Effectiveness of a Combined Toothbrushing Technique on Cariogenic Dental Biofilm in Relation to Stainless Steel and Elastomeric Ligatures in Orthodontic Patients: A Randomized Clinical Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants and Eligibility Criteria
2.3. Interventions
2.4. Outcomes
2.5. Sample Size Calculation
2.6. Randomization (Random Number Generation, Allocation Concealment, Implementation)
2.7. Blinding
2.8. Sample Description
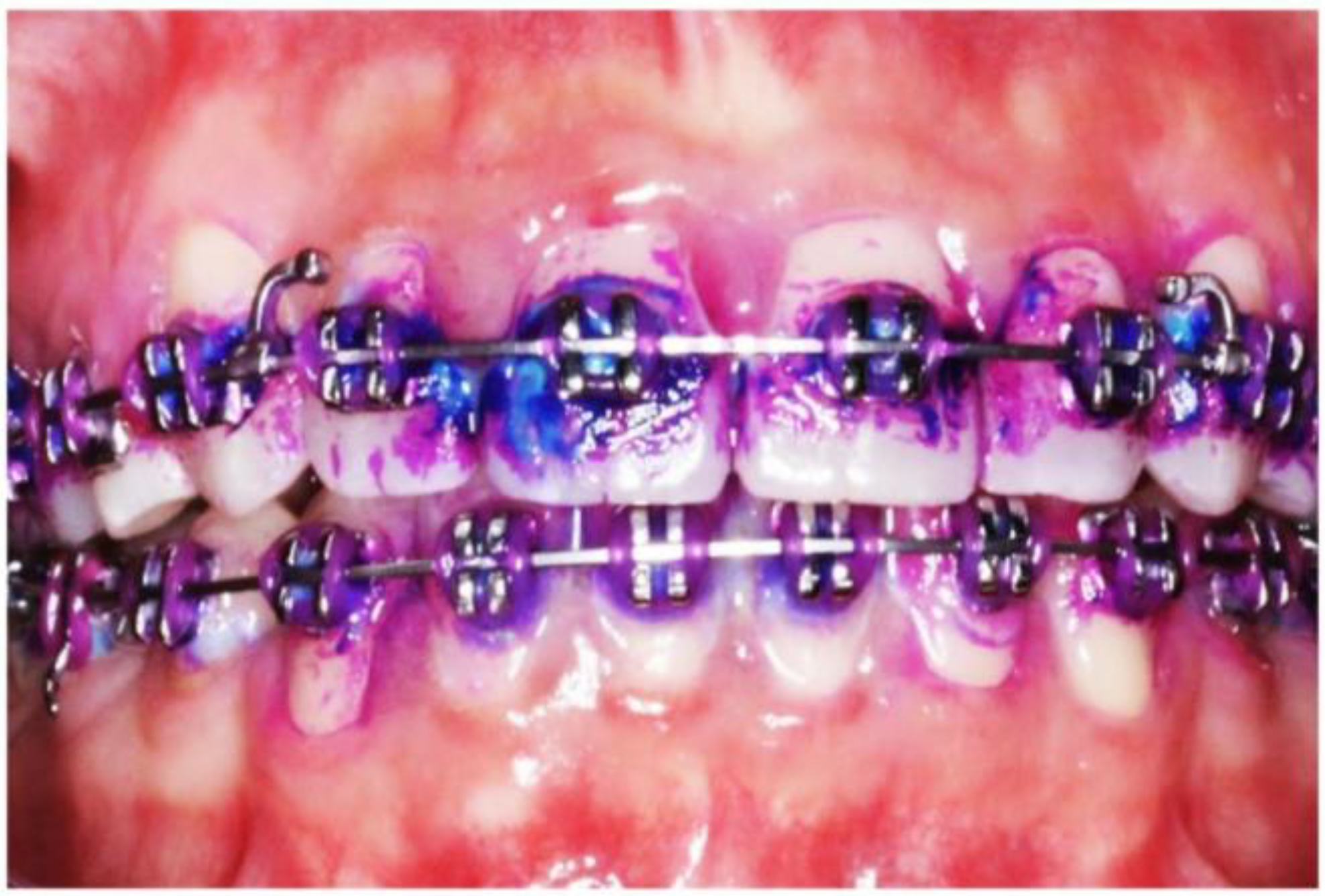
2.9. Dental Biofilm Maturity
2.10. Statistical Analysis
3. Results
3.1. Baseline Characteristics
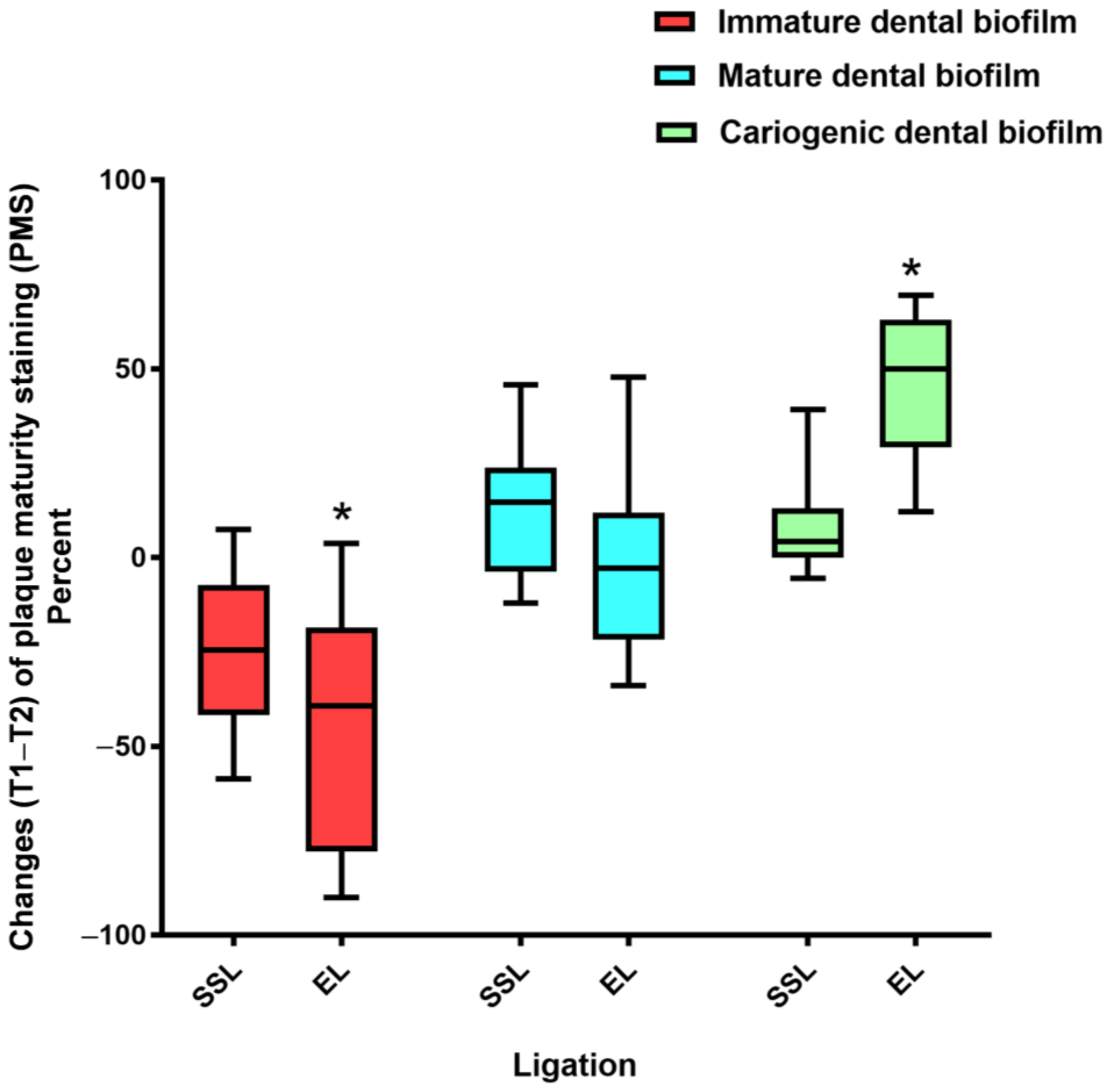
3.2. Effects of Toothbrushing on Dental Biofilm Maturity
3.3. Benefits and Harms
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marsh, P.D. Dental plaque as a biofilm and a microbial community—Implications for health and disease. BMC Oral Health 2006, 6, S14. [Google Scholar] [CrossRef] [Green Version]
- Ren, Y.; Jongsma, M.A.; Mei, L.; van der Mei, H.C.; Busscher, H.J. Orthodontic treatment with fixed appliances and biofilm formation—A potential public health threat? Clin. Oral Investig. 2014, 18, 1711–1718. [Google Scholar] [CrossRef] [PubMed]
- Freitas, A.O.; Marquezan, M.; Nojima Mda, C.; Alviano, D.S.; Maia, L.C. The influence of orthodontic fixed appliances on the oral microbiota: A systematic review. Dental Press J. Orthod. 2014, 19, 46–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulimani, P.; Popowics, T. Effect of orthodontic appliances on the oral environment and microbiome. Front. Dent. Med. 2022, 3, 924835. [Google Scholar] [CrossRef]
- Skilbeck, M.G.; Mei, L.; Mohammed, H.; Cannon, R.D.; Farella, M. The effect of ligation methods on biofilm formation in patients undergoing multi-bracketed fixed orthodontic therapy—A systematic review. Orthod. Craniofac. Res. 2021, 25, 14–30. [Google Scholar] [CrossRef] [PubMed]
- Arango Santander, S.; Luna Ossa, C.M. Stainless steel: Material facts for the orthodontic practitioner. Rev. Nac. Odontol. 2015, 11, 71–82. [Google Scholar] [CrossRef] [Green Version]
- Mapare, S.; Bansal, K.; Pawar, R.; Mishra, R.; Sthapak, A.; Khadri, S.F. Elastics and elastomeric in orthodontic practice. Int. J. Prev. Clin. Dent. Res. 2018, 5, S21–S30. [Google Scholar]
- Alves de Souza, R.; Borges de Araujo Magnani, M.B.; Nouer, D.F.; Oliveira da Silva, C.; Klein, M.I.; Sallum, E.A.; Gonçalves, R.B. Periodontal and microbiologic evaluation of 2 methods of archwire ligation: Ligature wires and elastomeric rings. Am. J. Orthod. Dentofacial Orthop. 2008, 134, 506–512. [Google Scholar] [CrossRef]
