Alzheimer’s Disease and Alzheimer’s Disease-Related Dementias in African Americans: Focus on Caregivers
Abstract
1. Introduction
1.1. Amyloid Beta (Aβ)
1.2. Tau Protein
1.3. Mitochondria and AD/ADRD
1.4. Mitophagy and AD/ADRD
2. Alzheimer’s Disease and Alzheimer’s Disease Related Dementias
3. African Americans—Origins and Background
African Americans—A Historical Background on Systematic Inequalities
4. Risk Factors/Susceptibility of AD in African Americans
4.1. Lifestyle, Diet, and Environmental Factors on AD/ADRD
4.2. Lifestyle, Diet, and Environmental Factors on AD/ADRD in African Americans
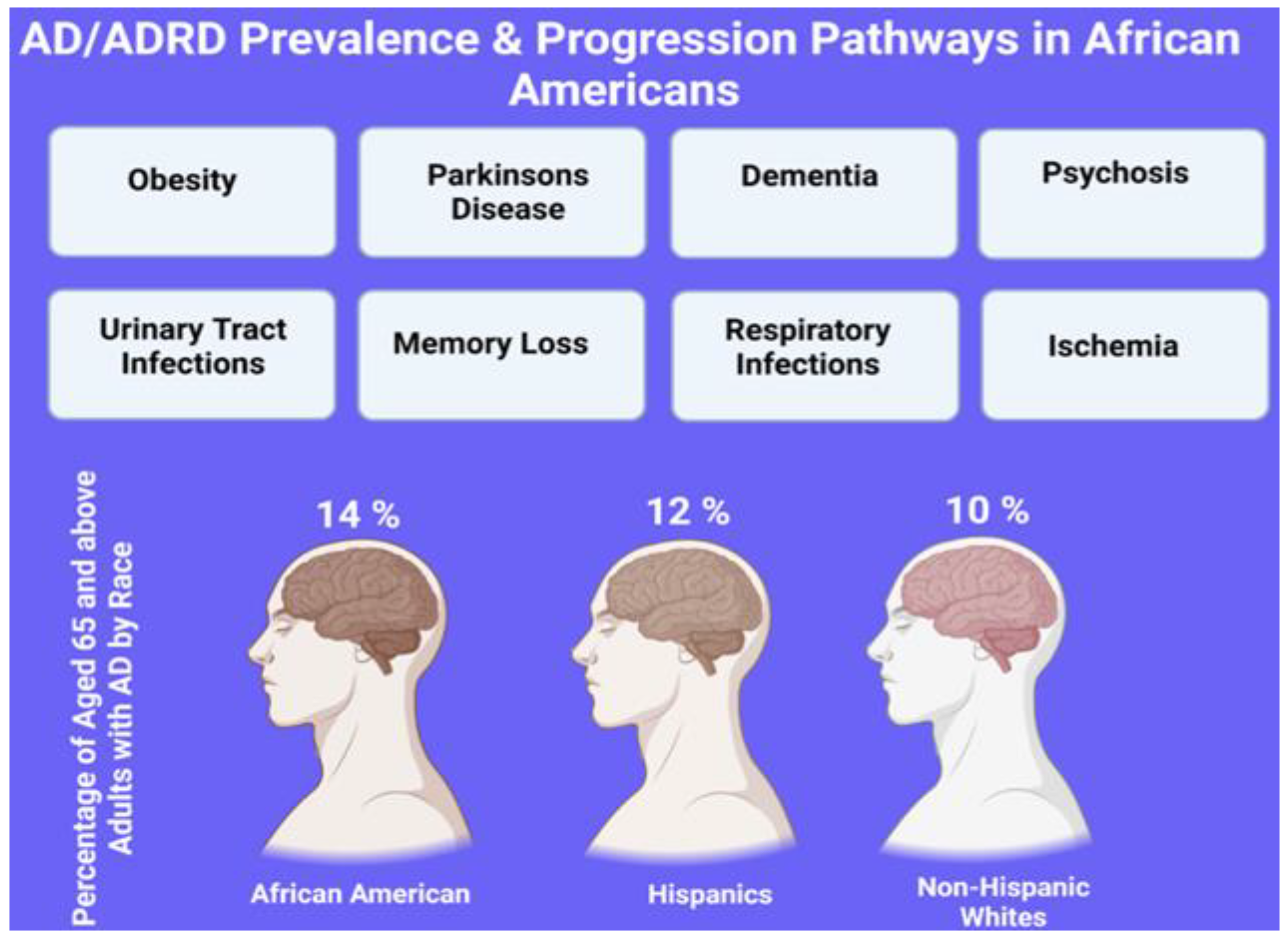
4.3. Prevalence of AD in African Americans
4.4. Status of Caregiving in AA and AD/ADRD
| Author, Year | Study Type | AA Sample Size Caregivers | Results |
|---|---|---|---|
| Lindauer et al. [161], 2015 | Interview | 22 |
|
| McLennon et al. [170], 2020 | Interview | 12 |
|
| Roth et al. [163], 2001 | Interview | 197 |
|
| Owen et al. [164], 2001 | Interview | 63 |
|
| Disbrow et al. [165], 2021 | Interview | 46 |
|
| Spurlock et al. [166], 2005 | Interview | 71 |
|
| Heo et al. [171], 2013 | Interview | 211 |
|
| Epps et al. [172], 2020 | Training Intervention | 202 |
|
| Wilks et al. [169], 2018 | Interview | 230 |
|
| Knight et al. [173], 2000 | Interview | 41 |
|
| Dilworth-Anderson et al. [174] 2005 | Interview | 48 |
|
| Samson et al. [175], 2016 | Interview | 32 |
|
| Dilworth-Anderson et al. [176], 2007 | Interview/Qualitative Study | 303 |
|
| Turner et al. [177], 2004 | Interview | 88 |
|
| Cothran et al. [178], 2022 | Interview | 21 |
|
| Guest et al. [179], 2021 | Intervention | 87 |
|
| Czaja et al. [180], 2013 | Intervention | 54 |
|
| Brewster et al. [181], 2020 | Interview | 142 |
|
| Wells et al. [182], 2017 | Randomized Control Trial | 109 |
|
4.5. Caregiving and AD/ADRD
4.6. Social, Cultural, and Religious Factors
4.7. Family Structure
4.8. Interventions (Education; Telemedicine; Sociological; Psychological)
4.9. Alzheimer’s Association Survey on Racial Disparities in AD among U.S. Adults
4.10. Alzheimer’s Association Survey on Racial Disparities among AD Caregivers
5. Recommendations from the Alzheimer’s Association Survey on Racial Disparities in AD and Caregivers
6. Reducing Racial Disparities
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- DeTure, M.A.; Dickson, D.W. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 32. [Google Scholar] [CrossRef]
- Jiang, T.; Yu, J.T.; Tian, Y.; Tan, L. Epidemiology and etiology of Alzheimer’s disease: From genetic to non-genetic factors. Curr. Alzheimer Res. 2013, 10, 852–867. [Google Scholar] [CrossRef]
- Kamboh, M.I. Molecular genetics of late-onset Alzheimer’s disease. Ann. Hum. Genet. 2004, 68, 381–404. [Google Scholar] [CrossRef]
- Sehar, U.; Rawat, P.; Reddy, A.P.; Kopel, J.; Reddy, P.H. Amyloid beta in aging and Alzheimer’s disease. Int. J. Mol. Sci. 2022, 23, 12924. [Google Scholar] [CrossRef] [PubMed]
- Citron, M. Alzheimer’s disease: Strategies for disease modification. Nat. Rev. Drug Discov. 2010, 9, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Rawat, P.; Sehar, U.; Bisht, J.; Selman, A.; Culberson, J.; Reddy, P.H. Phosphorylated tau in Alzheimer’s disease and other tauopathies. Int. J. Mol. Sci. 2022, 23, 12841. [Google Scholar] [CrossRef] [PubMed]
