed diffuse intrinsic pontine gliomas. Pediatr. Blood Cancer 2018, 65, e26832. [Google Scholar] [CrossRef] [PubMed]
- Veldhuijzen van Zanten, S.E.M.; El-Khouly, F.E.; Jansen, M.H.A.; Bakker, D.P.; Sanchez Aliaga, E.; Haasbeek, C.J.A.; Wolf, N.I.; Zwaan, C.M.; Vandertop, W.P.; van Vuurden, D.G. A phase I/II study of gemcitabine during radiotherapy in children with newly diagnosed diffuse intrinsic pontine glioma. J. Neurooncol. 2017, 135, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Manley, P.E.; Trippett, T.; Smith, A.A.; Macy, M.E.; Leary, S.E.S.; Boklan, J.; Cohen, K.J.; Goldman, S.; Kilburn, L.B.; Dhall, G. A phase 1/2 dose-finding, safety, and activity study of cabazitaxel in pediatric patients with refractory solid tumors including tumors of the central nervous system. Pediatr. Blood Cancer 2018, 65, e27217. [Google Scholar] [CrossRef]
- Fangusaro, J.; Cefalo, M.G.; Garré, M.L.; Marshall, L.V.; Massimino, M.; Benettaib, B.; Biserna, N.; Poon, J.; Quan, J.; Conlin, E. Phase 2 Study of Pomalidomide (CC-4047) Monotherapy for Children and Young Adults With Recurrent or Progressive Primary Brain Tumors. Front. Oncol. 2021, 11, 660892. [Google Scholar] [CrossRef] [PubMed]
- Schuelke, M.R.; Gundelach, J.H.; Coffey, M.; West, E.; Scott, K.; Johnson, D.R.; Samson, A.; Melcher, A.; Vile, R.G.; Bram, R.J. Phase I trial of sargramostim/pelareorep therapy in pediatric patients with recurrent or refractory high-grade brain tumors. Neuro-Oncol. Adv. 2022, 4, vdac085. [Google Scholar] [CrossRef] [PubMed]
- Tsvankin, V.; Hashizume, R.; Katagi, H.; Herndon, J.E.; Lascola, C.; Venkatraman, T.N.; Picard, D.; Burrus, B.; Becher, O.J.; Thompson, E.M. ABC transporter inhibition plus dexamethasone enhances the efficacy of convection enhanced delivery in H3. 3K27M mutant diffuse intrinsic pontine glioma. Neurosurgery 2020, 86, 742–751. [Google Scholar] [CrossRef] [PubMed]
- Hollingworth, M.; Zacharoulis, S. Infusion-related side-effects during convection enhanced delivery for brainstem-diffuse midline glioma/diffuse intrinsic pontine glioma. J. Neurooncol. 2022, 159, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Buczkowicz, P.; Hawkins, C. Pathology, molecular genetics, and epigenetics of diffuse intrinsic pontine glioma. Front. Oncol. 2015, 5, 147. [Google Scholar] [CrossRef]
- Sethi, R.; Allen, J.; Donahue, B.; Karajannis, M.; Gardner, S.; Wisoff, J.; Kunnakkat, S.; Mathew, J.; Zagzag, D.; Newman, K. Prospective neuraxis MRI surveillance reveals a high risk of leptomeningeal dissemination in diffuse intrinsic pontine glioma. J. Neurooncol. 2011, 102, 121–127. [Google Scholar] [CrossRef]
- Fowler, M.J.; Cotter, J.D.; Knight, B.E.; Sevick-Muraca, E.M.; Sandberg, D.I.; Sirianni, R.W. Intrathecal drug delivery in the era of nanomedicine. Adv. Drug Deliv. Rev. 2020, 165, 77–95. [Google Scholar] [CrossRef]
- Kline, C.; Liu, S.J.; Duriseti, S.; Banerjee, A.; Nicolaides, T.; Raber, S.; Gupta, N.; Haas-Kogan, D.; Braunstein, S.; Mueller, S. Reirradiation and PD-1 inhibition with nivolumab for the treatment of recurrent diffuse intrinsic pontine glioma: A single-institution experience. J. Neurooncol. 2018, 140, 629–638. [Google Scholar] [CrossRef]
- Cacciotti, C.; Liu, K.X.; Haas-Kogan, D.A.; Warren, K.E. Reirradiation practices for children with diffuse intrinsic pontine glioma. Neuro-Oncol. Pract. 2021, 8, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Puget, S.; Beccaria, K.; Blauwblomme, T.; Roujeau, T.; James, S.; Grill, J.; Zerah, M.; Varlet, P.; Sainte-Rose, C. Biopsy in a series of 130 pediatric diffuse intrinsic Pontine gliomas. Child’s Nerv. Syst. 2015, 31, 1773–1780. [Google Scholar] [CrossRef] [PubMed]
- Hamisch, C.; Kickingereder, P.; Fischer, M.; Simon, T.; Ruge, M.I. Update on the diagnostic value and safety of stereotactic biopsy for pediatric brainstem tumors: A systematic review and meta-analysis of 735 cases. J. Neurosurg. Pediatr. 2017, 20, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Somatic histone H3 alterations in pediatric diffuse intrinsic pontine gliomas and non-brainstem glioblastomas. Nat. Genet. 2012, 44, 251–253. [CrossRef]
- Mackay, A.; Burford, A.; Carvalho, D.; Izquierdo, E.; Fazal-Salom, J.; Taylor, K.R.; Bjerke, L.; Clarke, M.; Vinci, M.; Nandhabalan, M. Integrated molecular meta-analysis of 1000 pediatric high-grade and diffuse intrinsic pontine glioma. Cancer Cell 2017, 32, 520–537. [Google Scholar] [CrossRef] [PubMed]
- Schwartzentruber, J.; Korshunov, A.; Liu, X.-Y.; Jones, D.T.W.; Pfaff, E.; Jacob, K.; Sturm, D.; Fontebasso, A.M.; Quang, D.-A.K.; Tönjes, M. Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature 2012, 482, 226–231. [Google Scholar] [CrossRef]
- Jones, C.; Baker, S.J. Unique genetic and epigenetic mechanisms driving paediatric diffuse high-grade glioma. Nat. Rev. Cancer 2014, 14, 651–661. [Google Scholar] [CrossRef]
