A Novel Approach of Periodontal Osseous Wall Piezosplitting and Sequential Bone Expansion in Management of Localized Intra-Bony Defects with Wide Angulation—A Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Consideration and Patient Recruitment
2.2. Presurgical Therapy and Grouping
2.3. Randomization and Allocation Concealment
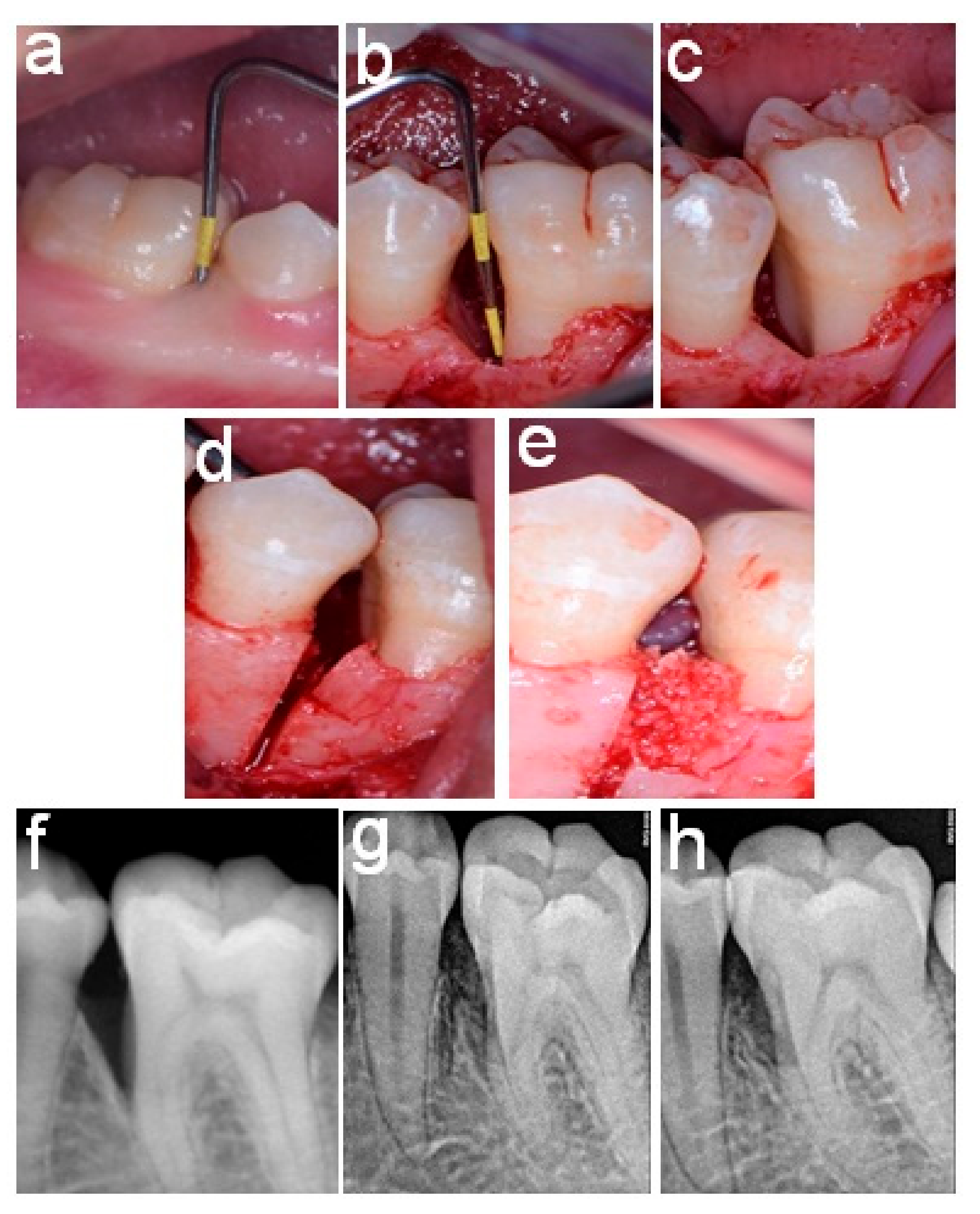
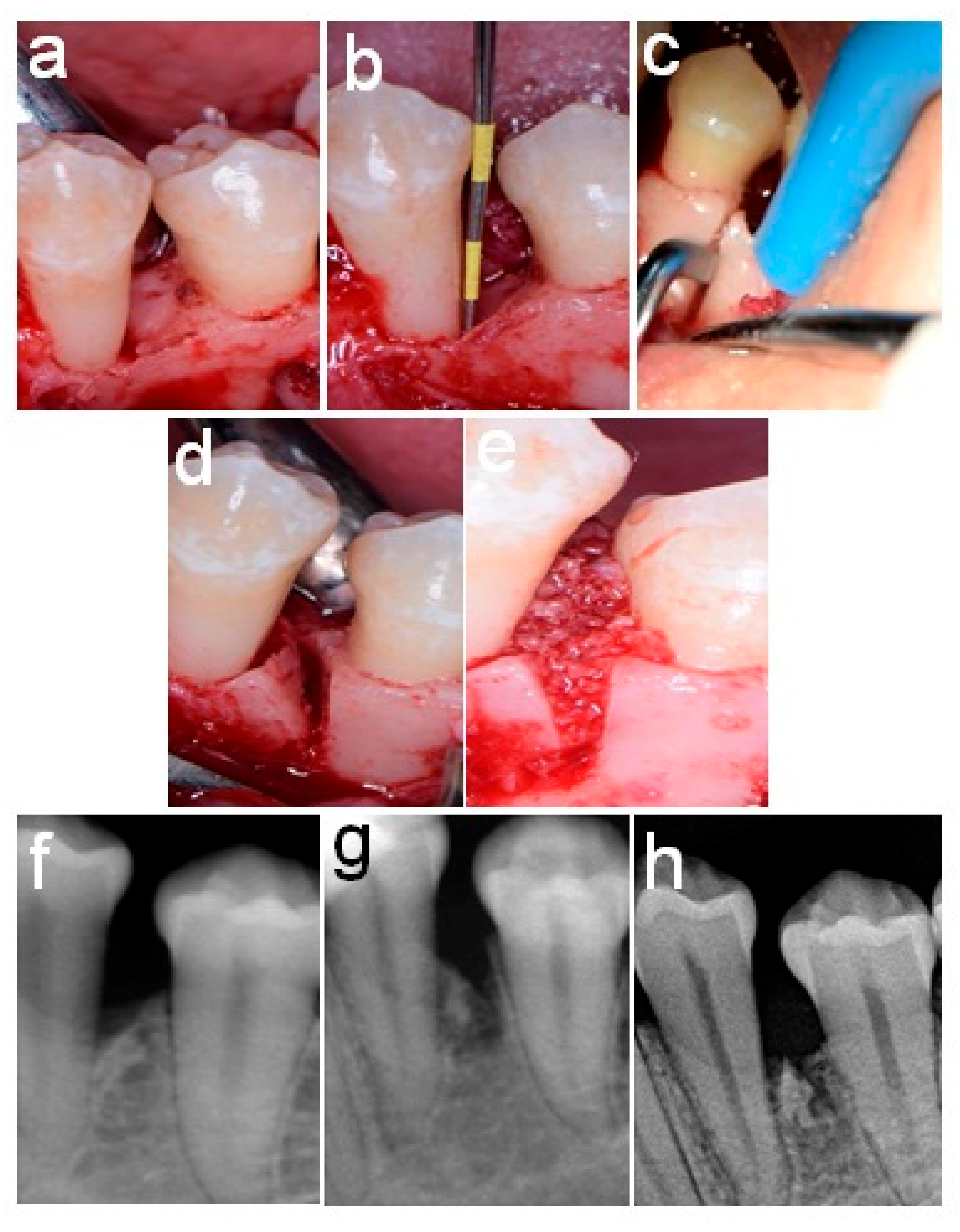
2.4. Surgical Procedures
2.5. Postoperative Care
2.6. Follow-Up and Re-Evaluation
2.7. Post-Surgical Measurements
2.8. Primary and Secondary Outcome Measures
2.9. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gamal, A.Y.; Mailhot, J.M. The effects of EDTA gel conditioning exposure time on periodontitis-affected human root surfaces: Surface topography and PDL cell adhesion. J. Int. Acad. Periodontol. 2003, 5, 11–22. [Google Scholar] [PubMed]
- Nilvféus, R.; Egelberg, J. The effect of topical citric acid application on the healing of experimental furcation defects in dogs. J. Periodontal Res. 1980, 15, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Wikesjö, U.M.; Crigger, M.; Nilvéus, R.; Selvig, K.A. Early healing events at the dentin-connective tissue interface. Light and transmission electron microscopy observations. J. Periodontol. 1991, 62, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Moraschini, V.; Fujioka-Kobayashi, M.; Zhang, Y.; Kawase, T.; Cosgarea, R.; Jepsen, S.; Bishara, M.; Canullo, L.; Shirakata, Y.; et al. Use of platelet-rich fibrin for the treatment of periodontal intrabony defects: A systematic review and meta-analysis. Clin. Oral Investig. 2021, 25, 2461–2478. [Google Scholar] [CrossRef]
- Kao, R.T.; Nares, S.; Reynolds, M.A. Periodontal regeneration—Intrabony defects: A systematic review from the AAP Regeneration Workshop. J. Periodontol. 2015, 86, S77–S104. [Google Scholar] [CrossRef]
- Aukhil, I. Biology of wound healing. Periodontol. 2000 2000, 22, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Caton, J.G.; Greenstein, G. Factors related to periodontal regeneration. Periodontol. 2000 1993, 1, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Eickholz, P.; Hörr, T.; Klein, F.; Hassfeld, S.; Kim, T.S. Radiographic parameters for prognosis of periodontal healing of infrabony defects: Two different definitions of defect depth. J. Periodontol. 2004, 75, 399–407. [Google Scholar] [CrossRef]