- Garcez, A.S.; Suzuki, S.S.; Ribeiro, M.S.; Mada, E.Y.; Freitas, A.Z.; Suzuki, H. Biofilm retention by 3 methods of ligation on orthodontic brackets: A microbiologic and optical coherence tomography analysis. Am. J. Orthod. Dentofacial Orthop. 2011, 140, e193–e198. [Google Scholar] [CrossRef]
- Forsberg, C.M.; Brattstrom, V.; Malmberg, E.; Nord, C.E. Ligature wires and elastomeric rings: Two methods of ligation, and their association with microbial colonization of Streptococcus mutans and lactobacilli. Eur. J. Orthod. 1991, 13, 416–420. [Google Scholar] [CrossRef]
- Bretas, S.M.; Macari, S.; Elias, A.M.; Ito, I.Y.; Matsumoto, M.A. Effect of 0.4% stannous fluoride gel on Streptococci mutans in relation to elastomeric rings and steel ligatures in orthodontic patients. Am. J. Orthod. Dentofacial Orthop. 2005, 127, 428–433. [Google Scholar] [CrossRef]
- Turkkahraman, H.; Sayin, M.O.; Bozkurt, F.Y.; Yetkin, Z.; Kaya, S.; Onal, S. Archwire ligation techniques, microbial colonization, and periodontal status in orthodontically treated patients. Angle Orthod. 2005, 75, 231–236. [Google Scholar] [PubMed]
- Walsh, L.J.; Healey, D.L. Prevention and caries risk management in teenage and orthodontic patients. Aust. Dent. J. 2019, 64, S37–S45. [Google Scholar] [CrossRef] [PubMed]
- Rajwani, A.R.; Hawes, S.N.D.; To, A.; Quaranta, A.; Rincon Aguilar, J.C. Effectiveness of manual toothbrushing techniques on plaque and gingivitis: A systematic review. Oral Health Prev. Dent. 2020, 18, 843–854. [Google Scholar] [PubMed]
- Wainwright, J.; Sheiham, A. An analysis of methods of toothbrushing recommended by dental associations, toothpaste and toothbrush companies and in dental texts. Br. Dent. J. 2014, 217, E5. [Google Scholar] [CrossRef]
- Bok, H.-J.; Lee, C.H. Proper tooth-brushing technique according to patient’s age and oral status. Int. J. Clin. Prev. Dent. 2020, 16, 149–153. [Google Scholar] [CrossRef]
- Nassar, P.O.; Bombardelli, C.G.; Walker, C.S.; Neves, K.V.; Tonet, K.; Nishi, R.N.; Bombonatti, R.; Nassar, C.A. Periodontal evaluation of different toothbrushing techniques in patients with fixed orthodontic appliances. Dental Press J. Orthod. 2013, 18, 76–80. [Google Scholar] [CrossRef] [Green Version]