- Grill, J.; Puget, S.; Andreiuolo, F.; Philippe, C.; MacConaill, L.; Kieran, M.W. Critical oncogenic mutations in newly diagnosed pediatric diffuse intrinsic pontine glioma. Pediatr. Blood Cancer 2012, 58, 489–491. [Google Scholar] [CrossRef]
- Puget, S.; Philippe, C.; Bax, D.A.; Job, B.; Varlet, P.; Junier, M.-P.; Andreiuolo, F.; Carvalho, D.; Reis, R.; Guerrini-Rousseau, L. Mesenchymal transition and PDGFRA amplification/mutation are key distinct oncogenic events in pediatric diffuse intrinsic pontine gliomas. PLoS ONE 2012, 7, e30313. [Google Scholar] [CrossRef]
- Buczkowicz, P.; Hoeman, C.; Rakopoulos, P.; Pajovic, S.; Letourneau, L.; Dzamba, M.; Morrison, A.; Lewis, P.; Bouffet, E.; Bartels, U. Genomic analysis of diffuse intrinsic pontine gliomas identifies three molecular subgroups and recurrent activating ACVR1 mutations. Nat. Genet. 2014, 46, 451–456. [Google Scholar] [CrossRef]
- Paugh, B.S.; Zhu, X.; Qu, C.; Endersby, R.; Diaz, A.K.; Zhang, J.; Bax, D.A.; Carvalho, D.; Reis, R.M.; Onar-Thomas, A. Novel Oncogenic PDGFRA Mutations in Pediatric High-Grade GliomasPDGFRA Mutations in Gliomagenesis. Cancer Res. 2013, 73, 6219–6229. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, L.M.; DeWire, M.; Ryall, S.; Buczkowicz, P.; Leach, J.; Miles, L.; Ramani, A.; Brudno, M.; Kumar, S.S.; Drissi, R. Spatial genomic heterogeneity in diffuse intrinsic pontine and midline high-grade glioma: Implications for diagnostic biopsy and targeted therapeutics. Acta Neuropathol. Commun. 2016, 4, 1–8. [Google Scholar] [CrossRef] [PubMed]

| Author | Year | Title | Journal | Country | Study Design | Inclusion Criteria | Intervention Given | Method of Administration |
| Blood-brain Barrier Bypass | ||||||||
| McCrea | 2021 [13] | Intraarterial delivery of bevacizumab and cetuximab utilizing blood-brain barrier disruption in children with high-grade glioma and diffuse intrinsic pontine glioma: results of a phase I trial | Journal of Neurosurgery | USA | Phase I trial (NCT01884740) | Patients aged <22 years with a histological diagnosis of relapsed or refractory HCG or radiological diagnosis of DIPG | Single intraarterial dose of 15 mg/kg bevacizumab and 200 mg/m2 cetuximab after BBBD with mannitol | Superselective intraarterial cerebral infusion |
| Heiss | 2018 [14] | Phase I trial of convection-enhanced delivery of IL13-Pseudomonas toxin in children with diffuse intrinsic pontine glioma | Journal of NeuroSurgery: Pediatrics | USA | Phase I dose-escalation trial (NCT00088061) | Patients aged <18 years with clinical and radiological evidence of progressive DIPG, >2 weeks after their last chemotherapy dose or neurosurgical procedure, and >4 weeks from the last dose of radiation | IL13-PE38QQR and surrogate marker of IL13-PE38QQR distribution, Gd-DTPA co-infused, initial concentration of 0.125 μg/mL followed by 0.25 μg/mL IL13-PE38QQR | Intratumoral CED |
| Pérez-Larraya | 2022 [15] | Oncolytic DNX-2401 Virus for Pediatric Diffuse Intrinsic Pontine Glioma | The New England Journal of Medicine | Spain | Phase 1 trial (NCT03178032) | Patients who were diagnosed with treatment-naive DIPG and aged 3–18 years | Single infusion of oncolytic adenovirus DNX-2401 (1 × 1010 or 5 × 1010 viral particles) followed by radiotherapy | CED, virus infused through a catheter placed in the cerebellar peduncle |
| Bander | 2020 [16] | Repeat convection-enhanced delivery for diffuse intrinsic pontine glioma | Journal of Neurosurgery | USA | Phase I trial (NCT01502917) | Patients who were radiographically diagnosed with DIPG and previously treated with external-beam radiation therapy; showed no dose-limiting toxicity or disease progression in the 30-day observation window after first round of CED | ≥2 infusions of I-8H9 monoclonal antibody (124I-omburtamab, Y-mAbs Therapeutics) via CED | CED through supratentorial trajectory with intraprocedural MRI-guided stereotactic placement |
| Majzner | 2022 [17] | GD2-CAR T cell therapy for H3K27M-mutated diffuse midline gliomas | Nature | USA | Phase I dose-escalation trial (NCT04196413) | Patients who had H3K27M-mutated DIPG at any stage and aged 5–25 years | GD2-CAR T cells at dose level 1 (1 × 106 GD2-CAR T cells per kg administered intravenously) and subsequent GD2-CAR T-cell infusions administered intracerebroventricularly if there was a clinical benefit with first dose | Intravenously followed by intracerebroventricularly |
| Author | Year | Title | Journal | Country | Study Design | Inclusion Criteria | Intervention Given | Method of Administration |
| Different Radiotherapy Regimens | ||||||||