- Fleischman, D.A.; Wilson, R.S.; Gabrieli, J.D.; Schneider, J.A.; Bienias, J.L.; Bennett, D.A. Implicit memory and Alzheimer’s disease neuropathology. Brain 2005, 128, 2006–2015. [Google Scholar] [CrossRef]
- Fan, L.; Mao, C.; Hu, X.; Zhang, S.; Yang, Z.; Hu, Z.; Sun, H.; Fan, Y.; Dong, Y.; Yang, J.; et al. New Insights Into the Pathogenesis of Alzheimer’s Disease. Front. Neurol. 2019, 10, 1312. [Google Scholar] [CrossRef]
- Sakamoto, S.; Ishii, K.; Sasaki, M.; Hosaka, K.; Mori, T.; Matsui, M.; Hirono, N.; Mori, E. Differences in cerebral metabolic impairment between early and late onset types of Alzheimer’s disease. J. Neurol. Sci. 2002, 200, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Alzheimer’s Association. Race, Ethnicity and Alzheimer’s in America. Alzheimers Dement. 2021, 17. [Google Scholar]
- Kopel, J.; Singh, S.P.; Hindle, A.; Quirch, M.; Bose, C.; Awasthi, S. Rlip Protein: A Potential Target for COVID-19. J. Community Hosp. Intern. Med. Perspect. 2022, 12, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Guo, Z. Alzheimer’s Aβ42 and Aβ40 peptides form interlaced amyloid fibrils. J. Neurochem. 2013, 126, 305–311. [Google Scholar] [CrossRef]
- Puzzo, D.; Privitera, L.; Leznik, E.; Fà, M.; Staniszewski, A.; Palmeri, A.; Arancio, O. Picomolar amyloid-beta positively modulates synaptic plasticity and memory in hippocampus. J. Neurosci. 2008, 28, 14537–14545. [Google Scholar] [CrossRef] [PubMed]
- Sadigh-Eteghad, S.; Sabermarouf, B.; Majdi, A.; Talebi, M.; Farhoudi, M.; Mahmoudi, J. Amyloid-beta: A crucial factor in Alzheimer’s disease. Med. Princ. Pract. 2015, 24, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.V.; Bailey, C.D. The p38 MAP kinase signaling pathway in Alzheimer’s disease. Exp. Neurol. 2003, 183, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Kummer, M.P.; Heneka, M.T. Truncated and modified amyloid-beta species. Alzheimers Res. Ther. 2014, 6, 28. [Google Scholar] [CrossRef] [PubMed]
- Götz, J.; Streffer, J.R.; David, D.; Schild, A.; Hoerndli, F.; Pennanen, L.; Kurosinski, P.; Chen, F. Transgenic animal models of Alzheimer’s disease and related disorders: Histopathology, behavior and therapy. Mol. Psychiatry 2004, 9, 664–683. [Google Scholar] [CrossRef] [PubMed]
- Stepler, K.E.; Gillyard, T.R.; Reed, C.B.; Avery, T.M.; Davis, J.S.; Robinson, R.A.S. ABCA7, a Genetic Risk Factor Associated with Alzheimer’s Disease Risk in African Americans. J. Alzheimer’s Dis. 2022, 86, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, C.; McCann, H.; Halliday, G.M. Variations in the neuropathology of familial Alzheimer’s disease. Acta Neuropathol. 2009, 118, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J. Alzheimer’s disease: Genes, proteins, and therapy. Physiol. Rev. 2001, 81, 741–766. [Google Scholar] [CrossRef]
- Goate, A.; Chartier-Harlin, M.C.; Mullan, M.; Brown, J.; Crawford, F.; Fidani, L.; Giuffra, L.; Haynes, A.; Irving, N.; James, L.; et al. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer’s disease. Nature 1991, 349, 704–706. [Google Scholar] [CrossRef]
- de la Torre, J.C. Three postulates to help identify the cause of Alzheimer’s disease. J. Alzheimers Dis. 2011, 24, 657–668. [Google Scholar] [CrossRef]
- Sims, R.; Hill, M.; Williams, J. The multiplex model of the genetics of Alzheimer’s disease. Nat. Neurosci. 2020, 23, 311–322. [Google Scholar] [CrossRef]
