A New Artificial Intelligence Approach Using Extreme Learning Machine as the Potentially Effective Model to Predict and Analyze the Diagnosis of Anemia
Abstract
1. Introduction

2. Related Work
3. Materials and Methods
3.1. Data Collection
3.2. Research Flow
3.3. Extreme Learning Machine
| Training Data | |||
| Step 1 | : | Prepare a training data matrix X of N number with features of d. | |
| Step 2 | : | Prepare training data target label . | |
| Step 3 | : | Determine the number of neurons H in the hidden layer. | |
| Step 4 | : | Create a matrix of initial weight values of size . | |
| Step 5 | : | Fill with a random value. | |
| Step 6 | : | Calculate the output hidden layer initialization matrix, | (8) |
| Step 7 | : | Calculate the hidden layer output matrix using a sigmoid function. | |
| Step 8 | : | Count , | (9) |
| Step 9 | : | Calculate output weight, | (10) |
| Step 10 | : | Calculate output value, | (11) |
| Testing Data | |||
| Step 1 | : | Prepare a testing data matrix X of N number with features of d. | |
| Step 2 | : | Calculate the output initialization matrix for the hidden layer using step 6. | |
| Step 3 | : | Calculate the output matrix for the hidden layer using step 7. | |
| Step 4 | : | Calculate the output value using step 10. | |
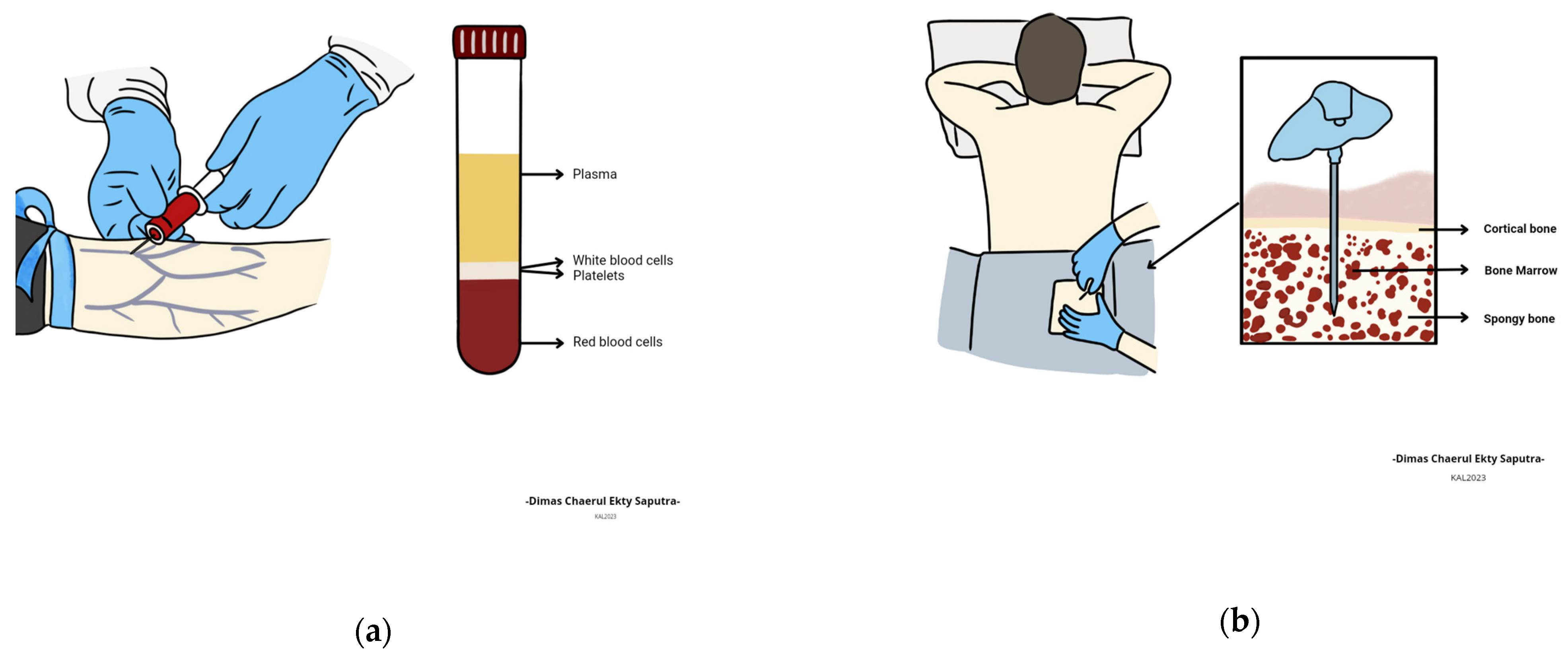
3.4. Blood
3.5. Anemia
4. Experimental Results
4.1. Evaluation Model
4.2. Experimental Results of Extreme Learning Machine
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chi, Z.; Beile, L.; Deyu, L.; Yubo, F. Application of multiscale coupling models in the numerical study of circulation system. Med. Nov. Technol. Devices 2022, 14, 100117. [Google Scholar] [CrossRef]
- Amin, J.; Sharif, M.; Anjum, M.A.; Yasmin, M.; Khattak, K.I.; Kadry, S.; Seo, S. An Integrated Design Based on Dual Thresholding and Features Optimization for White Blood Cells Detection. IEEE Access 2021, 9, 151421–151433. [Google Scholar] [CrossRef]
- Hézode, C.; Colombo, M.; Bourlière, M.; Spengler, U.; Ben-Ari, Z.; Strasser, S.I.; Lee, W.M.; Morgan, L.; Qiu, J.; Hwang, P.; et al. Elbasvir/Grazoprevir for Patients with Hepatitis C Virus Infection and Inherited Blood Disorders: A Phase III Study. Hepatology 2017, 66, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Tomasik, J.; Han, S.Y.S.; Barton-Owen, G.; Mirea, D.-M.; Martin-Key, N.A.; Rustogi, N.; Lago, S.G.; Olmert, T.; Cooper, J.D.; Ozcan, S.; et al. A machine learning algorithm to differentiate bipolar disorder from major depressive disorder using an online mental health questionnaire and blood biomarker data. Transl. Psychiatry 2021, 11, 41. [Google Scholar] [CrossRef]
- van Galen, K.P.M.; D’Oiron, R.; James, P.; Abdul-Kadir, R.; Kouides, P.A.; Kulkarni, R.; Mahlangu, J.N.; Othman, M.; Peyvandi, F.; Rotellini, D.; et al. A new hemophilia carrier nomenclature to define hemophilia in women and girls: Communication from the SSC of the ISTH. J. Thromb. Haemost. 2021, 19, 1883–1887. [Google Scholar] [CrossRef]
- Batsuli, G.; Greene, A.; Meeks, S.L.; Sidonio, R.F. Emicizumab in tolerized patients with hemophilia A with inhibitors: A single-institution pediatric cohort assessing inhibitor status. Res. Pract. Thromb. Haemost. 2021, 5, 342–348. [Google Scholar] [CrossRef]
- Pujhari, S.; Paul, S.; Ahluwalia, J.; Rasgon, J.L. Clotting disorder in severe acute respiratory syndrome coronavirus. Rev. Med Virol. 2020, 31, e2177. [Google Scholar] [CrossRef]
- Sun, Y.; Myers, D.R.; Nikolov, S.V.; Oshinowo, O.; Baek, J.; Bowie, S.M.; Lambert, T.P.; Woods, E.; Sakurai, Y.; Lam, W.A.; et al. Platelet heterogeneity enhances blood clot volumetric contraction: An example of asynchrono-mechanical amplification. Biomaterials 2021, 274, 120828. [Google Scholar] [CrossRef]
- Dese, K.; Raj, H.; Ayana, G.; Yemane, T.; Adissu, W.; Krishnamoorthy, J.; Kwa, T. Accurate Machine-Learning-Based classification of Leukemia from Blood Smear Images. Clin. Lymphoma Myeloma Leuk. 2021, 21, e903–e914. [Google Scholar] [CrossRef]
- Oldenburg, M.; Rüchel, N.; Janssen, S.; Borkhardt, A.; Gössling, K.L. The Microbiome in Childhood Acute Lymphoblastic Leukemia. Cancers 2021, 13, 4947. [Google Scholar] [CrossRef]
- Tensen, C.P.; Quint, K.D.; Vermeer, M.H. Genetic and epigenetic insights into cutaneous T-cell lymphoma. Blood 2022, 139, 15–33. [Google Scholar] [CrossRef] [PubMed]
- Labudde, E.J.; DeGroote, N.P.; Smith, S.; Ebelhar, J.; Allen, K.E.; Castellino, S.M.; Wasilewski-Masker, K.; Brock, K.E. Evaluating palliative opportunities in pediatric patients with leukemia and lymphoma. Cancer Med. 2021, 10, 2714–2722. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, E.K.; Shapiro, Y.; Nadeem, O.; Yee, A.J.; Laubach, J.P.; Branagan, A.R.; Anderson, K.C.; Mo, C.C.; Munshi, N.C.; Ghobrial, I.M.; et al. Quality of Life, Psychological Distress, and Prognostic Awareness in Caregivers of Patients with Multiple Myeloma. Blood 2021, 138, 3044. [Google Scholar] [CrossRef]