- Bhatia, N.; Ahluwalia, R.; Grewal, S.; Bisht, D. Effect of modified bass brushing technique and habitual brushing on the carriage of oral microbes in patients with fixed orthodontic appliances-A comparative study. Eur. J. Pharm. Med. Res. 2019, 6, 418–427. [Google Scholar]
- Jongsma, M.A.; van de Lagemaat, M.; Busscher, H.J.; Geertsema-Doornbusch, G.I.; Atema-Smit, J.; van der Mei, H.C.; Ren, Y. Synergy of brushing mode and antibacterial use on in vivo biofilm formation. J. Dent. 2015, 43, 1580–1586. [Google Scholar] [CrossRef]
- Al-Anezi, S.A.; Harradine, N.W. Quantifying plaque during orthodontic treatment. Angle Orthod. 2012, 82, 748–753. [Google Scholar] [CrossRef]
- Brostek, A.M.; Walsh, L.J. Minimal intervention dentistry in general practice. Oral Health Dent. Manag. 2014, 13, 285–294. [Google Scholar] [PubMed]
- Thanetchaloempong, W.; Koontongkaew, S.; Utispan, K. Fixed orthodontic treatment increases cariogenicity and virulence gene expression in dental biofilm. J. Clin. Med. 2022, 11, 5860. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, B.; Dunn, L. The Declaration of Helsinki on Medical Research involving Human Subjects: A Review of seventh revision. J. Nepal Health Res. Counc. 2020, 17, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Schulz, K.F.; Altman, D.G.; Mother, D. CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. J. Pharmacol. Pharmacother. 2010, 1, 100–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lakens, D. Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Front. Psychol. 2013, 4, 863. [Google Scholar] [CrossRef] [Green Version]
- Singh, G. Randomization made easy for small size controlled clinical trials. J. Int. Assoc. Med. Sci. Educ. 2006, 16, 75–78. [Google Scholar]
- Heymann, G.C.; Grauer, D. A contemporary review of white spot lesions in orthodontics. J. Esthet. Restor. Dent. 2013, 25, 85–95. [Google Scholar] [CrossRef]
- Ngo, H.C.; Wolff, M.S.; Hume, W.R. Dental caries: Activity and risk assessments as a logical and effective path to both prevention and cure. In Preservation and Restoration of Tooth Structure, 3rd ed.; John Wiley and Sons: Chichester, UK, 2016; pp. 33–49. [Google Scholar]
- Gorelick, L.; Geiger, A.M.; Gwinnett, J. Incidence of white spot formation after bonding and banding. Am. J. Orthod. 1982, 81, 93–98. [Google Scholar] [CrossRef]
- Widhianingsih, D.; Koontongkaew, S. Enhancement of cariogenic virulence properties of dental plaque in asthmatics. J. Asthma 2021, 58, 1051–1057. [Google Scholar] [CrossRef]