- Kelly, J.; Moyeed, R.; Carroll, C.; Luo, S.; Li, X. Genetic networks in Parkinson’s and Alzheimer’s disease. Aging 2020, 12, 5221–5243. [Google Scholar] [CrossRef] [PubMed]
- Michalicova, A.; Majerova, P.; Kovac, A. Tau Protein and Its Role in Blood-Brain Barrier Dysfunction. Front. Mol. Neurosci. 2020, 13, 570045. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Neve, R.L.; Kosik, K.S. The microtubule binding domain of tau protein. Neuron 1989, 2, 1615–1624. [Google Scholar] [CrossRef]
- Ballatore, C.; Lee, V.M.; Trojanowski, J.Q. Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat. Rev. Neurosci. 2007, 8, 663–672. [Google Scholar] [CrossRef]
- Hong, M.; Zhukareva, V.; Vogelsberg-Ragaglia, V.; Wszolek, Z.; Reed, L.; Miller, B.I.; Geschwind, D.H.; Bird, T.D.; McKeel, D.; Goate, A.; et al. Mutation-specific functional impairments in distinct tau isoforms of hereditary FTDP-17. Science 1998, 282, 1914–1917. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Cao, Y.; Ma, L.; Wei, Y.; Li, H. Possible Mechanisms of Tau Spread and Toxicity in Alzheimer’s Disease. Front. Cell Dev. Biol. 2021, 9, 707268. [Google Scholar] [CrossRef]
- d’Errico, P.; Meyer-Luehmann, M. Mechanisms of Pathogenic Tau and Aβ Protein Spreading in Alzheimer’s Disease. Front. Aging Neurosci. 2020, 12, 265. [Google Scholar] [CrossRef]
- Hagen, T.M.; Yowe, D.L.; Bartholomew, J.C.; Wehr, C.M.; Do, K.L.; Park, J.Y.; Ames, B.N. Mitochondrial decay in hepatocytes from old rats: Membrane potential declines, heterogeneity and oxidants increase. Proc. Natl. Acad. Sci. USA 1997, 94, 3064–3069. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zucker, R.S. Mitochondrial involvement in post-tetanic potentiation of synaptic transmission. Neuron 1997, 18, 483–491. [Google Scholar] [CrossRef]
- Reynolds, I.J.; Malaiyandi, L.M.; Coash, M.; Rintoul, G.L. Mitochondrial trafficking in neurons: A key variable in neurodegeneration? J. Bioenerg. Biomembr. 2004, 36, 283–286. [Google Scholar] [CrossRef]
- Wong-Riley, M.T. Cytochrome oxidase: An endogenous metabolic marker for neuronal activity. Trends Neurosci. 1989, 12, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.J. Neuronal Ca2+: Getting it up and keeping it up. Trends Neurosci. 1992, 15, 317–319. [Google Scholar] [CrossRef]
- Reddy, P.H. Mitochondrial dysfunction in aging and Alzheimer’s disease: Strategies to protect neurons. Antioxid. Redox Signal. 2007, 9, 1647–1658. [Google Scholar] [CrossRef]
- Gibson, G.E.; Sheu, K.F.; Blass, J.P. Abnormalities of mitochondrial enzymes in Alzheimer disease. J. Neural Transm. 1998, 105, 855–870. [Google Scholar] [CrossRef]
- Lin, M.T.; Beal, M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef]
- Pradeepkiran, J.A.; Baig, J.; Selman, A.; Reddy, P.H. Mitochondria in Aging and Alzheimer’s Disease: Focus on Mitophagy. Neuroscientist 2023, 10738584221139761. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Pal, D.; Sharma, U.; Kumar, A. Mitophagy: An Emergence of New Player in Alzheimer’s Disease. Front. Mol. Neurosci. 2022, 15, 921908. [Google Scholar] [CrossRef]
- Alves, L.; Correia, A.S.; Miguel, R.; Alegria, P.; Bugalho, P. Alzheimer’s disease: A clinical practice-oriented review. Front. Neurol. 2012, 3, 63. [Google Scholar] [CrossRef] [PubMed]
- Olney, N.T.; Spina, S.; Miller, B.L. Frontotemporal dementia. Neurol. Clin. 2017, 35, 339–374. [Google Scholar] [CrossRef] [PubMed]