- De Larrea, C.F.; Kyle, R.; Rosiñol, L.; Paiva, B.; Engelhardt, M.; Usmani, S.; Caers, J.; Gonsalves, W.; Schjesvold, F.; Merlini, G.; et al. Primary plasma cell leukemia: Consensus definition by the International Myeloma Working Group according to peripheral blood plasma cell percentage. Blood Cancer J. 2021, 11, 192. [Google Scholar] [CrossRef]
- Pu, J.; Liu, Y.; Zhang, H.; Tian, L.; Gui, S.; Yu, Y.; Chen, X.; Chen, Y.; Yang, L.; Ran, Y.; et al. An integrated meta-analysis of peripheral blood metabolites and biological functions in major depressive disorder. Mol. Psychiatry 2020, 26, 4265–4276. [Google Scholar] [CrossRef]
- Galea, I. The blood–brain barrier in systemic infection and inflammation. Cell. Mol. Immunol. 2021, 18, 2489–2501. [Google Scholar] [CrossRef]
- Banks, W.A.; Reed, M.J.; Logsdon, A.F.; Rhea, E.M.; Erickson, M.A. Healthy aging and the blood–brain barrier. Nat. Aging 2021, 1, 243–254. [Google Scholar] [CrossRef]
- Feusier, J.E.; Arunachalam, S.; Tashi, T.; Baker, M.J.; VanSant-Webb, C.; Ferdig, A.; Welm, B.E.; Rodriguez-Flores, J.L.; Ours, C.; Jorde, L.B.; et al. Large-scale Identification of Clonal Hematopoiesis and Mutations Recurrent in Blood Cancers. Blood Cancer Discov. 2021, 2, 226–237. [Google Scholar] [CrossRef]
- Bondyra-Wiśniewska, B.; Myszkowska-Ryciak, J.; Harton, A. Impact of Lifestyle Intervention Programs for Children and Adolescents with Overweight or Obesity on Body Weight and Selected Cardiometabolic Factors—A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 2061. [Google Scholar] [CrossRef]
- Shimano, K.A.; Narla, A.; Rose, M.J.; Gloude, N.J.; Allen, S.W.; Bergstrom, K.; Broglie, L.; Carella, B.A.; Castillo, P.; de Jong, J.L.O.; et al. Diagnostic work-up for severe aplastic anemia in children: Consensus of the North American Pediatric Aplastic Anemia Consortium. Am. J. Hematol. 2021, 96, 1491–1504. [Google Scholar] [CrossRef]
- DeZern, A.E.; Churpek, J.E. Approach to the diagnosis of aplastic anemia. Blood Adv. 2021, 5, 2660–2671. [Google Scholar] [CrossRef]
- Dong, M.; McGann, P.T. Changing the Clinical Paradigm of Hydroxyurea Treatment for Sickle Cell Anemia Through Precision Medicine. Clin. Pharmacol. Ther. 2020, 109, 73–81. [Google Scholar] [CrossRef]
- Safiri, S.; Kolahi, A.-A.; Noori, M.; Nejadghaderi, S.A.; Karamzad, N.; Bragazzi, N.L.; Sullman, M.J.M.; Abdollahi, M.; Collins, G.S.; Kaufman, J.S.; et al. Burden of anemia and its underlying causes in 204 countries and territories, 1990–2019: Results from the Global Burden of Disease Study. J. Hematol. Oncol. 2021, 14, 185. [Google Scholar] [CrossRef]
- Owais, A.; Merritt, C.; Lee, C.; Bhutta, Z. Anemia among Women of Reproductive Age: An Overview of Global Burden, Trends, Determinants, and Drivers of Progress in Low- and Middle-Income Countries. Nutrients 2021, 13, 2745. [Google Scholar] [CrossRef]
- Sahu, P.; Purohit, P.; Mantri, S.; Tudu, R.; Nayak, J.; Agrawalla, S.K.; Behera, S.K.; Patro, M.K.; Karmee, N.; Tripathy, D.; et al. Spectrum of hemoglobin disorders in southern Odisha, India: A hospital based study. Porto Biomed. J. 2021, 6, e126. [Google Scholar] [CrossRef]
- Sundararajan, S.; Rabe, H. Prevention of iron deficiency anemia in infants and toddlers. Pediatr. Res. 2020, 89, 63–73. [Google Scholar] [CrossRef]
- Zhang, Q.; Lu, X.-M.; Zhang, M.; Yang, C.-Y.; Lv, S.-Y.; Li, S.-F.; Zhong, C.-Y.; Geng, S.-S. Adverse effects of iron deficiency anemia on pregnancy outcome and offspring development and intervention of three iron supplements. Sci. Rep. 2021, 11, 1347. [Google Scholar] [CrossRef]
- Nowaczyk, J.; Poniedziałek, B.; Rzymski, P.; Sikora, D.; Ropacka-Lesiak, M. Platelets in Fetal Growth Restriction: Role of Reactive Oxygen Species, Oxygen Metabolism, and Aggregation. Cells 2022, 11, 724. [Google Scholar] [CrossRef]
- Zopfs, D.; Rinneburger, M.; dos Santos, D.P.; Reimer, R.P.; Laukamp, K.R.; Maintz, D.; Lennartz, S.; Hokamp, N.G. Evaluating anemia using contrast-enhanced spectral detector CT of the chest in a large cohort of 522 patients. Eur. Radiol. 2020, 31, 4350–4357. [Google Scholar] [CrossRef]
- Islam, M.; Rahman, J.; Roy, D.C.; Islam, M.; Tawabunnahar, M.; Ahmed, N.F.; Maniruzzaman, M. Risk Factors Identification and Prediction of Anemia among Women in Bangladesh using Machine Learning Techniques. Curr. Women’ s Health Rev. 2022, 18, 118–133. [Google Scholar] [CrossRef]
- Rusu, I.G.; Suharoschi, R.; Vodnar, D.C.; Pop, C.R.; Socaci, S.A.; Vulturar, R.; Istrati, M.; Moroșan, I.; Fărcaș, A.C.; Kerezsi, A.D.; et al. Iron Supplementation Influence on the Gut Microbiota and Probiotic Intake Effect in Iron Deficiency—A Literature-Based Review. Nutrients 2020, 12, 1993. [Google Scholar] [CrossRef]
- Prevalence of Anemia in Women of Reproductive Age, Our World in Data. Available online: https://ourworldindata.org/grapher/prevalence-of-anemia-in-women-of-reproductive-age-aged-15-29 (accessed on 28 November 2022).