- Quintanilha, L.E.L.P.; Vianna, R.B.C.; Luiz, R.R.; Maia, L.C.; Kelly, A.; Antonio, A.G. Reliability assessment of a plaque scoring index using photographs. Methods Inf. Med. 2018, 47, 443–447. [Google Scholar]
- Koo, T.K.; Li, M.Y. A Guideline of selecting and reporting intraclass correlation coefficients for reliability research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, P.; Pandey, C.M.; Singh, U.; Gupta, A.; Sahu, C.; Keshri, A. Descriptive statistics and normality tests for statistical data. Ann. Card. Anaesth. 2019, 22, 67–72. [Google Scholar] [PubMed]
- Lee, S.W. Methods for testing statistical differences between groups in medical research: Statistical standard and guideline of Life Cycle Committee. Life Cycle 2022, 2, e1. [Google Scholar] [CrossRef]
- Usha, C.; Sathyanarayanan, R. Dental caries—A complete changeover (Part I). J. Conserv. Dent. 2009, 12, 46–54. [Google Scholar] [CrossRef] [Green Version]
- Cvikl, B.; Lussi, A. Supragingival biofilm: Toothpaste and toothbrushes. Monogr. Oral Sci. 2021, 29, 65–73. [Google Scholar]
- Yu, O.Y.; Lam, W.Y.; Wong, A.W.; Duangthip, D.; Chu, C.H. Nonrestorative management of dental caries. Dent. J. 2021, 9, 121. [Google Scholar] [CrossRef]
- Claydon, N.C. Current concepts in toothbrushing and interdental cleaning. Periodontology 2000 2008, 48, 10–22. [Google Scholar] [CrossRef]
- Paraskevas, S.; Rosema, N.A.; Versteeg, P.; Timmerman, M.F.; van der Velden, U.; van der Weijden, G.A. The additional effect of a dentifrice on the instant efficacy of toothbrushing: A crossover study. J. Periodontol. 2007, 78, 1011–1016. [Google Scholar] [CrossRef]
- Kozak, U.; Lasota, A.; Chalas, R. Changes in distribution of dental biofilm after insertion of fixed orthodontic appliances. J. Clin. Med. 2021, 10, 5638. [Google Scholar] [CrossRef] [PubMed]
- Philip, N.; Suneja, B.; Walsh, L.J. Ecological approaches to dental caries prevention: Paradigm shift or shibboleth? Caries Res. 2018, 52, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Walsh, L.J. Dental plaque fermentation and its role in caries risk. Int. Dent. S. Afr. 2006, 8, 34–40. [Google Scholar]
- Popova, C.; Dosseva-Panova, V.; Panov, V. Microbiology of periodontal diseases. A review. Biotechnol. Biotechnol. Equip. 2014, 27, 3754–3759. [Google Scholar] [CrossRef]
- Teughels, W.; Van Assche, N.; Sliepen, I.; Quirynen, M. Effect of material characteristics and/or surface topography on biofilm development. Clin. Oral Imp. Res. 2006, 17 (Suppl. S2), 68–81. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, M.; Maaly, T.; El-Nagar, R. Evaluation of surface roughness and microbial biofilm adhesion of different orthodontic arch-wires. Egypt. Dent. J. 2020, 66, 727–736. [Google Scholar] [CrossRef]