- Burns, S.; Selman, A.; Sehar, U.; Rawat, P.; Reddy, A.P.; Reddy, P.H. Therapeutics of Alzheimer’s Disease: Recent Developments. Antioxidants 2022, 11, 2402. [Google Scholar] [CrossRef]
- Thijssen, E.H.; Verberk, I.M.; Kindermans, J.; Abramian, A.; Vanbrabant, J.; Ball, A.J.; Pijnenburg, Y.; Lemstra, A.W.; van der Flier, W.M.; Stoops, E. Differential diagnostic performance of a panel of plasma biomarkers for different types of dementia. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2022, 14, e12285. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Miller, B. Frontotemporal Dementia: Epidemiology, Pathology, and Pathogenesis. 2016. Available online: https://www.medilib.ir/uptodate/show/14138 (accessed on 6 March 2022).
- Garcia-Esparcia, P.; López-González, I.; Grau-Rivera, O.; García-Garrido, M.F.; Konetti, A.; Llorens, F.; Zafar, S.; Carmona, M.; Del Rio, J.A.; Zerr, I. Dementia with Lewy bodies: Molecular pathology in the frontal cortex in typical and rapidly progressive forms. Front. Neurol. 2017, 8, 89. [Google Scholar] [CrossRef] [PubMed]
- Custodio, N.; Montesinos, R.; Lira, D.; Herrera-Pérez, E.; Bardales, Y.; Valeriano-Lorenzo, L. Mixed dementia: A review of the evidence. Dement. Neuropsychol. 2017, 11, 364–370. [Google Scholar] [CrossRef]
- Cechetto, D.F.; Weishaupt, N. The Cerebral Cortex in Neurodegenerative and Neuropsychiatric Disorders: Experimental Approaches to Clinical Issues; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Kapasi, A.; Schneider, J. Vascular contributions to cognitive impairment, clinical Alzheimer’s disease, and dementia in older persons. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2016, 1862, 878–886. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, J. Lipid metabolism in Alzheimer’s disease. Neurosci. Bull. 2014, 30, 331–345. [Google Scholar] [CrossRef]
- Liu, J.; Dietz, K.; DeLoyht, J.M.; Pedre, X.; Kelkar, D.; Kaur, J.; Vialou, V.; Lobo, M.K.; Dietz, D.M.; Nestler, E.J. Impaired adult myelination in the prefrontal cortex of socially isolated mice. Nat. Neurosci. 2012, 15, 1621–1623. [Google Scholar] [CrossRef]
- Rojo, A.I.; Pajares, M.; Rada, P.; Nuñez, A.; Nevado-Holgado, A.J.; Killik, R.; Van Leuven, F.; Ribe, E.; Lovestone, S.; Yamamoto, M. NRF2 deficiency replicates transcriptomic changes in Alzheimer’s patients and worsens APP and TAU pathology. Redox Biol. 2017, 13, 444–451. [Google Scholar] [CrossRef]
- Kashiwaya, Y.; Takeshima, T.; Mori, N.; Nakashima, K.; Clarke, K.; Veech, R.L. d-β-Hydroxybutyrate protects neurons in models of Alzheimer’s and Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2000, 97, 5440–5444. [Google Scholar] [CrossRef] [PubMed]
- Galton, C.J.; Gomez-Anson, B.; Antoun, N.; Scheltens, P.; Patterson, K.; Graves, M.; Sahakian, B.J.; Hodges, J.R. Temporal lobe rating scale: Application to Alzheimer’s disease and frontotemporal dementia. J. Neurol. Neurosurg. Psychiatry 2001, 70, 165–173. [Google Scholar] [CrossRef]
- Benson, D.F.; Davis, R.J.; Snyder, B.D. Posterior cortical atrophy. Arch. Neurol. 1988, 45, 789–793. [Google Scholar] [CrossRef] [PubMed]
- Alladi, S.; Xuereb, J.; Bak, T.; Nestor, P.; Knibb, J.; Patterson, K.; Hodges, J.R. Focal cortical presentations of Alzheimer’s disease. Brain 2007, 130, 2636–2645. [Google Scholar] [CrossRef]