- Knijff, M.; Roshita, A.; Suryantan, J.; Izwardy, D.; Rah, J.H. Frequent Consumption of Micronutrient-Rich Foods Is Associated with Reduced Risk of Anemia among Adolescent Girls and Boys in Indonesia: A Cross-Sectional Study. Food Nutr. Bull. 2021, 42, S59–S71. [Google Scholar] [CrossRef] [PubMed]
- Vogt, A.-C.; Arsiwala, T.; Mohsen, M.; Vogel, M.; Manolova, V.; Bachmann, M. On Iron Metabolism and Its Regulation. Int. J. Mol. Sci. 2021, 22, 4591. [Google Scholar] [CrossRef]
- Ayyıldız, H.; Tuncer, S.A. Determination of the effect of red blood cell parameters in the discrimination of iron deficiency anemia and beta thalassemia via Neighborhood Component Analysis Feature Selection-Based machine learning. Chemom. Intell. Lab. Syst. 2019, 196, 103886. [Google Scholar] [CrossRef]
- Ali, S.; Mumtaz, S.; Shakir, H.A.; Khan, M.; Tahir, H.M.; Mumtaz, S.; Mughal, T.A.; Hassan, A.; Kazmi, S.A.R.; Sadia; et al. Current status of beta-thalassemia and its treatment strategies. Mol. Genet. Genom. Med. 2021, 9, e1788. [Google Scholar] [CrossRef]
- Amjad, F.; Fatima, T.; Fayyaz, T.; Khan, M.A.; Qadeer, M.I. Novel genetic therapeutic approaches for modulating the severity of β-thalassemia (Review). Biomed. Rep. 2020, 13, 48. [Google Scholar] [CrossRef] [PubMed]
- Dignass, A.; Farrag, K.; Stein, J. Limitations of Serum Ferritin in Diagnosing Iron Deficiency in Inflammatory Conditions. Int. J. Chronic Dis. 2018, 2018, 9394060. [Google Scholar] [CrossRef]
- Luo, Q.; Zhang, H.; Wang, H.; Ma, L.; Huang, M.; Niu, J.; Luo, B.; Yan, J.; Li, X. The Effects of Lead and Cadmium Co-exposure on Serum Ions in Residents Living Near a Mining and Smelting Area in Northwest China. Biol. Trace Element Res. 2021, 200, 3932–3940. [Google Scholar] [CrossRef]
- Joerling, J.; Doll, K. Monitoring of iron deficiency in calves by determination of serum ferritin in comparison with serum iron: A preliminary study. Open Vet. J. 2019, 9, 177–184. [Google Scholar] [CrossRef]
- Sezgin, G.; Monagle, P.; Loh, T.P.; Ignjatovic, V.; Hoq, M.; Pearce, C.; McLeod, A.; Westbrook, J.; Li, L.; Georgiou, A. Clinical thresholds for diagnosing iron deficiency: Comparison of functional assessment of serum ferritin to population based centiles. Sci. Rep. 2020, 10, 18233. [Google Scholar] [CrossRef]
- Zou, J.; Huang, S.; Xi, H.; Huang, C.; Zou, L.; Qiu, L.; Nie, X.; Zhou, J.; Zhuang, Y.; Chen, Y.; et al. Application of an optimized interpretation model in capillary hemoglobin electrophoresis for newborn thalassemia screening. Int. J. Lab. Hematol. 2021, 44, 223–228. [Google Scholar] [CrossRef]
- Noor, F.A.; Sultana, N.; Bhuyan, G.S.; Islam, T.; Hossain, M.; Sarker, S.K.; Islam, K.; Khan, W.A.; Rahman, M.; Qadri, S.K.; et al. Nationwide carrier detection and molecular characterization of β-thalassemia and hemoglobin E variants in Bangladeshi population. Orphanet J. Rare Dis. 2020, 15, 15. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, S.; Khakwani, M.; Fayyaz, A.; Taj, N. Role of Mentzer index for differentiating iron deficiency anemia and beta thalassemia trait in pregnant women. Pak. J. Med Sci. 2022, 38, 878. [Google Scholar] [CrossRef] [PubMed]
- Prod’Homme, C.; Touzet, L.; Pierrat, M.; Chevalier, L.; Lesaffre, H.; Berthon, C.; Coiteux, V.; Barbieux, S.; Beauvais, D.; Bauschert, L.; et al. Can palliative care consultation increase integration of palliative care for patients with hematologic malignancies? Blood Adv. 2021, 5, 2123–2127. [Google Scholar] [CrossRef] [PubMed]
- Anaemia in Women and Children, The Global Health Observatory: Explore a World of Health Data. Available online: https://www.who.int/data/gho/data/themes/topics/anaemia_in_women_and_children (accessed on 28 November 2022).
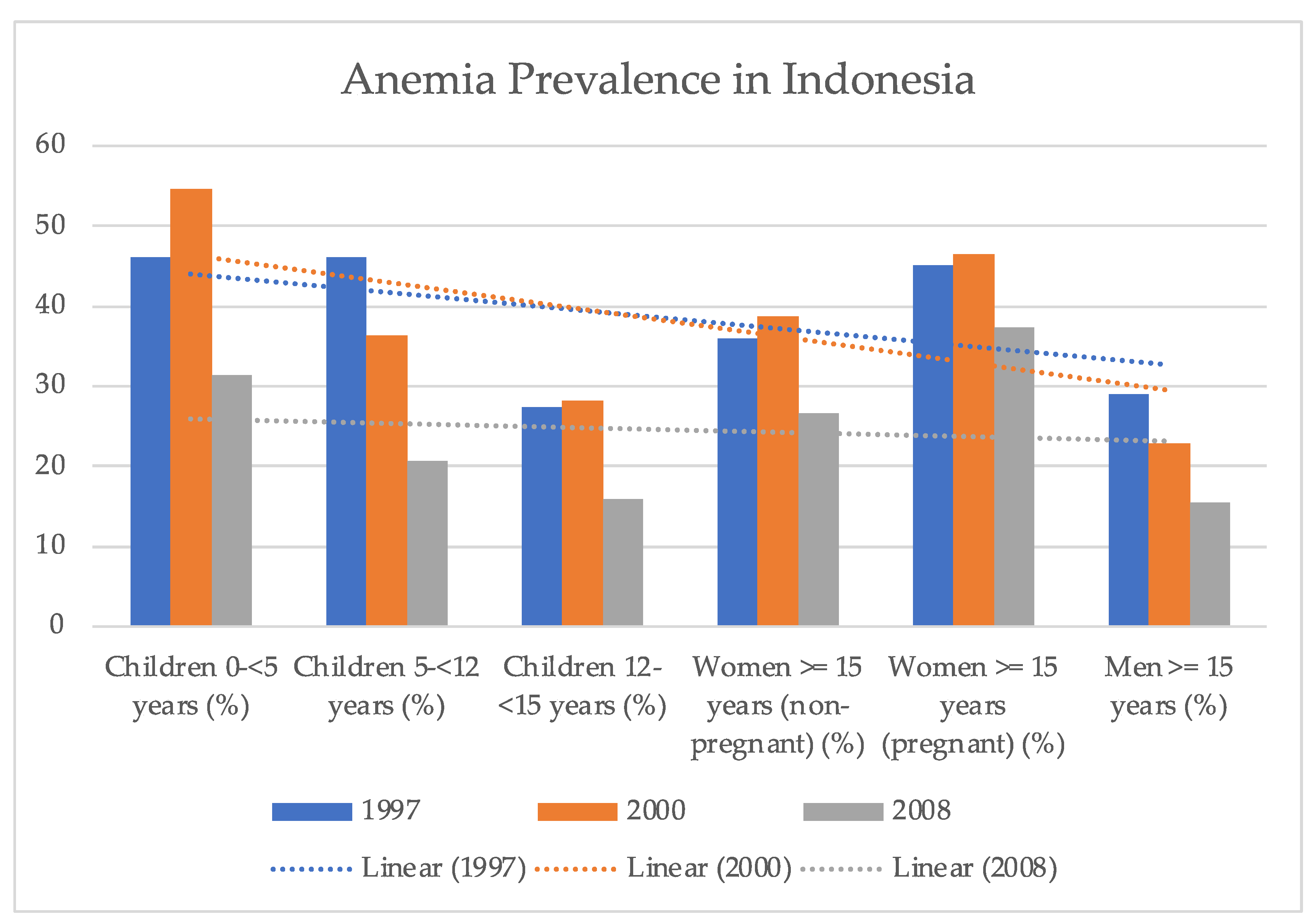
- Barkley, J.S.; Kendrick, K.L.; Codling, K.; Muslimatun, S.; Pachón, H. Anaemia prevalence over time in Indonesia: Estimates from the 1997, 2000, and 2008 Indonesia Family Life Surveys. Asia Pac. J. Clin. Nutr. 2015, 24, 452–455. [Google Scholar] [CrossRef]
- Martens, P.; Nijst, P.; Verbrugge, F.H.; Smeets, K.; Dupont, M.; Mullens, W. Impact of iron deficiency on exercise capacity and outcome in heart failure with reduced, mid-range and preserved ejection fraction. Acta Cardiol. 2017, 73, 115–123. [Google Scholar] [CrossRef]
- Prefumo, F.; Fichera, A.; Fratelli, N.; Sartori, E. Fetal anemia: Diagnosis and management. Best Pract. Res. Clin. Obstet. Gynaecol. 2019, 58, 2–14. [Google Scholar] [CrossRef]
- Young, M.F.; Oaks, B.M.; Tandon, S.; Martorell, R.; Dewey, K.G.; Wendt, A. Maternal hemoglobin concentrations across pregnancy and maternal and child health: A systematic review and meta-analysis. Ann. N. Y. Acad. Sci. 2019, 1450, 47–68. [Google Scholar] [CrossRef] [PubMed]
- Girelli, D.; Marchi, G.; Camaschella, C. Anemia in the Elderly. Hemasphere 2018, 2, e40. [Google Scholar] [CrossRef]
- Provan, D.; Baglin, T.; Dokal, I.; de Vos, J. Oxford Handbook of Clinical Haematology, 4th ed.; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- Alsaeed, M.; Ahmed, S.S.; Seyadi, K.; Ahmed, A.J.; Alawi, A.S.; Abulsaad, K. The prevalence and impact of anemia in hospitalized older adults: A single center experience from Bahrain. J. Taibah Univ. Med Sci. 2022, 17, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Shalby, K.Y.; Alradhi, A.Y.; Holdar, S.J.; Alghamdi, A.S.; Alduilej, S.A.; Albuainain, S.; Al Zaghal, A.M.; Sadiq, N.A. Extremes of Anemia: The Lowest Hemoglobin Values Probably Ever Reported in the Pediatric Literature Attributed to Iron Deficiency Anemia. Am. J. Case Rep. 2022, 23, e936252-1. [Google Scholar] [CrossRef] [PubMed]
- Tvedten, H. Classification and Laboratory Evaluation of Anemia. Schalm’s Vet. Hematol. 2022, 35, 198–208. [Google Scholar] [CrossRef]