- Arora, P.; Garg, H.; Bohidar, H.B. Surface topography and composition of As-received and-retrieved initial archwires: A comparative study. World J. Dent. 2019, 10, 144–149. [Google Scholar] [CrossRef]
- Harikrishnan, P.; Subha, T.S.; Kavitha, V.; Gnanamani, A. Microbial adhesion on orthodontic ligating materials: An in vitro assessment. Adv. Microbiol. 2013, 3, 108–114. [Google Scholar] [CrossRef] [Green Version]
- Atkinson, F.S.; Khan, J.H.; Brand-Miller, J.C.; Eberhard, J. The impact of carbohydrate quality on dental plaque pH: Does the glycemic index of starchy foods matter for dental health? Nutrients 2021, 13, 2711. [Google Scholar] [CrossRef]
- Moynihan, P.; Petersen, P.E. Diet, nutrition and the prevention of dental diseases. Public Health Nutr. 2004, 7, 201–226. [Google Scholar] [CrossRef] [Green Version]
- Kahan, B.C.; Cro, S.; Doré, C.J.; Bratton, D.J.; Rehal, S.; Maskell, N.A.; Rahman, N.; Jairath, V. Reducing bias in open-label trials where blinded outcome assessment is not feasibles: Strategies from two randomised trials. Trials 2014, 15, 456. [Google Scholar] [CrossRef] [Green Version]



| Variable | SSL | EL |
|---|---|---|
| Socio-demography | ||
| Age (years) | ||
| Median | 28 | 28 |
| Min–max | 13–43 | 15–53 |
| Sex | ||
| Male, n (%) | 5 (14.29) | 8 (22.86) |
| Female, n (%) | 30 (85.71) | 27 (71.14) |
| Educational level, n (%) | ||
| <Diploma | 11 (31.43) | 9 (25.71) |
| ≥Diploma | 24 (68.57) | 26 (74.29) |
| White spot lesion (%) | ||
| No [Median (min–max)] | 100 (83.33–100) | 96.42 (76.16–100) |
| Slight [Median (min–max)] | 0 (0–16.16) | 3.58 (0–16.16) |
| Severe [Median (min–max)] | 0 (0–4.16) | 0 (0–4.16) |
| Sugary intake between meals (time/day) | ||
| Median | 0.2 | 0.2 |
| Min-max | 0–2.8 | 0–1.4 |
| Food acid intake between meals (time/day) | ||
| Median | 0 | 0 |
| Min–max | 0–2.0 | 0–1.6 |
| Variable | SSL | EL |
|---|---|---|
| Systemic disease, n (%) | ||
| Yes | 0 (0) | 0 (0) |
| No | 35 (100) | 35 (100) |
| Medication-induced hyposalivation, n (%) | ||
| Yes | 0 (0) | 0 (0) |
| No | 35 (100) | 35 (100) |
| Dry mouth, n (%) | ||
| Yes | 0 (0) | 0 (0) |
| No | 35 (100) | 35 (100) |
| Difficulty swallowing, n (%) | ||
| Yes | 0 (0) | 0 (0) |
| No | 35 (100) | 35 (100) |
| Feeling thirsty, n (%) | ||
| Yes | 0 (0) | 0 (0) |