| Zaghloul | 2022 [18] | Hypofractionated Radiation Therapy For Diffuse Intrinsic Pontine Glioma: A Noninferiority Randomized Study Including 253 Children | International Journal of Radiation Oncology | Egypt | Randomized clinical trial | Patients with diagnosed DIPG | HF radiotherapy regimens: HF1, receiving 39 Gy in 13 fractions; HF2, receiving 45 Gy in 15 fractions; and CF, receiving 54 Gy in 30 fractions | External beam radiotherapy |
| Izzuddeen | 2019 [19] | Hypofractionated radiotherapy with temozolomide in diffuse intrinsic pontine gliomas: a randomized controlled trial | Journal of Neuro-Oncology | India | Phase II randomized trial | Patients aged 3 to 40 years with newly diagnosed patients with DIPG, confirmed radiologically on MRI with involvement of more than half of pons and basilar artery | Arm A received conventional fractionated RT of 60 Gy in 30 fractions over 6 weeks while patients in arm B received hypo-fractionated radiotherapy of 39 Gy in 13 fractions over 2.6 weeks along with concurrent TMZ 75 mg/m2 from day 1 to day 17 followed by adjuvant TMZ for six cycles. | Orally and external beam radiotherapy |
| Amsbaugh | 2019 [20] | A Phase 1/2 Trial of Reirradiation for Diffuse Intrinsic Pontine Glioma | International Journal of Radiation Oncology | India | Phase I/II trial | Patients with radiologically confirmed DIPG by MRI who received radiation therapy at least 10 months previously with progressive disease confirmed clinically and radiologically | Re-irradiation at three doses, dose level 1: 24 Gy in 12 fractions, dose level 2: 26.4 Gy in 12 fractions, dose level 3: 30.8 Gy in 14 fractions | External beam radiotherapy |
| Author | Year | Title | Journal | Country | Study Design | Inclusion Criteria | Intervention Given | Method of Administration |
| Non-Chemotherapeutic Agent Regimens | ||||||||
| Fleischhack | 2019 [21] | Nimotuzumab and radiotherapy for treatment of newly diagnosed diffuse intrinsic pontine glioma (DIPG): a phase III clinical study | Journal of Neuro-Oncology | Germany, Italy, Russia | Phase III trial | Patients between 3 and 20 years with clinically and radiologically confirmed DIPG, diagnosed in the last 3 months | Nimotuzumab was given intravenously at 150 mg/m2 weekly for 12 weeks and radiotherapy at a total dose of 54 Gy between week 3 and week 9 | Intravenously and external beam radiotherapy |
| Su | 2022 [22] | Phase I/II trial of vorinostat and radiation and maintenance vorinostat in children with diffuse intrinsic pontine glioma: A Children’s Oncology Group report | Neuro-Oncology | USA | Phase I/II trial | Children aged 3 to 21 years with newly diagnosed DIPG, radiographically defined as tumors with a pontine epicenter and diffuse involvement of at least two-thirds of the pons; if aforementioned criteria were not met then tumors were biopsied, children who had anaplastic astrocytoma, glioblastoma, gliosarcoma, or anaplastic mixed glioma were included | Vorinostat once daily from Monday till Friday, during radiation therapy (54 Gy in 30 fractions), then 230 mg/m2 daily for a maximum of twelve 28-day cycles | Orally and external beam radiotherapy |
| Mueller | 2022 [23] | Wee1 kinase inhibitor adavosertib with radiation in newly diagnosed diffuse intrinsic pontine glioma: A Children’s Oncology Group phase I consortium study | Neuro-Oncology Advances | USA | Phase I trial (NCT01922076) | Patients aged 3–21 years with newly diagnosed DIPG | On days of cranial radiation therapy, 7 adavosertib DLs (50 mg/m2 alternating weeks, 50 mg/m2 alternating with weeks of every other day, 50 mg/m2, then 95, 130, 160, 200 mg/m2) | Orally |
| Su | 2020 [24] | A phase 2 study of valproic acid and radiation, followed by maintenance valproic acid and bevacizumab in children with newly diagnosed diffuse intrinsic pontine glioma or high-grade glioma | Pediatric Blood & Cancer | USA | Phase II trial (NCT00879437) | Patients between 3 and 21 years with newly diagnosed radiologically confirmed DIPG | Radiation therapy and VPA (15 mg/kg/day and dose adjustment to maintain a trough range of 85 to 115 μg/mL), VPA continued post-radiation, bevacizumab (10 mg/kg) intravenously biweekly, four weeks after completing radiation therapy | Intravenously and external beam radiotherapy |
| Author | Year | Title | Journal | Country | Study Design | Inclusion Criteria | Intervention Given | Method of Administration |
| Chemotherapeutic Agent Regimens | ||||||||
| Macy | 2017 [25] | A pediatric trial of radiation/cetuximab followed by irinotecan/cetuximab in newly diagnosed diffuse pontine gliomas and high-grade astrocytomas: A Pediatric Oncology Experimental Therapeutics Investigators’ Consortium study | Pediatric Blood & Cancer | USA | Phase II trial | Patients 3 to 21 years of age with newly diagnosed DIPG confirmed clinically and radiologically (MRI) | EBT (5940 cGy in 33 fractions (180 cGy)) with concomitant cetuximab (250 mg/m2 IV weekly for six doses) with a recovery period of 26–52 days and followed by irinotecan (16 mg/m2/day over one hour for five days, given two consecutive weeks) and cetuximab once weekly (250 mg/m2/dose IV in 21-day cycles) | Intravenously and external beam radiotherapy |