- Kang, W.; Barad, A.; Clark, A.G.; Wang, Y.; Lin, X.; Gu, Z.; O O’Brien, K. Ethnic Differences in Iron Status. Adv. Nutr. Int. Rev. J. 2021, 12, 1838–1853. [Google Scholar] [CrossRef] [PubMed]
- Nalado, A.M.; Mahlangu, J.N.; Waziri, B.; Duarte, R.; Paget, G.; Olorunfemi, G.; Naicker, S. Ethnic prevalence of anemia and predictors of anemia among chronic kidney disease patients at a tertiary hospital in Johannesburg, South Africa. Int. J. Nephrol. Renov. Dis. 2019, 12, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Çil, B.; Ayyıldız, H.; Tuncer, T. Discrimination of β-thalassemia and iron deficiency anemia through extreme learning machine and regularized extreme learning machine based decision support system. Med. Hypotheses 2020, 138, 109611. [Google Scholar] [CrossRef]
- Helm, J.M.; Swiergosz, A.M.; Haeberle, H.S.; Karnuta, J.M.; Schaffer, J.L.; Krebs, V.E.; Spitzer, A.I.; Ramkumar, P.N. Machine Learning and Artificial Intelligence: Definitions, Applications, and Future Directions. Curr. Rev. Musculoskelet. Med. 2020, 13, 69–76. [Google Scholar] [CrossRef]
- Holzinger, A.; Langs, G.; Denk, H.; Zatloukal, K.; Müller, H. Causability and explainability of artificial intelligence in medicine. WIREs Data Min. Knowl. Discov. 2019, 9, e1312. [Google Scholar] [CrossRef]
- Briganti, G.; Le Moine, O. Artificial Intelligence in Medicine: Today and Tomorrow. Front. Med. 2020, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Dimiduk, D.M.; Holm, E.A.; Niezgoda, S.R. Perspectives on the Impact of Machine Learning, Deep Learning, and Artificial Intelligence on Materials, Processes, and Structures Engineering. Integr. Mater. Manuf. Innov. 2018, 7, 157–172. [Google Scholar] [CrossRef]
- Lamy, J.-B.; Sekar, B.; Guezennec, G.; Bouaud, J.; Séroussi, B. Explainable artificial intelligence for breast cancer: A visual case-based reasoning approach. Artif. Intell. Med. 2019, 94, 42–53. [Google Scholar] [CrossRef]
- Jin, Q.; Cui, H.; Sun, C.; Meng, Z.; Wei, L.; Su, R. Domain adaptation based self-correction model for COVID-19 infection segmentation in CT images. Expert Syst. Appl. 2021, 176, 114848. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, A.; Mahmood, A. Review of Deep Learning Algorithms and Architectures. IEEE Access 2019, 7, 53040–53065. [Google Scholar] [CrossRef]
- Garcia, C.G.; Núñez-Valdez, E.; García-Díaz, V.; G-Bustelo, C.P.; Cueva-Lovelle, J.M. A Review of Artificial Intelligence in the Internet of Things. Int. J. Interact. Multimedia Artif. Intell. 2019, 5, 9–20. [Google Scholar] [CrossRef]
- Laengsri, V.; Shoombuatong, W.; Adirojananon, W.; Nantasenamart, C.; Prachayasittikul, V.; Nuchnoi, P. ThalPred: A web-based prediction tool for discriminating thalassemia trait and iron deficiency anemia. BMC Med. Inform. Decis. Mak. 2019, 19, 212. [Google Scholar] [CrossRef]
- El-kenawy, E.M.T. A Machine Learning Model for Hemoglobin Estimation and Anemia Classification. Int. J. Comput. Sci. Inf. Secur. 2019, 17, 100–108. [Google Scholar]
- Khan, J.R.; Chowdhury, S.; Islam, H.; Raheem, E. Machine Learning Algorithms to Predict the Childhood Anemia in Bangladesh. J. Data Sci. 2019, 17, 195–218. [Google Scholar] [CrossRef]
- Jaiswal, M.; Srivastava, A.; Siddiqui, T.J. Machine Learning Algorithms for Anemia Disease Prediction; Springer Singapore: Singapore, 2018; pp. 463–469. [Google Scholar] [CrossRef]
- Khan, M.A.; Muhammad, K.; Sharif, M.; Akram, T.; Kadry, S. Intelligent fusion-assisted skin lesion localization and classification for smart healthcare. Neural Comput. Appl. 2021, 2021, 1–16. [Google Scholar] [CrossRef]
- Cichy, R.M.; Kaiser, D. Deep Neural Networks as Scientific Models. Trends Cogn. Sci. 2019, 23, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Abdulkarim, H.A.; Razak, M.A.A.; Sudirman, R.; Ramli, N. A deep learning AlexNet model for classification of red blood cells in sickle cell anemia. IAES Int. J. Artif. Intell. (IJ-AI) 2020, 9, 221–228. [Google Scholar] [CrossRef]
- Kilicarslan, S.; Celik, M.; Sahin, Ş. Hybrid models based on genetic algorithm and deep learning algorithms for nutritional Anemia disease classification. Biomed. Signal Process. Control 2020, 63, 102231. [Google Scholar] [CrossRef]
- Yeruva, S.; Varalakshmi, M.S.; Gowtham, B.P.; Chandana, Y.H.; Prasad, P.K. Identification of Sickle Cell Anemia Using Deep Neural Networks. Emerg. Sci. J. 2021, 5, 200–210. [Google Scholar] [CrossRef]
- Esteva, A.; Robicquet, A.; Ramsundar, B.; Kuleshov, V.; Depristo, M.; Chou, K.; Cui, C.; Corrado, G.; Thrun, S.; Dean, J. A guide to deep learning in healthcare. Nat. Med. 2019, 25, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Valentine, S.L.; Bembea, M.; Muszynski, J.A.; Cholette, J.M.; Doctor, A.; Spinella, P.C.; Steiner, M.E.; Tucci, M.; Hassan, N.E.; Parker, R.I.; et al. Consensus Recommendations for RBC Transfusion Practice in Critically Ill Children from the Pediatric Critical Care Transfusion and Anemia Expertise Initiative. Pediatr. Crit. Care Med. 2018, 19, 884–898. [Google Scholar] [CrossRef]
- Michel, J.-B.; Martin-Ventura, J.L. Red Blood Cells and Hemoglobin in Human Atherosclerosis and Related Arterial Diseases. Int. J. Mol. Sci. 2020, 21, 6756. [Google Scholar] [CrossRef]
- Hornik, K. Approximation capabilities of multilayer feedforward networks. Neural Netw. 1991, 4, 251–257. [Google Scholar] [CrossRef]
- Leshno, M.; Lin, V.Y.; Pinkus, A.; Schocken, S. Multilayer feedforward networks with a nonpolynomial activation function can approximate any function. Neural Netw. 1993, 6, 861–867. [Google Scholar] [CrossRef]
- Ito, Y. Approximation of continuous functions on Rd by linear combinations of shifted rotations of a sigmoid function with and without scaling. Neural Netw. 1992, 5, 105–115. [Google Scholar] [CrossRef]