- McMonagle, P.; Deering, F.; Berliner, Y.; Kertesz, A. The cognitive profile of posterior cortical atrophy. Neurology 2006, 66, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Tang-Wai, D.F.; Graff-Radford, N.R.; Boeve, B.F.; Dickson, D.W.; Parisi, J.E.; Crook, R.; Caselli, R.J.; Knopman, D.S.; Petersen, R.C. Clinical, genetic, and neuropathologic characteristics of posterior cortical atrophy. Neurology 2004, 63, 1168–1174. [Google Scholar] [CrossRef] [PubMed]
- Roongroj Bhidayasiri, M. Atypical dementia: When it is not Alzheimer’s disease. J. Med. Assoc. Thai 2007, 90, 2222–2232. [Google Scholar]
- Henry, M.L.; Gorno-Tempini, M.L. The logopenic variant of primary progressive aphasia. Curr. Opin. Neurol. 2010, 23, 633–637. [Google Scholar] [CrossRef] [PubMed]
- Byrd, W.M.; Clayton, L.A. Race, medicine, and health care in the United States: A historical survey. J. Natl. Med. Assoc. 2001, 93, 11s–34s. [Google Scholar]
- Agyemang, C.; Bhopal, R.; Bruijnzeels, M. Negro, Black, Black African, African Caribbean, African American or what? Labelling African origin populations in the health arena in the 21st century. J. Epidemiol. Community Health 2005, 59, 1014. [Google Scholar] [CrossRef]
- Mays, V.M. Identity development of black Americans: The role of history and the importance of ethnicity. Am. J. Psychother. 1986, 40, 582–593. [Google Scholar] [CrossRef]
- Erby, B.M. Surviving the Jim Crow South: “The Talk” as an African American Rhetorical Form. J. Hist. Rhetor. 2021, 24, 24–38. [Google Scholar] [CrossRef]
- Tolnay, S.E. The African American “Great Migration” and Beyond. Annu. Rev. Sociol. 2003, 29, 209–232. [Google Scholar] [CrossRef]
- Collins, W.J. The Great Migration of Black Americans from the US South: A guide and interpretation. Explor. Econ. Hist. 2021, 80, 101382. [Google Scholar] [CrossRef]
- Leibbrand, C.; Massey, C.; Alexander, J.T.; Genadek, K.R.; Tolnay, S. The Great Migration and Residential Segregation in American Cities during the Twentieth Century. Soc. Sci. Hist. 2020, 44, 19–55. [Google Scholar] [CrossRef]
- Hudson-Weems, C. Resurrecting Emmett Till: The Catalyst of the Modern Civil Rights Movement. J. Black Stud. 1998, 29, 179–188. [Google Scholar] [CrossRef]
- Institute of Medicine Committee. Eliminating Ethnic Disparities in Health Care. In Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care; Smedley, B.D., Stith, A.Y., Nelson, A.R., Eds.; National Academies Press (US): Washington, DC, USA, 2003. [Google Scholar]
- Salles, A.; Arora, V.M.; Mitchell, K.-A. Everyone Must Address Anti-Black Racism in Health Care: Steps for Non-Black Health Care Professionals to Take. JAMA 2021, 326, 601–602. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.B.; Casper, E. The Burdens of Race and History on Black People’s Health 400 Years After Jamestown. Am. J. Public Health 2019, 109, 1346–1347. [Google Scholar] [CrossRef]
- Scharff, D.P.; Mathews, K.J.; Jackson, P.; Hoffsuemmer, J.; Martin, E.; Edwards, D. More than Tuskegee: Understanding mistrust about research participation. J. Health Care Poor Underserved 2010, 21, 879–897. [Google Scholar] [CrossRef] [PubMed]
- Jackman, M.R.; Shauman, K.A. The toll of inequality: Excess African American deaths in the United States over the twentieth century. Du Bois Review: Social Science Research on Race 2019, 16, 291–340. [Google Scholar] [CrossRef]
- Funk, C. Black Americans’ Views of and Engagement with Science; Pew Research Center: Washington, DC, USA, 2022. [Google Scholar]