| El-Khouly | 2021 [26] | A phase I/II study of bevacizumab, irinotecan, and erlotinib in children with progressive diffuse intrinsic pontine glioma | Journal of Neuro-Oncology | The Netherlands | Phase I/II trial (EudraCT 2009-016080-11, Dutch Trial Register NTR2391) | Patients aged 3–18 years with progressive DIPG (clinical or radiological) after initial radiotherapy | Biweekly bevacizumab (10 mg/kg) and irinotecan (125 mg/m2) combined with daily erlotinib, dose increased for erlotinib for 2 cohorts (65 and 85 mg/m2) following a 3 + 3 dose-escalation schedule, until disease progression for a maximum of one year | Through central venous catheter and intravenously |
| DeWire | 2022 [27] | Phase I study of ribociclib and everolimus in children with newly diagnosed DIPG and high-grade glioma: A CONNECT pediatric neuro-oncology consortium report | Neuro-Oncology Advances | USA | Phase I trial (NCT02607124) | Patients with newly diagnosed DIPG or HIG who initiated radiotherapy within 30 days of radiographic diagnosis or definitive surgery (whichever was later) | Ribociclib 170 mg/m2 daily for 21 days and everolimus 1.5 mg/m2 daily for 28 days | Orally, via g-tube, or nasogastric tube |
| DeWire | 2020 [28] | A phase I/II study of ribociclib following radiation therapy in children with newly diagnosed diffuse intrinsic pontine glioma (DIPG) | Journal of Neuro-Oncology | USA | Phase I/II trial (NCT02607124) | Patients aged 1–30 years with newly-diagnosed DIPG confirmed radiologically or histologically, within 30 days of radiographic diagnosis or definitive surgery | 350 mg/m2 ribociclib daily for 21 days/7 days of every 28 days for up to 12 courses, 2–4 weeks after radiotherapy | Orally or via nasogastric/gastric tube |
| Baxter | 2020 [29] | A phase I/II study of veliparib (ABT-888) with radiation and temozolomide in newly diagnosed diffuse pontine glioma: a Pediatric Brain Tumor Consortium study | Neuro-Oncology | USA | Phase I/II trial (NCT01514201) | Children aged 21 years or younger with newly diagnosed DIPG, defined as tumors with a pontine epicenter and diffuse intrinsic involvement of the pons | Veliparib was given Monday through Friday (50 mg/m2/dose twice daily with 2 planned dose escalations, 65 and 85 mg/m2/dose twice daily and 1 planned de-escalation (35 mg/m2/dose twice daily) during radiation (5400 cGy in 30 fractions over 6 weeks) and a 4-week gap, followed by veliparib at 25 mg/m2 b.i.d. and TMZ 135 mg/m2 daily for 5 days every 28 days | Orally |
| Kilburn | 2018 [30] | A pediatric brain tumor consortium phase II trial of capecitabine rapidly disintegrating tablets with concomitant radiation therapy in children with newly diagnosed diffuse intrinsic pontine gliomas | Pediatric Blood & Cancer | USA | Phase II trial | Children aged 3 to 17 years with newly diagnosed DIPG | Capecitabine, 650 mg/m2/dose BID (MTD in children with concurrent radiation) was administered for 9 weeks starting the first day of RT. Following a 2-week break, 3 courses of capecitabine, 1250 mg/m2/dose BID for 14 days followed by a 7-day rest, were administered | Orally and external beam radiation |
| Zanten | 2017 [31] | A phase I/II study of gemcitabine during radiotherapy in children with newly diagnosed diffuse intrinsic pontine glioma | Journal of Neuro-Oncology | The Netherlands | Phase I/II trial | Patients with newly-diagnosed DIPG | Gemcitabine (weekly dose for 6 weeks, increasing doses of 140, 175, and 200 mg/m2 gemcitabine, respectively, following a 3 + 3 dose-escalation schedule) concomitant to 6 weeks of hyper fractionated radiotherapy | Intravenously and external beam radiation |
| Manley | 2018 [32] | A phase 1/2 dose-finding, safety, and activity study of cabazitaxel in pediatric patients with refractory solid tumors including tumors of the central nervous system | Pediatric Blood & Cancer | USA | Phase I/II dose-escalating trial (NCT01751308) | Patients aged 2–18 years old with progressive DIPG and recovered from any acute toxic effects of all prior therapy to grade ≤1 before enrollment | Cabazitaxel infused over 1 h (20 mg/m2 initially and if tolerated, escalated to 25, 30, 35, and 40 mg/m2) on day 1 of every 21-day cycle | Intravenously |
| Author | Year | Title | Journal | Country | Study design | Inclusion criteria | Intervention given | Method of administration |
| Immunotherapy | ||||||||