- Huang, G.B.; Zhu, Q.Y.; Siew, C.K. Extreme learning machine: A new learning scheme of feedforward neural networks. Neurocomputing 2004, 2, 985–990. [Google Scholar]
- Horata, P.; Chiewchanwattana, S.; Sunat, K. Robust extreme learning machine. Neurocomputing 2013, 102, 31–44. [Google Scholar] [CrossRef]
- Huang, G.-B.; Babri, H. Upper bounds on the number of hidden neurons in feedforward networks with arbitrary bounded nonlinear activation functions. IEEE Trans. Neural Netw. 1998, 9, 224–229. [Google Scholar] [CrossRef]
- Qin, Q.; Huang, Z.; Zhou, Z.; Chen, Y.; Zhao, W. Hodrick–Prescott filter-based hybrid ARIMA–SLFNs model with residual decomposition scheme for carbon price forecasting. Appl. Soft Comput. 2022, 119, 108560. [Google Scholar] [CrossRef]
- Yonglong, C.; Jinda, Z.; Dongmei, Y.; Xinping, W. An Improved Quantum Particle Swarm Algorithm Optimized Regularized Extreme Learning Machine for Short-Term Load Forecasting. In Proceedings of the 2020 5th Asia Conference on Power and Electrical Engineering, Chengdu, China, 4–7 June 2020; pp. 87–93. [Google Scholar] [CrossRef]
- Gupta, U.; Gupta, D. Regularized based implicit Lagrangian twin extreme learning machine in primal for pattern classification. Int. J. Mach. Learn. Cybern. 2021, 12, 1311–1342. [Google Scholar] [CrossRef]
- Li, S.; Huang, X.; Wang, D. Stochastic configuration networks for multi-dimensional integral evaluation. Inf. Sci. 2022, 601, 323–339. [Google Scholar] [CrossRef]
- Huang, G.-B.; Zhu, Q.-Y.; Siew, C.-K. Extreme learning machine: Theory and applications. Neurocomputing 2006, 70, 489–501. [Google Scholar] [CrossRef]
- Zhou, S.; Chen, L.; Sugumaran, V. Hidden Two-Stream Collaborative Learning Network for Action Recognition. CMC-Comput. Mater. Contin. 2020, 63, 1545–1561. [Google Scholar] [CrossRef]
- Savkovic, B.; Kovac, P.; Dudic, B.; Gregus, M.; Rodic, D.; Strbac, B.; Ducic, N. Comparative Characteristics of Ductile Iron and Austempered Ductile Iron Modeled by Neural Network. Materials 2019, 12, 2864. [Google Scholar] [CrossRef] [PubMed]
- Duan, M.; Li, K.; Liao, X.; Li, K. A Parallel Multiclassification Algorithm for Big Data Using an Extreme Learning Machine. IEEE Trans. Neural Netw. Learn. Syst. 2017, 29, 2337–2351. [Google Scholar] [CrossRef]
- Yoshida, T.; Prudent, M.; D’Alessandro, A. Red blood cell storage lesion: Causes and potential clinical consequences. Blood Transfus. = Trasfus. Sangue 2019, 17, 27–52. [Google Scholar] [CrossRef]
- Andrade, A.R.; Vogado, L.H.; Veras, R.D.M.; Silva, R.R.; Araujo, F.H.; Medeiros, F.N. Recent computational methods for white blood cell nuclei segmentation: A comparative study. Comput. Methods Programs Biomed. 2019, 173, 1–14. [Google Scholar] [CrossRef]
- Chavez, J.C.; Bachmeier, C.; Kharfan-Dabaja, M.A. CAR T-cell therapy for B-cell lymphomas: Clinical trial results of available products. Ther. Adv. Hematol. 2019, 10, 2040620719841581. [Google Scholar] [CrossRef]
- Jiang, L.; Tang, C.; Zhou, H. White Blood Cell Classification via DiscriminativeRegion Detection Assisted Feature Aggregation Network. Biomed. Opt. Express 2022, 13, 5246–5260. [Google Scholar] [CrossRef] [PubMed]
- Hoffbrand, A.V.; Moss, P.A.H. Hoffbrand’s Essential Haematology, 7th ed.; Wiley Blackwell: West Sussex, UK, 2016. [Google Scholar]
- Han, X.; Wang, C.; Liu, Z. Red Blood Cells as Smart Delivery Systems. Bioconjug. Chem. 2018, 29, 852–860. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Zhang, Y.; Li, Z.; Hou, X.; Feng, N. Red blood cell membrane-camouflaged nanoparticles: A novel drug delivery system for antitumor application. Acta Pharm. Sin. B 2019, 9, 675–689. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Rondina, M.T. The Era of Thromboinflammation: Platelets Are Dynamic Sensors and Effector Cells During Infectious Diseases. Front. Immunol. 2019, 10, 2204. [Google Scholar] [CrossRef]
- Anz, A.W.; Hubbard, R.; Rendos, N.; Everts, P.A.; Andrews, J.R.; Hackel, J.G. Bone Marrow Aspirate Concentrate Is Equivalent to Platelet-Rich Plasma for the Treatment of Knee Osteoarthritis at 1 Year: A Prospective, Randomized Trial. Orthop. J. Sports Med. 2020, 8, 2325967119900958. [Google Scholar] [CrossRef]
- Markowicz-Piasecka, M.; Huttunen, K.M.; Sadkowska, A.; Sikora, J. Pleiotropic Activity of Metformin and Its Sulfonamide Derivatives on Vascular and Platelet Haemostasis. Molecules 2019, 25, 125. [Google Scholar] [CrossRef]
- Izzi, B.; Tirozzi, A.; Cerletti, C.; Donati, M.B.; De Gaetano, G.; Hoylaerts, M.F.; Iacoviello, L.; Gialluisi, A. Beyond Haemostasis and Thrombosis: Platelets in Depression and Its Co-Morbidities. Int. J. Mol. Sci. 2020, 21, 8817. [Google Scholar] [CrossRef]
- Crotti, C.; Agape, E.; Becciolini, A.; Biggioggero, M.; Favalli, E.G. Targeting Granulocyte-Monocyte Colony-Stimulating Factor Signaling in Rheumatoid Arthritis: Future Prospects. Drugs 2019, 79, 1741–1755. [Google Scholar] [CrossRef]
- Silvestre-Roig, C.; Fridlender, Z.G.; Glogauer, M.; Scapini, P. Neutrophil Diversity in Health and Disease. Trends Immunol. 2019, 40, 565–583. [Google Scholar] [CrossRef]
- Narasimhan, P.B.; Marcovecchio, P.; Hamers, A.A.; Hedrick, C.C. Nonclassical Monocytes in Health and Disease. Annu. Rev. Immunol. 2019, 37, 439–456. [Google Scholar] [CrossRef]
- Rooney, B.V.; Bigley, A.B.; LaVoy, E.; Laughlin, M.; Pedlar, C.; Simpson, R.J. Lymphocytes and monocytes egress peripheral blood within minutes after cessation of steady state exercise: A detailed temporal analysis of leukocyte extravasation. Physiol. Behav. 2018, 194, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Florentin, J.; Coppin, E.; Vasamsetti, S.B.; Zhao, J.; Tai, Y.-Y.; Tang, Y.; Zhang, Y.; Watson, A.; Sembrat, J.; Rojas, M.; et al. Inflammatory Macrophage Expansion in Pulmonary Hypertension Depends upon Mobilization of Blood-Borne Monocytes. J. Immunol. 2018, 200, 3612–3625. [Google Scholar] [CrossRef]