- Tsitoura, E.; Tucker, R.; Suvan, J.; Laurell, L.; Cortellini, P.; Tonetti, M. Baseline radiographic defect angle of the intrabony defect as a prognostic indicator in regenerative periodontal surgery with enamel matrix derivative. J. Clin. Periodontol. 2004, 31, 643–647. [Google Scholar] [CrossRef]
- Cortellini, P.; Tonetti, M. Radiographic Defect Angle Influences the Outcomes of GTR Therapy in Intrabony Defects. In Proceedings of the 77th General Session of the IADR, Vancouver, BC, Canada, 10–13 March 1999. [Google Scholar]
- Gamal, A.Y.; Abdel Ghaffar, K.A.; Alghezwy, O.A. Crevicular Fluid Growth Factors Release Profile Following the Use of Platelet-Rich Fibrin and Plasma Rich Growth Factors in Treating Periodontal Intrabony Defects: A Randomized Clinical Trial. J. Periodontol. 2016, 87, 654–662. [Google Scholar] [CrossRef]
- Gamal, A.Y.; Abdel-Ghaffar, K.A.; Zouair, M.G.; Salama, M.H.; El Destawy, M.T. Dimensional evaluation of blood clot gap distances within intrabony defects following grafting and EDTA root surface treatment-experimental study in dogs. J. Periodontol. 2018, 89, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Minenna, L.; Herrero, F.; Sanz, M.; Trombelli, L. Adjunctive effect of a polylactide/polyglycolide copolymer in the treatment of deep periodontal intra-osseous defects: A randomized clinical trial. J. Clin. Periodontol. 2005, 32, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Nevins, M.; Camelo, M.; Nevins, M.L.; Schenk, R.K.; Lynch, S.E. Periodontal regeneration in humans using recombinant human platelet-derived growth factor-BB (rhPDGF-BB) and allogenic bone. J. Periodontol. 2003, 74, 1282–1292. [Google Scholar] [CrossRef]
- Gamal, A.Y.; Mailhot, J.M. Effects of EDTA gel preconditioning of periodontally affected human root surfaces on chlorhexidine substantivity—An SEM study. J. Periodontol. 2007, 78, 1759–1766. [Google Scholar] [CrossRef]