| Fangusaro | 2021 [33] | Phase 2 Study of Pomalidomide (CC-4047) Monotherapy for Children and Young Adults With Recurrent or Progressive Primary Brain Tumors | Frontiers in Oncology | USA, France, Italy, Spain, UK | Phase II trial (NCT03257631) | Patients aged 1 to <21 years with a diagnosis of recurrent or progressive DIPG, must have received ≥1 prior standard therapy | Pomalidomide (2.6 mg/m2/day once daily) on days 1–21 of each 28-day treatment cycle, followed by a 7-day rest period for up to 24 cycles | Orally |
| Schuelke | 2022 [34] | Phase I trial of sargramostim/pelareorep therapy in pediatric patients with recurrent or refractory high-grade brain tumors | Neuro-Oncology Advances | USA | Phase I trial (NCT02444546) | Patients aged 10 to 21 years with progressive high-grade DPIG and life expectancy >3 months | Sargramostim (subcutaneously at 250 mcg/m2) for 2 days followed by 3 days of pelareorep (IV over 60 min) | Intravenously and subcutaneously |
| Author | Number of Patients with DIPG | Age at Diagnosis (Years) | Sex (% Male) | Previous Treatment | Outcome Measures |
| Blood–Brain Barrier Bypass | |||||
| McCrea | 10 patients | 5.5 years (5–7) | 5 patients (50%) | All patients had received standard radiotherapy, 6 patients (60%) had received immunotherapy, convection-enhanced delivery, MK-1775/Wee1 inhibitor, and oral panobinostat | Clinical response, safety, objective response (T1-weighted pre- and postcontrast sequences and T2-weighted FLAIR sequences) |
| Heiss | 5 patients | Mean: 13 years (SD: 5) | 3 patients (60%) | Standard radiotherapy | Clinical response, radiological response, corticosteroid dose, QoL |
| Pérez-Larraya | 12 patients | NR | NR | None | Safety, overall survival, quality of life, objective response, tumor biopsy and peripheral-blood samples for correlative studies of the molecular features of DIPG and antitumor immune responses |
| Bander | 7 patients | Mean: 5.4 years | 4 patients (57.14%) | All patients received external-beam RT | Postinfusion deficits, distribution volume, targeting accuracy |
| Majzner | 3 patients | Mean: 13.3 years | 1 patient (33.3%) | All patients received standard radiotherapy ≥ 6 months before enrollment | Safety, clinical improvement, radiological improvement |
| Author | Number of Patients with DIPG | Age at Diagnosis (Years) | Sex (% Male) | Previous Treatment | Outcome Measures |
| Different Radiotherapy Regimens | |||||
| Zaghloul | 253 patients | NR | NR | NR | OS, PFS |
| Izzuddeen | 33 patients (conventional treatment arm: n = 16, experimental arm: n = 17) | <7 years: conventional arm: 9 patients (52%), experimental arm: 9 patients (50%); 8–18 years: conventional arm: 5 patients (29%), experimental arm: 5 patients (27%); >18 years: conventional arm: 3 patients (17%), experimental arm: 4 patients (22%) | Conventional arm: 8 patients (47%), treatment arm: 7 patients (38%) | None | Toxicities, PFS, OS |
| Amsbaugh | 12 patients (group 1: n = 6, group 2: n = 4, group 3: n = 2) | Group 1: 5.5 years (4–20), group 2: 10 years (5–26) group 3: 6 years (5–7) | 7 patients (58.3%) | Radiotherapy (at least 10 months before) | Toxicities, OS, PFS, clinical improvement, radiological response, QoL |
| Author | Number of Patients with DIPG | Age at Diagnosis (Years) | Sex (% Male) | Previous Treatment | Outcome Measures |
| Non-Chemotherapeutic Agent Regimens | |||||
| Su | 61 patients | 7.1 years (3.3–19.4) | 32 patients (45.7%) | None | Toxicities, EFS, OS |
| Fleischhack | 42 patients | Median: 7.4 years (3–15) | 16 patients (38.1%) | None | PFS, clinical response, radiological response (RECIST criteria), adverse events |
| Mueller | 46 patients | Median: 6 years (3–21) | 22 patients (48%) | None (except surgery) | Tolerability, pharmacokinetics, OS, radiological response, peripheral blood γH2AX levels |
| Su | 20 patients | Median: 7.69 years (5.2–9.9) | 10 patients (50%) | May have received surgery and/or corticosteroids | Safety, radiological response (WHO bidimensional criteria), EFS, OS |
| Author | Number of Patients with DIPG | Age at Diagnosis (Years) | Sex (% Male) | Previous Treatment | Outcome Measures |
| Chemotherapeutic Agent Regimens | |||||
| Macy | 25 patients | Median: 8 years (3–19) | 21 patients (47%) | None (except surgery) | Tolerability, OS, PFS, TTP |
| El-Khouly | 9 patients | Mean: 9.39 years | 5 patients (55.5%) | Radiotherapy (all 9 patients, 100%) combined with gemcitabine (4 patients, 44.4%) in the previous phase I of the trial, re-irradiation during the current trial (4 patients, 44.4%) | Safety (DLTs), sPFS, OS, radiological response (MRI images scored with the modified RANO-criteria), QoL |
| DeWire | 15 patients | Median: 6.5 years (2–15) | 5 patients (26%) | Patients were eligible if they received 10% of standard dose of radiotherapy (54 Gy across 1.8 Gy daily fractions over 6 weeks to the planning target volume) | Toxicities, OS, radiological response (MRI based on RANO criteria) |