- Alzheimer’s Association. African-Americans and Alzheimer’s Disease: The Silent Epidemic; Alzheimer’s Association: Chicago, IL, USA, 2002. [Google Scholar]
- Barnes, L.L.; Bennett, D.A. Alzheimer’s disease in African Americans: Risk factors and challenges for the future. Health Aff. 2014, 33, 580–586. [Google Scholar] [CrossRef]
- Logue, M.W.; Schu, M.; Vardarajan, B.N.; Buros, J.; Green, R.C.; Go, R.C.; Griffith, P.; Obisesan, T.O.; Shatz, R.; Borenstein, A.; et al. A comprehensive genetic association study of Alzheimer disease in African Americans. Arch. Neurol. 2011, 68, 1569–1579. [Google Scholar] [CrossRef] [PubMed]
- Reitz, C.; Jun, G.; Naj, A.; Rajbhandary, R.; Vardarajan, B.N.; Wang, L.S.; Valladares, O.; Lin, C.F.; Larson, E.B.; Graff-Radford, N.R.; et al. Variants in the ATP-binding cassette transporter (ABCA7), apolipoprotein E ϵ4,and the risk of late-onset Alzheimer disease in African Americans. JAMA 2013, 309, 1483–1492. [Google Scholar] [CrossRef] [PubMed]
- Michaelson, D.M. APOE ε4: The most prevalent yet understudied risk factor for Alzheimer’s disease. Alzheimers Dement. 2014, 10, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Schultz, S.A.; Boots, E.A.; Darst, B.F.; Zetterberg, H.; Blennow, K.; Edwards, D.F.; Koscik, R.L.; Carlsson, C.M.; Gallagher, C.L.; Bendlin, B.B.; et al. Cardiorespiratory fitness alters the influence of a polygenic risk score on biomarkers of AD. Neurology 2017, 88, 1650–1658. [Google Scholar] [CrossRef] [PubMed]
- Hendrie, H.C.; Murrell, J.; Baiyewu, O.; Lane, K.A.; Purnell, C.; Ogunniyi, A.; Unverzagt, F.W.; Hall, K.; Callahan, C.M.; Saykin, A.J.; et al. APOE ε4 and the risk for Alzheimer disease and cognitive decline in African Americans and Yoruba. Int. Psychogeriatr. 2014, 26, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Wesnes, K.A.; Annas, P.; Basun, H.; Edgar, C.; Blennow, K. Performance on a pattern separation task by Alzheimer’s patients shows possible links between disrupted dentate gyrus activity and apolipoprotein E ∈4 status and cerebrospinal fluid amyloid-β42 levels. Alzheimers Res. Ther. 2014, 6, 20. [Google Scholar] [CrossRef]
- Potter, H.; Wisniewski, T. Apolipoprotein e: Essential catalyst of the Alzheimer amyloid cascade. Int. J. Alzheimers Dis. 2012, 2012, 489428. [Google Scholar] [CrossRef]
- Bookheimer, S.Y.; Strojwas, M.H.; Cohen, M.S.; Saunders, A.M.; Pericak-Vance, M.A.; Mazziotta, J.C.; Small, G.W. Patterns of brain activation in people at risk for Alzheimer’s disease. N. Engl. J. Med. 2000, 343, 450–456. [Google Scholar] [CrossRef]
- Filippini, N.; MacIntosh, B.J.; Hough, M.G.; Goodwin, G.M.; Frisoni, G.B.; Smith, S.M.; Matthews, P.M.; Beckmann, C.F.; Mackay, C.E. Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proc. Natl. Acad. Sci. USA 2009, 106, 7209–7214. [Google Scholar] [CrossRef]
- Dennis, N.A.; Browndyke, J.N.; Stokes, J.; Need, A.; Burke, J.R.; Welsh-Bohmer, K.A.; Cabeza, R. Temporal lobe functional activity and connectivity in young adult APOE varepsilon4 carriers. Alzheimers Dement. 2010, 6, 303–311. [Google Scholar] [CrossRef]
- Huang, Y.-W.A.; Zhou, B.; Wernig, M.; Südhof, T.C. ApoE2, ApoE3, and ApoE4 differentially stimulate APP transcription and Aβ secretion. Cell 2017, 168, 427–441.e421. [Google Scholar] [CrossRef] [PubMed]
- Bretsky, P.; Guralnik, J.; Launer, L.; Albert, M.; Seeman, T. The role of APOE-ε4 in longitudinal cognitive decline: MacArthur Studies of Successful Aging. Neurology 2003, 60, 1077–1081. [Google Scholar] [CrossRef]