- Esposito, M.; Grusovin, M.G.; Papanikolaou, N.; Coulthard, P.; Worthington, H.V. Enamel matrix derivative (Emdogain) for periodontal tissue regeneration in intrabony defects. A Cochrane systematic review. Eur. J. Oral Implantol. 2009, 2, 247–266. [Google Scholar] [PubMed]
- Passanezi, E.; Damante, C.A.; de Rezende, M.L.; Greghi, S.L. Lasers in periodontal therapy. Periodontol. 2000 2015, 67, 268–291. [Google Scholar] [CrossRef]
- Ewen, S.J. Bone Swaging. J. Periodontol. 1965, 36, 57–63. [Google Scholar] [CrossRef]
- Ross, S.E.; Malamed, E.H.; Amsterdam, M. The contiguous autogenous transplant—Its rationale, indications and technique. Periodontics 1966, 4, 246–255. [Google Scholar]
- Kodama, T.; Minabe, M.; Sugiyama, T.; Mitarai, E.; Fushimi, H.; Kitsugi, D.; Tsutsumi, K.; Katsuki, M. Guided tissue regeneration using a collagen barrier and bone swaging technique in noncontained infrabony defects. Int. J. Periodontics Restor. Dent. 2013, 33, 805–812. [Google Scholar] [CrossRef]
- Zubery, Y.; Kozlovsky, A.; Tal, H. Histologic assessment of a contiguous autogenous transplant in a human intrabony defect. A case report. J. Periodontol. 1993, 64, 66–71. [Google Scholar] [CrossRef]
- Armitage, G.C. Development of a classification system for periodontal diseases and conditions. Ann. Periodontol. 1999, 4, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Natto, Z.S.; Abu Ahmad, R.H.; Alsharif, L.T.; Alrowithi, H.F.; Alsini, D.A.; Salih, H.A.; Bissada, N.F. Chronic Periodontitis Case Definitions and Confounders in Periodontal Research: A Systematic Assessment. BioMed Res. Int. 2018, 2018, 4578782. [Google Scholar] [CrossRef] [PubMed]
- Silness, J.; Loe, H. Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condition. Acta Odontol. Scand. 1964, 22, 121–135. [Google Scholar] [CrossRef]
- Loe, H.; Silness, J. Periodontal disease in pregnancy. i. prevalence and severity. Acta Odontol. Scand. 1963, 21, 533–551. [Google Scholar] [CrossRef]
- Polson, A.M.; Caton, J.G.; Yeaple, R.N.; Zander, H.A. Histological determination of probe tip penetration into gingival sulcus of humans using an electronic pressure-sensitive probe. J. Clin. Periodontol. 1980, 7, 479–488. [Google Scholar] [CrossRef]