| DeWire | 9 patients | 7.3 years (5–14.7) | 4 patients (40%) | Patients were eligible if they received 10% of standard dose of radiotherapy (54 Gy across 1.8 Gy daily fractions over 6 weeks to the planning target volume) | Safety, OS, feasibility |
| Baxter | 65 patients | 6.6 years (2.2–15.8) | 40 (61.5%) | None | OS, radiological response, DLT |
| Kilburn | 44 patients | 7.2 (3.4–16.2) | 22 (50%) | None (except surgery and corticosteroid therapy) | PFS, safety |
| Zanten | 9 patients | 10.8 years (7.5–17.3) | 5 patients (55.5%) | None | DLTs, radiological response (based on modified RANO criteria), PFS, OS, QoL |
| Manley | 12 patients | Phase 1: 9.0 years (4–18), phase 2: 9.5 years (3–16) | Phase 1: 15 patients (65%), phase 2: 8 patients (50%) | Systemic anticancer therapy within ≤3 weeks, investigational agents, or small field radiotherapy ≤4 weeks, craniospinal radiation therapy ≤6 months | Radiological response (as per the modified RANO criteria), PFS, DLTs |
| Author | Number of Patients with DIPG | Age at Diagnosis (Years) | Sex (% Male) | Previous Treatment | Outcome Measures |
| Immunotherapy | |||||
| Fangusaro | 9 patients | 7.0 (4–12) | 7 (63.6%) | Radiation (11 patients, 100%), surgery (5 patients, 55.55%), systemic therapy (7 patients, 63.6%) | OR, LTSD, OS, PFS, safety |
| Schuelke | 2 patients | 10 and 17 years | 0 | Radiation (2 patients, 100%), chemotherapy (2 patients, 100%) | DLTs, radiological response, OS |
| Author | Median OS (Months) | Median EFS/PFS (Months) | Radiological Response (%) | Clinical Improvement (n, %) | Tolerance and Safety | Steroid Use Discontinuation | Concluding Remarks |
| Blood–Brain Barrier Bypass | |||||||
| McCrea | 17.3 months (221–761 days) | NR | T1-weighted postcontrast sequence: progressive disease (4 patients, 40%), stable disease (2 patients, 20%), partial response (2 patients, 20%), complete response (1 patient, 10%); T2-weighted FLAIR imaging: progressive disease (5 patients, 50%), stable disease (5 patients, 50%) | 6 patients (60%) | Well-tolerated; 4 (40%) patients had minor adverse events (grade I epistaxis in 2 patients and grade I rash in 2 patients) | 2 patients (20%) | Intraarterial therapy of bevacizumab and cetuximab is well-tolerated in children with DIPG and warrants further investigation |
| Heiss | NR | NR | Disease progression: 3 patients (60%) | 1 patient (20%) | Elevated serum creatine kinase (2 patients, 40%), renal calculi (1 patient, 20%), somnolence (1 patient, 20%), suspected aspiration prompting hospitalization (1 patient, 20%) | 4 patients (80%) | Direct brainstem infusion of IL13-PE using CED temporarily arrested disease progression in 2 out of 5 patients and adverse events were due to infusion-related brainstem edema with no signs of toxicity noted |
| Pérez-Larraya | 17.8 months (5.9–33.5) | NR | MRI: complete response (9 patients, 75%) partial response (3 patients, 25%), stable disease (8 patients, 66.7%) | NR | Grade 1 and 2 events: headache, nausea, vomiting, fatigue, hemiparesis (1 patient, 8.3%), tetraparesis (1 patient, 8.3%) | NR | Intratumoral infusion of oncolytic virus DNX-2401 followed by radiotherapy in pediatric patients with DIPG has molecular changes including T-cell activity and a reduction in or stabilization of tumor size but there may be adverse events |
| Bander | NR | NR | NR | NR | Grade 1 and 2 events: contralateral hemiparesis (5 patients, 71.4%), nystagmus (2 patients, 28.6%), dysmetria (1 patient, 14.3%), cranial nerve VI and/or VII palsy (4 patients, 51.1%) | NR | Repeated CED in the brainstem for children with DIPG is safe |
| Majzner | Patient 1: 13 months, Patient 2: 20 months, Patient 3: 26 months | NR | MRI: 20% enlargement (1 patient, 33.3%), improved T2/FLAIR signal (2 patients, 66.7%), 17% smaller tumor volume (1 patient, 33.3%) | 2 patients (66.7%) | CAR T cell-mediated inflammation at the local tumor site, termed TIAN in all 3 patients (100%) | 3 patients (100%) | Toxicity management algorithm for TIAN offers potentially safe delivery of targeted CAR T-cell therapy locally |
| Author | Median OS (Months) | Median EFS/PFS (Months) | Radiological Response (%) | Clinical Improvement (n, %) | Tolerance and Safety | Steroid Use Discontinuation | Concluding Remarks |
| Different Radiotherapy Regimens | |||||||
| Zaghloul | HF1: 9.6 months, HF2: 8.2 months, CF: 8.7 months | NR | NR | NR | Well-tolerated | NR | Hypofractioned radiation therapy is non-inferior to conventional fractionation with younger age (2–5 years) showing superiority with HF1 (low hypofractionated therapy dose of 39 Gy in 13 fractions) |