- Klion, A.D.; Ackerman, S.J.; Bochner, B.S. Contributions of Eosinophils to Human Health and Disease. Annu. Rev. Pathol. Mech. Dis. 2020, 15, 179–209. [Google Scholar] [CrossRef]
- Nakagome, K.; Nagata, M. Involvement and Possible Role of Eosinophils in Asthma Exacerbation. Front. Immunol. 2018, 9, 2220. [Google Scholar] [CrossRef] [PubMed]
- Kubo, M. Mast cells and basophils in allergic inflammation. Curr. Opin. Immunol. 2018, 54, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Luo, D.; Cai, S.; Li, Q.; Li, X. Circulating basophil count as a prognostic marker of tumor aggressiveness and survival outcomes in colorectal cancer. Clin. Transl. Med. 2020, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Mariyum, S.; Gulfraz, S.; Sultana, T.; Masood, K. Analysis of White Blood Cells Using Hematology Counts. In Intelligent Technologies and Applications; Bajwa, I.S., Sibalija, T., Jawawi, D.N.A., Eds.; Springer Singapore: Singapore, 2020; Volume 1198, pp. 210–221. [Google Scholar] [CrossRef]
- Idos, G.E.; Kwok, J.; Bonthala, N.; Kysh, L.; Gruber, S.B.; Qu, C. The Prognostic Implications of Tumor Infiltrating Lymphocytes in Colorectal Cancer: A Systematic Review and Meta-Analysis. Sci. Rep. 2020, 10, 3360. [Google Scholar] [CrossRef]
- Gavrilov, S.; Zhudenkov, K.; Helmlinger, G.; Dunyak, J.; Peskov, K.; Aksenov, S. Longitudinal Tumor Size and Neutrophil-to-Lymphocyte Ratio Are Prognostic Biomarkers for Overall Survival in Patients with Advanced Non-Small Cell Lung Cancer Treated with Durvalumab. CPT Pharmacomet. Syst. Pharmacol. 2020, 10, 67–74. [Google Scholar] [CrossRef]
- Cohen, A.D.; Garfall, A.L.; Stadtmauer, E.A.; Melenhorst, J.J.; Lacey, S.F.; Lancaster, E.; Vogl, D.T.; Weiss, B.M.; Dengel, K.; Nelson, A.; et al. B cell maturation antigen–specific CAR T cells are clinically active in multiple myeloma. J. Clin. Investig. 2019, 129, 2210–2221. [Google Scholar] [CrossRef]
- Lu, D.R.; McDavid, A.N.; Kongpachith, S.; Lingampalli, N.; Glanville, J.; Ju, C.-H.; Gottardo, R.; Robinson, W.H. T Cell-Dependent Affinity Maturation and Innate Immune Pathways Differentially Drive Autoreactive B Cell Responses in Rheumatoid Arthritis. Arthritis Rheumatol. 2018, 70, 1732–1744. [Google Scholar] [CrossRef]
- Lee-Six, H.; Øbro, N.F.; Shepherd, M.S.; Grossmann, S.; Dawson, K.; Belmonte, M.; Osborne, R.J.; Huntly, B.J.P.; Martincorena, I.; Anderson, E.; et al. Population dynamics of normal human blood inferred from somatic mutations. Nature 2018, 561, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Chaparro, C.M.; Suchdev, P.S. Anemia epidemiology, pathophysiology, and etiology in low- and middle-income countries. Ann. N. Y. Acad. Sci. 2019, 1450, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Tiruneh, T.; Shiferaw, E.; Enawgaw, B. Prevalence and associated factors of anemia among full-term newborn babies at University of Gondar comprehensive specialized hospital, Northwest Ethiopia: A cross-sectional study. Ital. J. Pediatr. 2020, 46, nyas.14092. [Google Scholar] [CrossRef] [PubMed]
- Srour, M.A.; Aqel, S.S.; Srour, K.M.; Younis, K.R.; Samarah, F. Prevalence of Anemia and Iron Deficiency among Palestinian Pregnant Women and Its Association with Pregnancy Outcome. Anemia 2018, 2018, 9135625. [Google Scholar] [CrossRef] [PubMed]
- Mansour, D.; Hofmann, A.; Gemzell-Danielsson, K. A Review of Clinical Guidelines on the Management of Iron Deficiency and Iron-Deficiency Anemia in Women with Heavy Menstrual Bleeding. Adv. Ther. 2020, 38, 201–225. [Google Scholar] [CrossRef]
- Ganz, T. Anemia of Inflammation. N. Engl. J. Med. 2019, 381, 1148–1157. [Google Scholar] [CrossRef]
- Okocha, O.; Dand, H.; Avram, M.J.; Sweitzer, B. An Effective and Efficient Testing Protocol for Diagnosing Iron-deficiency Anemia Preoperatively. Anesthesiology 2020, 133, 109–118. [Google Scholar] [CrossRef]
- Timilsina, S.; Karki, S.; Gautam, A.; Bhusal, P.; Paudel, G.; Sharma, D. Correlation between maternal and umbilical cord blood in pregnant women of Pokhara Valley: A cross sectional study. BMC Pregnancy Childbirth 2018, 18, 70. [Google Scholar] [CrossRef]
- Jayasuriya, N.A.; Kjaergaard, A.D.; Pedersen, K.M.; Sørensen, A.L.; Bak, M.; Larsen, M.K.; Nordestgaard, B.G.; Bojesen, S.E.; Çolak, Y.; Skov, V.; et al. Smoking, blood cells and myeloproliferative neoplasms: Meta-analysis and Mendelian randomization of 2.3 million people. Br. J. Haematol. 2019, 189, 323–334. [Google Scholar] [CrossRef]
- Luo, X.; Feng, L.; Bai, X.; Zhu, J.; Zhao, G.; Wu, M.; Yan, Z. Continuous changes in biological levels of complete blood count in a high altitude area of China. SAGE Open Med. 2020, 8, 2050312120931334. [Google Scholar] [CrossRef]
- Hortinela, C.C.; Balbin, J.R.; Fausto, J.C.; Divina, P.D.C.; Felices, J.P.T. Identification of Abnormal Red Blood Cells and Diagnosing Specific Types of Anemia Using Image Processing and Support Vector Machine. In Proceedings of the 2019 IEEE 11th International Conference on Humanoid, Nanotechnology, Information Technology, Communication and Control, Environment, and Management (HNICEM), Laoag, Philippines, 29 November–1 December 2019; pp. 1–6. [Google Scholar] [CrossRef]
- Sithika, T.A.; Prasaad, P.R.; Sivastava, V. A Bone Marrow Aspiration Study in Evaluation of Severe Anemia in Adults. J. Pharm. Res. Int. 2021, 33, 33–43. [Google Scholar] [CrossRef]
- Definition of CBC—NCI Dictionary of Cancer Terms—NCI. 2 February 2011. Available online: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/cbc (accessed on 1 December 2022).