- Ramfjord, S.P. The Periodontal Disease Index (PDI). J. Periodontol. 1967, 38, 602–610. [Google Scholar] [CrossRef]
- Becker, W.; Becker, B.E. Periodontal regeneration: A contemporary re-evaluation. Periodontol. 2000 1999, 19, 104–114. [Google Scholar] [CrossRef]
- Sculean, A.; Stavropoulos, A.; Windisch, P.; Keglevich, T.; Karring, T.; Gera, I. Healing of human intrabony defects following regenerative periodontal therapy with a bovine-derived xenograft and guided tissue regeneration. Clin. Oral Investig. 2004, 8, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Shirakata, Y.; Yoshimoto, T.; Takeuchi, N.; Taniyama, K.; Noguchi, K. Effects of EMD in combination with bone swaging and calcium phosphate bone cement on periodontal regeneration in one-wall intrabony defects in dogs. J. Periodontal Res. 2013, 48, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Hennet, P. Piezoelectric Bone Surgery: A Review of the Literature and Potential Applications in Veterinary Oromaxillofacial Surgery. Front. Vet. Sci. 2015, 2, 8. [Google Scholar] [CrossRef]
- Lin, K.Y.; Bartlett, S.P.; Yaremchuk, M.J.; Fallon, M.; Grossman, R.F.; Whitaker, L.A. The effect of rigid fixation on the survival of onlay bone grafts: An experimental study. Plast. Reconstr. Surg. 1990, 86, 449–456. [Google Scholar] [CrossRef]
- Polson, A.M.; Proye, M.P. Fibrin linkage: A precursor for new attachment. J. Periodontol. 1983, 54, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Wikesjö, U.M.; Selvig, K.A. Periodontal wound healing and regeneration. Periodontol. 2000 1999, 19, 21–39. [Google Scholar] [CrossRef]
- Nevins, M.L.; Camelo, M.; Lynch, S.E.; Schenk, R.K.; Nevins, M. Evaluation of periodontal regeneration following grafting intrabony defects with bio-oss collagen: A human histologic report. Int. J. Periodontics Restor. Dent. 2003, 23, 9–17. [Google Scholar]
- Stahl, S.S.; Froum, S. Histological evaluation of human intraosseous healing responses to the placement of tricalcium phosphate ceramic implants. I. Three to eight months. J. Periodontol. 1986, 57, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Gamal, A.Y. Enhanced β-tricalcium phosphate blended clot adhesion to EDTA biomodulated periodontally affected root surfaces: In vivo scanning electron microscopy evaluation. J. Periodontol. 2011, 82, 1587–1595. [Google Scholar] [CrossRef] [PubMed]