| Izzuddeen | Conventional arm: 11 months (95% CI: 7.5–14.5), experimental arm: 12 months (95% CI: 10.5–13.5) | PFS: conventional arm—7 months (95% CI: 3.6–10.3), experimental arm—8 months (95% CI: 6.7–9.3) | NR | NR | 5 patients (28%) in the experimental arm developed ≥ grade 3 hematological toxicity; 1 patient (7%) developed ≥ grade 3 toxicity | NR | Hypofractionated radiotherapy with concurrent and adjuvant temozolomide did not improve survival rates and has higher hematological toxicity |
| Amsbaugh | 19.5 months (95% CI: 15.6–21.1) | PFS: 4.5 months | Improvement: 8 patients (66.7%) | 11 (91.7%) | Dose level 3: ≥Grade 3 events: hypoxia and dysphagia (1 patient, 50%) | NR | Re-irradiation was safe and had improved survival outcomes among patients with progressive DIPG |
| Author | Median OS (Months) | Median EFS/PFS (Months) | Radiological Response (%) | Clinical Improvement (n, %) | Tolerance and Safety | Steroid Use Discontinuation | Concluding Remarks |
| Non-Chemotherapeutic Agent Regimens | |||||||
| Su | 1-year OS: 39.2% (95% CI: 27.8–50.5%). | 1-year EFS: 5.85% (95% CI 1.89–13.1%) | NR | NR | 42 patients (60%) had at least 1 DLT | NR | Vorinostat given together with radiation and afterward was well-tolerated but did not improve survival outcomes |
| Fleischhack | 9.4 months | PFS: 5.8 months | ORR: 4.2%, Partial response (2 patients, 4.8%), stable disease (27 patients, 64.3%), progressive disease (10 patients, 23.8%) | NR | Alopecia (6 patients, 14.3%), vomiting (3 patients, 7.1%), headache (3 patients, 7.1%) radiation skin injury (3 patients, 7.1%), intra-tumoral bleeding (1 patient, 2.4%), acute respiratory failure (1 patient, 2.4%) | NR | Nimotuzumab combined with RT is well-tolerated and has comparable efficacy with RT and intensive chemotherapy without requiring prolonged hospitalization among children with newly diagnosed DIPG |
| Mueller | 11.8 months (9–13.9) | NR | Stable disease: 33 patients (80.5%), progressive disease: 8 patients (19.5%) | NR | ≥Grade 3 events: ALT elevation (1 patient, 6.7%), neutropenia (1 patient, 6.7%) | NR | Adavosertib in combination with CRT is well tolerated in children with newly diagnosed DIPG, however, compared to historical controls, did not improve OS. These results can inform future trial designs in children with high-risk cancer. |
| Su | 10.3 (7.4–13.4) months | EFS: 7.8 (95% CI 5.6–8.2) | Partial response (8 patients, 40%), minor response (9 patients, 45%), stable disease (1 patient, 5%), not available (2 patients, 10%) | NR | ≥Grade 3 events with VPA and RT: thrombocytopenia (3 patients), somnolence (1 patient), fatigue (3 patients), weight gain (2 patients); ≥grade 3 events with VPA and bevacizumab maintenance: thrombocytopenia (3 patients), intracranial/intratumoral hemorrhage (1 patient), hypertension (4 patients), subacute bone infarction (1 patient), fatigue (3 patients), weight gain (2 patients) | NR | VPA and bevacizumab given in combination with radiation is well-tolerated but there is no improvement in EFS or OS among children with newly diagnosed DIPG |
| Author | Median OS (Months) | Median EFS/PFS (Months) | Radiological Response (%) | Clinical Improvement (n, %) | Tolerance and Safety | Steroid Use Discontinuation | Concluding Remarks |
| Chemotherapeutic Agent Regimens | |||||||
| Macy | 12.1 months (95% CI: 9.93, 18) | PFS: 7.12 months (95% CI: 6.89, 12.5) | Stable disease: 6 patients (24%) | NR | ≥Grade 3 events: lymphopenia (26 patients, 57.7%), hypokalemia (18 patients, 40%), neutropenia (6 patients, 13.3%), anorexia (9 patients, 20%) | NR | Combination of EGFR inhibitor to radiation and irinotecan is a treatment regimen that may improve progression-free survival and is well-tolerated but did not improve survival rates |
| El-Khouly | Overall: 13.8 months (9.3–33.0), patients who were re-irradiated: 16.2 months (12.8–20.0) | PFS: 7.3 months (3.5–10.0), sPFS: 3.2 months (1.0–10.9) | 3 months: Partial response (3 patients, 33.3%), stable disease (1 patient, 11.1%), progressive disease (5 patients, 55.5%); 6 months: progressive disease (2 patients, 50%). stable disease (2 patients, 50%) | 4 patients (44.4%) | Grade III acute diarrhea (1 patient, 11/1%), grade II acute secretory diarrhea (1 patient, 11.1%). grade I/II late-onset diarrhea (5 patients, 55.5%), grade I/II nausea and vomiting (4 patients, 44.4%), grade I acneiform rash (5 patients, 55.5%), grade I/II mucositis (1 patient, 11.1%), grade I/II constipation (1 patient, 11.1%), grade II keratitis (1 patient, 11.1%), grade II urinary tract infection (2 patients, 22.2%), and grade II adrenal insufficiency as a result of chronic dexamethasone use (2 patients, 22.2%) | NR | Daily erlotinib (up to 85 mg/m2) with biweekly bevacizumab and irinotecan is safe and improves median OS in children with progressive DIPG |