- Kuller, L.H.; Shemanski, L.; Manolio, T.; Haan, M.; Fried, L.; Bryan, N.; Burke, G.L.; Tracy, R.; Bhadelia, R. Relationship between ApoE, MRI findings, and cognitive function in the Cardiovascular Health Study. Stroke 1998, 29, 388–398. [Google Scholar] [CrossRef]
- Di Paolo, G.; Kim, T.W. Linking lipids to Alzheimer’s disease: Cholesterol and beyond. Nat. Rev. Neurosci. 2011, 12, 284–296. [Google Scholar] [CrossRef]
- Anuurad, E.; Rubin, J.; Lu, G.; Pearson, T.A.; Holleran, S.; Ramakrishnan, R.; Berglund, L. Protective effect of apolipoprotein E2 on coronary artery disease in African Americans is mediated through lipoprotein cholesterol. J. Lipid Res. 2006, 47, 2475–2481. [Google Scholar] [CrossRef] [PubMed]
- Palmer, A.; Good, M. Hippocampal synaptic activity, pattern separation and episodic-like memory: Implications for mouse models of Alzheimer’s disease pathology. Biochem. Soc. Trans. 2011, 39, 902–909. [Google Scholar] [CrossRef] [PubMed]
- Rajabli, F.; Feliciano, B.E.; Celis, K.; Hamilton-Nelson, K.L.; Whitehead, P.L.; Adams, L.D.; Bussies, P.L.; Manrique, C.P.; Rodriguez, A.; Rodriguez, V.; et al. Ancestral origin of ApoE ε4 Alzheimer disease risk in Puerto Rican and African American populations. PLoS Genet. 2018, 14, e1007791. [Google Scholar] [CrossRef]
- Tang, M.X.; Cross, P.; Andrews, H.; Jacobs, D.M.; Small, S.; Bell, K.; Merchant, C.; Lantigua, R.; Costa, R.; Stern, Y.; et al. Incidence of AD in African-Americans, Caribbean Hispanics, and Caucasians in northern Manhattan. Neurology 2001, 56, 49–56. [Google Scholar] [CrossRef]
- Farrer, L.A.; Cupples, L.A.; Haines, J.L.; Hyman, B.; Kukull, W.A.; Mayeux, R.; Myers, R.H.; Pericak-Vance, M.A.; Risch, N.; van Duijn, C.M. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA 1997, 278, 1349–1356. [Google Scholar] [CrossRef]
- Cukier, H.N.; Kunkle, B.W.; Vardarajan, B.N.; Rolati, S.; Hamilton-Nelson, K.L.; Kohli, M.A.; Whitehead, P.L.; Dombroski, B.A.; Van Booven, D.; Lang, R.; et al. ABCA7 frameshift deletion associated with Alzheimer disease in African Americans. Neurol. Genet. 2016, 2, e79. [Google Scholar] [CrossRef] [PubMed]
- Pooler, A.M.; Polydoro, M.; Maury, E.A.; Nicholls, S.B.; Reddy, S.M.; Wegmann, S.; William, C.; Saqran, L.; Cagsal-Getkin, O.; Pitstick, R.; et al. Amyloid accelerates tau propagation and toxicity in a model of early Alzheimer’s disease. Acta Neuropathol. Commun. 2015, 3, 14. [Google Scholar] [CrossRef]
- Selkoe, D.J. The molecular pathology of Alzheimer’s disease. Neuron 1991, 6, 487–498. [Google Scholar] [CrossRef]
- Sinha, N.; Reagh, Z.M.; Tustison, N.J.; Berg, C.N.; Shaw, A.; Myers, C.E.; Hill, D.; Yassa, M.A.; Gluck, M.A. ABCA7 risk variant in healthy older African Americans is associated with a functionally isolated entorhinal cortex mediating deficient generalization of prior discrimination training. Hippocampus 2019, 29, 527–538. [Google Scholar] [CrossRef]
- Sinha, N.; Berg, C.N.; Tustison, N.J.; Shaw, A.; Hill, D.; Yassa, M.A.; Gluck, M.A. APOE ε4 status in healthy older African Americans is associated with deficits in pattern separation and hippocampal hyperactivation. Neurobiol. Aging 2018, 69, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Maesako, M.; Uemura, K.; Kubota, M.; Kuzuya, A.; Sasaki, K.; Hayashida, N.; Asada-Utsugi, M.; Watanabe, K.; Uemura, M.; Kihara, T.; et al. Exercise is more effective than diet control in preventing high fat diet-induced β-amyloid deposition and memory deficit in amyloid precursor protein transgenic mice. J. Biol. Chem. 2012, 287, 23024–23033. [Google Scholar] [CrossRef]