| No | 35 (100) | 35 (100) |
| Toothbrushing frequency, n (%) | ||
| <2 times a day | 2 (5.72) | 2 (5.72) |
| ≥2 times a day | 33 (94.28) | 33 (94.28) |
| Occasion of toothbrushing, n (%) | ||
| In the morning | 34 (97.14) | 32 (91.43) |
| After lunch | 34 (97.14) | 32 (91.43) |
| Before bedtime | 34 (97.14) | 32 (91.43) |
| Other times | 32 (91.43) | 31 (88.57) |
| Toothbrushing technique, n (%) | ||
| Horizontal, vertical, or circular | 4 (11.43) | 7 (20) |
| Combined | 31 (88.57) | 28 (80) |
| Toothbrushing time, n (%) | ||
| <2 min | 8 (22.86) | 5 (14.29) |
| ≥2 min | 27 (77.14) | 30 (85.71) |
| Use of dental floss, n (%) | ||
| Yes | 26 (74.29) | 29 (82.86) |
| No | 9 (25.71) | 6 (17.14) |
| Use of interdental brush, n (%) | ||
| Yes | 25 (71.43) | 33 (94.29) |
| No | 10 (28.57) | 2 (5.71) |
| Use of other oral healthcare products, n (%) | ||
| Yes | 3 (8.58) | 7 (20) |
| No | 32 (91.42) | 28 (80) |
| Use of mouthwash, n (%) | ||
| Yes | 20 (57.14) | 26 (74.29) |
| No | 15 (42.86) | 9 (25.71) |
| Professional fluoride application, n (%) | ||
| Yes | 4 (11.43) | 8 (22.86) |
| No | 31 (88.57) | 27 (77.14) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saengphen, T.; Koontongkaew, S.; Utispan, K. Effectiveness of a Combined Toothbrushing Technique on Cariogenic Dental Biofilm in Relation to Stainless Steel and Elastomeric Ligatures in Orthodontic Patients: A Randomized Clinical Trial. Healthcare 2023, 11, 731. https://doi.org/10.3390/healthcare11050731
Saengphen T, Koontongkaew S, Utispan K. Effectiveness of a Combined Toothbrushing Technique on Cariogenic Dental Biofilm in Relation to Stainless Steel and Elastomeric Ligatures in Orthodontic Patients: A Randomized Clinical Trial. Healthcare. 2023; 11(5):731. https://doi.org/10.3390/healthcare11050731
Chicago/Turabian StyleSaengphen, Thanakorn, Sittichai Koontongkaew, and Kusumawadee Utispan. 2023. "Effectiveness of a Combined Toothbrushing Technique on Cariogenic Dental Biofilm in Relation to Stainless Steel and Elastomeric Ligatures in Orthodontic Patients: A Randomized Clinical Trial" Healthcare 11, no. 5: 731. https://doi.org/10.3390/healthcare11050731
APA StyleSaengphen, T., Koontongkaew, S., & Utispan, K. (2023). Effectiveness of a Combined Toothbrushing Technique on Cariogenic Dental Biofilm in Relation to Stainless Steel and Elastomeric Ligatures in Orthodontic Patients: A Randomized Clinical Trial. Healthcare, 11(5), 731. https://doi.org/10.3390/healthcare11050731