- Shirakata, Y.; Taniyama, K.; Yoshimoto, T.; Takeuchi, N.; Noguchi, K. Effect of bone swaging with calcium phosphate bone cement on periodontal regeneration in dogs. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 114, 35–42. [Google Scholar] [CrossRef]
- Alberius, P.; Dahlin, C.; Linde, A. Role of osteopromotion in experimental bone grafting to the skull: A study in adult rats using a membrane technique. J. Oral Maxillofac. Surg. 1992, 50, 829–834. [Google Scholar] [CrossRef]
- Donos, N.; Kostopoulos, L.; Karring, T. Augmentation of the rat jaw with autogeneic cortico-cancellous bone grafts and guided tissue regeneration. Clin. Oral Implant. Res. 2002, 13, 192–202. [Google Scholar] [CrossRef]
- Donos, N.; Kostopoulos, L.; Tonetti, M.; Karring, T. Long-term stability of autogenous bone grafts following combined application with guided bone regeneration. Clin. Oral Implant. Res. 2005, 16, 133–139. [Google Scholar] [CrossRef] [PubMed]

| Patient No. | Group 1 | Group 2 | ||||
|---|---|---|---|---|---|---|
| Tooth Number and Surface | Defect Type | Bony Walls Present | Tooth Number and Surface | Defect Type | Bony Walls Present | |
| 1 | 5 M | 2-Wall | DL | 5 M | 2-Wall | BLD |
| 2 | 4 M | 3-Wall | BLD | 13 M | 2-Wall | BL |
| 3 | 3 M | 3-Wall | BLD | 14 D | 3-Wall | BLD |
| 4 | 20 D | 2-Wall | BL | 14 M | 2-Wall | BM |
| 5 | 19 M | 2-Wall | BD | 28 M | 3-Wall | BLD |
| 6 | 30 M | 3-Wall | BLD | 29 D | 3-Wall | BLD |
| 7 | 30 M | 2-Wall | MDL | 30 D | 3-Wall | BLD |
| 8 | 31 D | 2-Wall | BD | 21 D | 2-Wall | BD |
| Parameter | Group 1 | Group 2 | ||||
|---|---|---|---|---|---|---|
| Baseline | 6 Months | p-Value | Baseline | 6 Months | p-Value | |
| Clinical parameters | ||||||
| PD (mm) | 6.2 ± 0.3 a | 3.6 ± 0.4 b | <0.001 * | 5.9 ± 0.6 a | 2.4 ± 0.4 b | <0.001 * |
| CAL (mm) | 5.7 ± 0.5 a | 3.7 ± 0.5 b | <0.001 * | 5.1 ± 0.4 a | 2.2 ± 0.3 b | <0.001 * |