- Children’s Oncology Group. Bone Marrow Aspirate. Available online: https://www.childrensoncologygroup.org/testsandprocedures/bonemarrowaspirate (accessed on 1 December 2022).
- Soppi, E.T. Iron deficiency without anemia—A clinical challenge. Clin. Case Rep. 2018, 6, 1082–1086. [Google Scholar] [CrossRef]
- Mattiello, V.; on behalf of the SPOG Pediatric Hematology Working Group; Schmugge, M.; Hengartner, H.; von der Weid, N.; Renella, R. Diagnosis and management of iron deficiency in children with or without anemia: Consensus recommendations of the SPOG Pediatric Hematology Working Group. Eur. J. Pediatr. 2020, 179, 527–545. [Google Scholar] [CrossRef] [PubMed]
- Jäger, U.; Barcellini, W.; Broome, C.M.; Gertz, M.A.; Hill, A.; Hill, Q.A.; Jilma, B.; Kuter, D.J.; Michel, M.; Montillo, M.; et al. Diagnosis and treatment of autoimmune hemolytic anemia in adults: Recommendations from the First International Consensus Meeting. Blood Rev. 2019, 41, 100648. [Google Scholar] [CrossRef] [PubMed]
- Metelitsina, T.I.; Sheth, V.S.; Patel, S.B.; Grassi, M.A. Peripheral retinopathy associated with aplastic anemia. Retin. Cases Brief Rep. 2017, 11, 108–110. [Google Scholar] [CrossRef] [PubMed]
- Torrez, M.; Chabot-Richards, D.; Babu, D.; Lockhart, E.; Foucar, K. How I investigate acquired megaloblastic anemia. Int. J. Lab. Hematol. 2022, 44, 236–247. [Google Scholar] [CrossRef]
- Kallen, M.E.; Dulau-Florea, A.; Wang, W.; Calvo, K.R. Acquired and germline predisposition to bone marrow failure: Diagnostic features and clinical implications. Semin. Hematol. 2019, 56, 69–82. [Google Scholar] [CrossRef]
- Mohandas, N. Inherited hemolytic anemia: A possessive beginner’s guide. Hematology 2018, 2018, 377–381. [Google Scholar] [CrossRef]
- Bahr, T.M.; Lozano-Chinga, M.; Agarwal, A.M.; Meznarich, J.A.; Gerday, E.; Smoot, J.L.; Taylor, A.; Christensen, R.D. Dizygotic twins with prolonged jaundice and microcytic, hypochromic, hemolytic anemia with pyropoikilocytosis. Blood Cells Mol. Dis. 2020, 85, 102462. [Google Scholar] [CrossRef]
- Takeshima, M.; Ishikawa, H.; Kitadate, A.; Sasaki, R.; Kobayashi, T.; Nanjyo, H.; Kanbayashi, T.; Shimizu, T. Anorexia nervosa-associated pancytopenia mimicking idiopathic aplastic anemia: A case report. BMC Psychiatry 2018, 18, 150. [Google Scholar] [CrossRef]
- Lee, S.; Park, D. A Real-Time Abnormal Beat Detection Method Using a Template Cluster for the ECG Diagnosis of IoT Devices. Hum.-Cent. Comput. Inf. Sci. 2021, 1, 1–15. [Google Scholar] [CrossRef]
- Kale, G.A.; Karakuzu, C. Multilayer extreme learning machines and their modeling performance on dynamical systems. Appl. Soft Comput. 2022, 122, 108861. [Google Scholar] [CrossRef]
- Meena, K.; Tayal, D.K.; Gupta, V.; Fatima, A. Using classification techniques for statistical analysis of Anemia. Artif. Intell. Med. 2019, 94, 138–152. [Google Scholar] [CrossRef] [PubMed]
- Sow, B.; Mukhtar, H.; Ahmad, H.F.; Suguri, H. Assessing the relative importance of social determinants of health in malaria and anemia classification based on machine learning techniques. Inform. Health Soc. Care 2019, 45, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Tyas, D.A.; Hartati, S.; Harjoko, A.; Ratnaningsih, T. Morphological, Texture, and Color Feature Analysis for Erythrocyte Classification in Thalassemia Cases. IEEE Access 2020, 8, 69849–69860. [Google Scholar] [CrossRef]
- De, S.; Chakraborty, B. Case-Based Reasoning (CBR)-Based Anemia Severity Detection System (ASDS) Using Machine Learning Algorithm. In Advanced Machine Learning Technologies and Applications; Hassanien, A.E., Bhatnagar, R., Darwish, A., Eds.; Springer Singapore: Singapore, 2020; Volume 1141, pp. 621–632. [Google Scholar] [CrossRef]
- Fu, Y.-K.; Liu, H.-M.; Lee, L.-H.; Chen, Y.-J.; Chien, S.-H.; Lin, J.-S.; Chen, W.-C.; Cheng, M.-H.; Lin, P.-H.; Lai, J.-Y.; et al. The TVGH-NYCU Thal-Classifier: Development of a Machine-Learning Classifier for Differentiating Thalassemia and Non-Thalassemia Patients. Diagnostics 2021, 11, 1725. [Google Scholar] [CrossRef]
- Dejene, B.E.; Abuhay, T.M.; Bogale, D.S. Predicting the level of anemia among Ethiopian pregnant women using homogeneous ensemble machine learning algorithm. BMC Med. Inform. Decis. Mak. 2022, 22, 247. [Google Scholar] [CrossRef]
- Memmolo, P.; Aprea, G.; Bianco, V.; Russo, R.; Andolfo, I.; Mugnano, M.; Merola, F.; Miccio, L.; Iolascon, A.; Ferraro, P. Differential diagnosis of hereditary anemias from a fraction of blood drop by digital holography and hierarchical machine learning. Biosens. Bioelectron. 2022, 201, 113945. [Google Scholar] [CrossRef]




| Type | Analyte |
|---|---|
| Anemia | Blood Hemoglobin |
| Hemoglobinopathy: Thalassemia Trait; Hemoglobin E; Hemoglobin S | Hemoglobin/Capillary Electrophoresis |
| Iron Deficiency | Serum Ferritin and Serum Transferrin Receptor (sTfR) |
| Folate Deficiency | Erythrocyte Folate |
| Vitamin B12 Deficiency | Serum Cyanocobalamin |
| Inflammation | Serum C-Reactive Protein (CRP) |
| Full Name | Abbreviation |
|---|---|
| Red Blood Cell | RBC |
| Hemoglobin | Hb |
| Hematocrit | HCT |
| Mean Corpuscular Volume | MCV |
| Mean Corpuscular Hemoglobin | MCH |
| Mean Corpuscular Hemoglobin Concentration | MCHC |
| Red-Cell Distribution Width | RDW |
| -Thalassemia Trait | BTT |
| Iron Deficiency Anemia | IDA |
| Hemoglobin E | HbE |
| Variable | Definition |
|---|---|
| Output layers | |
| Weight of output layer | |
| Activation functions | |
| Weight vector between the input and hidden layers | |
| Input vector | |
| Threshold functions | |
| Number of neurons in hidden layers | |
| Target class | |
| Output hidden layer initialization matrix | |
| Cell Type | Diameter | Lifespan in Blood | Number of Cells | Function |
|---|---|---|---|---|