| DeWire | 13.9 months | NR | Pseudoprogression: 4 patients (8.3%), progression: 2 patients (4.2%) | NR | ≥Grade 3 events: neutropenia (6 patients, 33%), leucopenia (3 patients, 17%), lymphopenia (2 patients, 11%), pulmonary infection (1 patient, 6%), elevated ALT and hypokalemia (1 patient, 6%), cardiac toxicity (1 patient, 6%) | NR | Ribociclib and everolimus following radiotherapy in children with newly diagnosed DIPG is well-tolerated and requires further exploration for efficacy potential |
| DeWire | 16.1 months (10–30) | NR | Disease progression: 9 patients (90%) | NR | ≥grade 3 events: neutropenia (9 patients, 90%), lymphopenia (5 patients, 50%), and leukopenia (7 patients, 70%) | NR | Ribociclib administered following radiotherapy has survival benefits but increased tumor necrosis may be a treatment effect represent a treatment effect |
| Baxter | One-year OS: 37.2% (SE 7%) | NR | PR: 7 patients (14%) | NR | DLTs: 4 patients (33.3%)during radiation in phase I (Grade 2 intratumoral hemorrhage (n = 1), grade 3 maculopapular rash (n = 2), and grade 3 nervous system disorder (generalized neurologic deterioration) (n = 1)), 4 patients (50%) during intrapatient dose escalation | NR | Veliparib used in combination with radiation followed by TMZ and veliparib was well-tolerated and did not improve survival rates in patients with newly diagnosed DIPG |
| Kilburn | NR | 6-month PFS: 33.7% (SE = 7.1%), 1-year PFS: 7.2% (SE = 3.5%) | Progressive disease (8 patients, 18.2%) | Deterioration (3 patients, 6.8%) | DLTs: 5 patients (grade 4 neutropenia (n = 1), grade 2 CNS necrosis (n = 2), grade 4 neutropenia that did not resolve within 7 days (n = 1), and persistent toe infection (n = 1) | NR | Concomitant and adjuvant Capecitabine with radiotherapy was well-tolerated but did not improve survival outcomes for children with newly-diagnosed DIPG |
| Zanten | Intermediate risk: 12.4 months, high risk: 8.1 months | PFS for intermediate risk: 6.4 months, PFS for high risk: 4.5 months | Stable disease: 2 patients, progressive disease: 3 patients, pseudoprogression: 4 patients | 9 patients (100%) | Grade 3 hepatotoxicity (2 patients, 22.2%), grade 3 neutropenia (1 patient, 11.1%) | 3 patients (75%) | Gemcitabine in combination with radiotherapy is well-tolerated but does not improve survival outcomes in patients with newly-diagnosed DIPG |
| Manley | 2.7 months (95% CI: 1.7–4.5) | Median PFS: 1.3 months (95% CI: 0.6–2.1) | Progressive disease 25 patients (75.8%), stable disease: 6 patients (18.2%), partial response: 1 patient (3%), complete response: 1 patient (3%) | NR | ≥grade 3 events: 12 patients (52%) | Steroid treatment used as part of protocol | Cabazitaxel in pediatric patients with progressive DIPG does not improve survival outcomes but is well-tolerated and safe at the established MTD |
| Author | Median OS (Months) | Median EFS/PFS (Months) | Radiological Response (%) | Clinical Improvement (n, %) | Tolerance and Safety | Steroid Use Discontinuation | Concluding Remarks |
| Immunotherapy | |||||||
| Fangusaro | 3.78 months | Median PFS: 2.6 months | Disease progression: 6 (66.7%) | NR | ≥grade 3 events: neutropenia (3 patients, 27.3%), lymphopenia (1 patient, 9.1%), leucopenia (1 patient, 9.1%), vertigo (1 patient, 9.1%) | NR | Pomalidomide monotherapy in progressive DIPG did not improve survival rates |
| Schuelke | 1.1 months | NR | Disease progression: 2 patients (100%) | Death: 82 and 60 days | No DLTs | NR | Sargramostim/pelareorep was well-tolerated but did not improve survival rates |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farrukh, S.; Habib, S.; Rafaqat, A.; Sarfraz, Z.; Sarfraz, A.; Sarfraz, M.; Robles-Velasco, K.; Felix, M.; Cherrez-Ojeda, I. Emerging Therapeutic Strategies for Diffuse Intrinsic Pontine Glioma: A Systematic Review. Healthcare 2023, 11, 559. https://doi.org/10.3390/healthcare11040559
Farrukh S, Habib S, Rafaqat A, Sarfraz Z, Sarfraz A, Sarfraz M, Robles-Velasco K, Felix M, Cherrez-Ojeda I. Emerging Therapeutic Strategies for Diffuse Intrinsic Pontine Glioma: A Systematic Review. Healthcare. 2023; 11(4):559. https://doi.org/10.3390/healthcare11040559
Chicago/Turabian StyleFarrukh, Shahrukh, Shagufta Habib, Amna Rafaqat, Zouina Sarfraz, Azza Sarfraz, Muzna Sarfraz, Karla Robles-Velasco, Miguel Felix, and Ivan Cherrez-Ojeda. 2023. "Emerging Therapeutic Strategies for Diffuse Intrinsic Pontine Glioma: A Systematic Review" Healthcare 11, no. 4: 559. https://doi.org/10.3390/healthcare11040559
APA StyleFarrukh, S., Habib, S., Rafaqat, A., Sarfraz, Z., Sarfraz, A., Sarfraz, M., Robles-Velasco, K., Felix, M., & Cherrez-Ojeda, I. (2023). Emerging Therapeutic Strategies for Diffuse Intrinsic Pontine Glioma: A Systematic Review. Healthcare, 11(4), 559. https://doi.org/10.3390/healthcare11040559