| PI | 0.4 ± 0.1 | 0.3 ± 0.2 | 0.427 | 0.5 ± 0.2 | 0.5 ± 0.2 | 0.233 |
| GI | 0.4 ± 03 | 0.5 ± 0.2 | 0.521 | 0.4 ± 0.2 | 0.3 ± 0.3 | 0.239 |
| Radiographic parameters | ||||||
| DBL (mm) | 8.7 ± 0.6 a | 6.7 ± 1 b | <0.001 * | 9.2 ± 0.7 a | 3.1 ± 0.4 b | <0.001 * |
| CBL (mm) | 5.6 ± 0.7 b | 6.5 ± 1.2 a | 0.02 * | 5.8 ± 0.8 a | 3.2 ± 0.3 b | <0.001 * |
| CIBW | 1.2 ± 0.3 | 1.4 ± 0.1 | 0.233 | 1.4 ± 0.3 a | 3.4 ± 0.4 b | <0.001 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Destawy, M.T.; Khedr, M.F.; Hosny, M.M.; Bilal, A.M.; Elshamy, A.M.; El sayed, I.S.; Borhamy, A.e.-l.g.; Aboamo, A.a.-a.k.; Gamal, A.Y. A Novel Approach of Periodontal Osseous Wall Piezosplitting and Sequential Bone Expansion in Management of Localized Intra-Bony Defects with Wide Angulation—A Randomized Controlled Trial. Healthcare 2023, 11, 791. https://doi.org/10.3390/healthcare11060791
El-Destawy MT, Khedr MF, Hosny MM, Bilal AM, Elshamy AM, El sayed IS, Borhamy Ae-lg, Aboamo Aa-ak, Gamal AY. A Novel Approach of Periodontal Osseous Wall Piezosplitting and Sequential Bone Expansion in Management of Localized Intra-Bony Defects with Wide Angulation—A Randomized Controlled Trial. Healthcare. 2023; 11(6):791. https://doi.org/10.3390/healthcare11060791
Chicago/Turabian StyleEl-Destawy, Mahmoud Taha, Mohamed Fekry Khedr, Mostafa Mohamed Hosny, Ahmed Mohamed Bilal, Ahmed Mohamed Elshamy, Ibrahim Sabry El sayed, Abd el-latif galal Borhamy, Abd al-aziz kamal Aboamo, and Ahmed Yousef Gamal. 2023. "A Novel Approach of Periodontal Osseous Wall Piezosplitting and Sequential Bone Expansion in Management of Localized Intra-Bony Defects with Wide Angulation—A Randomized Controlled Trial" Healthcare 11, no. 6: 791. https://doi.org/10.3390/healthcare11060791
APA StyleEl-Destawy, M. T., Khedr, M. F., Hosny, M. M., Bilal, A. M., Elshamy, A. M., El sayed, I. S., Borhamy, A. e.-l. g., Aboamo, A. a.-a. k., & Gamal, A. Y. (2023). A Novel Approach of Periodontal Osseous Wall Piezosplitting and Sequential Bone Expansion in Management of Localized Intra-Bony Defects with Wide Angulation—A Randomized Controlled Trial. Healthcare, 11(6), 791. https://doi.org/10.3390/healthcare11060791