Red cells | 6–8 | 120 days | Male: Female: 3.9 | Conveyance of oxygen and carbon dioxide |
Platelets | 0.5–3.0 | 10 days | Hemostasis | |
| Phagocytes | ||||
Neutrophils | 12–15 | 6–10 h | (48–76%) | Protection against organisms such as bacteria and fungi |
Monocytes | 12–20 | 20–40 h | (2.5–8.5%) | Protection against organisms such as bacteria and fungi |
Eosinophils | 12–15 | Days | (<5%) | Protection against parasites |
Basophils | 12–15 | Days | (<1.5%) | Release histamine for inflammatory responses |
Lymphocyte B T | 7–9 (resting) 12–20 (active) | Weeks or years | (18–41%) | B-cells: Releases antibodies and assists activation of T-cells. T-cells: Protection against viruses; immune function. |
| Type | Males | Females |
|---|---|---|
| Hemoglobin | 135.0–175.0 | 115.0–155.0 |
| Erythrocytes | 4.5–6.5 | 3.9–5.6 |
| Hematocrit | 40–52 | 36–48 |
| Mean Corpuscular Volume | 80–95 | |
| Mean Corpuscular Hemoglobin | 27–34 | |
| Leucocytes | 50–150 | |
| Total | 4.0–11.0 | |
| Neutrophils | 1.8–7.5 | |
| Monocytes | 0.2–0.8 | |
| Eosinophils | 0.04–0.44 | |
| Basophils | 0.01–0.1 | |
| Lymphocyte | 1.5–3.5 | |
| Platelets | 150–400 | |
| Serum Ferritin | 40–340 | 14–150 |
| Serum Vitamin B12 | 160–925 (20–680 ) | |
| Serum Folate | 3.0–15.0 (4–30 n) | |
| Red Cell Folate | 160–640 (360–1460 n) | |
| Real | True | False | |
|---|---|---|---|
| Class Prediction | |||
| True | TP | FN | |
| False | FP | TN | |
| Parameters | Extreme Learning Machine |
|---|---|
| Target (RMSE) | 0.001 |
| Inputs | 7 |
| Outputs | 4 |
| Hidden layers | 1 |
| Training data | 128 |
| Testing data | 62 |
| Hidden layer neurons | 9 |
| Output layer neurons | 4 |
| Activation function | Sigmoid |
| Split Data | Model | Accuracy (%) | Precision (%) | Sensitivity (%) | F1 Score (%) |
|---|---|---|---|---|---|
| 67% (128 Data) Train–33% (62 Data) Test | Extreme Learning Machine | 99.21 | 99.30 | 98.44 | 98.84 |
| Model | Classes | BTT | IDA | HbE | Combination |
|---|---|---|---|---|---|
| Random Forest | BTT | 6 | 0 | 0 | 0 |
| IDA | 0 | 13 | 4 | 0 | |
| HbE | 1 | 0 | 34 | 0 | |
| Combination | 0 | 1 | 2 | 2 | |
| K-Nearest Neighbor | BTT | 5 | 1 | 0 | 0 |
| IDA | 0 | 13 | 4 | 0 | |
| HbE | 1 | 2 | 32 | 0 | |
| Combination | 0 | 2 | 3 | 0 | |
| Support Vector Machine | BTT | 6 | 1 | 0 | 4 |
| IDA | 0 | 13 | 2 | 1 | |
| HbE | 1 | 1 | 27 | 5 | |
| Combination | 0 | 0 | 2 | 0 | |
| Extreme Learning Machine | BTT | 5 | 0 | 0 | 0 |
| IDA | 0 | 15 | 1 | 0 | |
| HbE | 0 | 0 | 35 | 0 | |
| Combination | 0 | 0 | 0 | 7 |
| Model | Classes | Accuracy (%) | Precision (%) | Sensitivity (%) | F1 Score (%) |
|---|---|---|---|---|---|
| Random Forest | BTT | 100 | 85.71 | 100 | 92.30 |
| IDA | 88.57 | 92.86 | 76.47 | 83.87 | |
| HbE | 96.55 | 85 | 97.14 | 90.66 | |
| Combination | 90.91 | 100 | 40 | 57.14 | |
| K-Nearest Neighbor | BTT | 89.70 | 83.34 | 83.34 | 83.34 |
| IDA | 87.88 | 72.23 | 76.47 | 37.14 | |
| HbE | 89.28 | 82.05 | 91.43 | 86.49 | |
| Combination | 85.29 | 0 | 0 | 0 | |
| Support Vector Machine | BTT | 96.61 | 85.71 | 54.54 | 66.61 |
| IDA | 93.44 | 86.67 | 81.25 | 83.87 | |
| HbE | 82.54 | 87.10 | 79.41 | 83.08 | |
| Combination | 80.95 | 0 | 0 | 0 | |
| Extreme Learning Machine | BTT | 100 | 100 | 100 | 100 |
| IDA | 98.44 | 100 | 93.75 | 96.77 | |
| HbE | 98.41 | 97.22 | 100 | 98.59 | |
| Combination | 100 | 100 | 100 | 100 |
| Authors | Year | Data Size | Number of Classes | Method | Accuracy (%) |
|---|---|---|---|---|---|
| Meena et al. [143] | 2019 | 259,627 | 4 | Decision Tree | 97.35 |
| Sow et al. [144] | 2019 | 6935 | 4 | ANN, SVM, RF, and NB | 94.74 |
| Laengsri et al. [67] | 2019 | 186 | 2 | DT, KNN, RF, ANN, and SVM | 98.03 |
| Ayyildiz and Tuncer [35] | 2019 | 342 | 2 | SVM and KNN | 96.20 |
| Kilicarslan et al. [74] | 2020 | 15,300 | 5 | GA-CNN and GA-SAE | 98.50 |
| Çil et al. [58] | 2020 | 342 | 2 | ELM, RELM, SVM, and KNN | 95.59 |
| Tyas et al. [145] | 2020 | 7108 | 9 | Multilayer Perceptron | 93.77 |
| De and Chakraborty [146] | 2021 | 200 | 2 | LR, RF, NB, MLP, DT, and KNN | 92.00 |
| Fu Yi-Kai et al. [147] | 2021 | 350 | 3 | Support Vector Machine | 76.00 |
| Dejene et al. [148] | 2022 | 11,174 | 4 | RF, Extreme Gradient Boosting, and Cat Boost | 97.56 |
| Memmolo et al. [149] | 2022 | 1000 | 2 | DT, DA, NB, SVM, KNN, and Ensemble Learning | 84.30 |
| Memmolo et al. [149] | 2022 | 1000 | 5 | DT, DA, NB, SVM, KNN, and Ensemble Learning | 69.50 |
| Islam et al. [30] | 2022 | 3020 | 2 | LR, LDA, KNN, SVM, QDA, NN, CART, and RF | 81.29 |
| Proposed Model | 2023 | 190 | 4 | ELM | 99.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saputra, D.C.E.; Sunat, K.; Ratnaningsih, T. A New Artificial Intelligence Approach Using Extreme Learning Machine as the Potentially Effective Model to Predict and Analyze the Diagnosis of Anemia. Healthcare 2023, 11, 697. https://doi.org/10.3390/healthcare11050697
Saputra DCE, Sunat K, Ratnaningsih T. A New Artificial Intelligence Approach Using Extreme Learning Machine as the Potentially Effective Model to Predict and Analyze the Diagnosis of Anemia. Healthcare. 2023; 11(5):697. https://doi.org/10.3390/healthcare11050697
Chicago/Turabian StyleSaputra, Dimas Chaerul Ekty, Khamron Sunat, and Tri Ratnaningsih. 2023. "A New Artificial Intelligence Approach Using Extreme Learning Machine as the Potentially Effective Model to Predict and Analyze the Diagnosis of Anemia" Healthcare 11, no. 5: 697. https://doi.org/10.3390/healthcare11050697
APA StyleSaputra, D. C. E., Sunat, K., & Ratnaningsih, T. (2023). A New Artificial Intelligence Approach Using Extreme Learning Machine as the Potentially Effective Model to Predict and Analyze the Diagnosis of Anemia. Healthcare, 11(5), 697. https://doi.org/10.3390/healthcare11050697