- Houdebine, L.; Gallelli, C.A.; Rastelli, M.; Sampathkumar, N.K.; Grenier, J. Effect of physical exercise on brain and lipid metabolism in mouse models of multiple sclerosis. Chem. Phys. Lipids 2017, 207, 127–134. [Google Scholar] [CrossRef] [PubMed]
- He, X.F.; Liu, D.X.; Zhang, Q.; Liang, F.Y.; Dai, G.Y.; Zeng, J.S.; Pei, Z.; Xu, G.Q.; Lan, Y. Voluntary Exercise Promotes Glymphatic Clearance of Amyloid Beta and Reduces the Activation of Astrocytes and Microglia in Aged Mice. Front. Mol. Neurosci. 2017, 10, 144. [Google Scholar] [CrossRef]
- Sayal, N. Exercise training increases size of hippocampus and improves memory. Ann. Neurosci. 2015, 22. [Google Scholar] [CrossRef]
- Allard, J.S.; Ntekim, O.; Johnson, S.P.; Ngwa, J.S.; Bond, V.; Pinder, D.; Gillum, R.F.; Fungwe, T.V.; Kwagyan, J.; Obisesan, T.O. APOEε4 impacts up-regulation of brain-derived neurotrophic factor after a six-month stretch and aerobic exercise intervention in mild cognitively impaired elderly African Americans: A pilot study. Exp. Gerontol. 2017, 87, 129–136. [Google Scholar] [CrossRef]
- Yang, J.J.; Keohane, L.M.; Pan, X.F.; Qu, R.; Shu, X.O.; Lipworth, L.; Braun, K.; Steinwandel, M.D.; Dai, Q.; Shrubsole, M.; et al. Association of Healthy Lifestyles With Risk of Alzheimer Disease and Related Dementias in Low-Income Black and White Americans. Neurology 2022, 99, e944–e953. [Google Scholar] [CrossRef] [PubMed]
- McGrattan, A.M.; McGuinness, B.; McKinley, M.C.; Kee, F.; Passmore, P.; Woodside, J.V.; McEvoy, C.T. Diet and Inflammation in Cognitive Ageing and Alzheimer’s Disease. Curr. Nutr. Rep. 2019, 8, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Canevelli, M.; Lucchini, F.; Quarata, F.; Bruno, G.; Cesari, M. Nutrition and Dementia: Evidence for Preventive Approaches? Nutrients 2016, 8, 144. [Google Scholar] [CrossRef]
- Wilson, D.W.; Nash, P.; Buttar, H.S.; Griffiths, K.; Singh, R.; De Meester, F.; Horiuchi, R.; Takahashi, T. The Role of Food Antioxidants, Benefits of Functional Foods, and Influence of Feeding Habits on the Health of the Older Person: An Overview. Antioxidants 2017, 6, 81. [Google Scholar] [CrossRef] [PubMed]
- Caspersen, C.J.; Powell, K.E.; Christenson, G.M. Physical activity, exercise, and physical fitness: Definitions and distinctions for health-related research. Public Health Rep. 1985, 100, 126–131. [Google Scholar] [PubMed]
- Colberg, S.R.; Sigal, R.J.; Yardley, J.E.; Riddell, M.C.; Dunstan, D.W.; Dempsey, P.C.; Horton, E.S.; Castorino, K.; Tate, D.F. Physical Activity/Exercise and Diabetes: A Position Statement of the American Diabetes Association. Diabetes Care 2016, 39, 2065–2079. [Google Scholar] [CrossRef]
- Ashby-Mitchell, K.; Burns, R.; Shaw, J.; Anstey, K.J. Proportion of dementia in Australia explained by common modifiable risk factors. Alzheimers Res. Ther. 2017, 9, 11. [Google Scholar] [CrossRef]
- Pietropaolo, S.; Sun, Y.; Li, R.; Brana, C.; Feldon, J.; Yee, B.K. The impact of voluntary exercise on mental health in rodents: A neuroplasticity perspective. Behav. Brain Res. 2008, 192, 42–60. [Google Scholar] [CrossRef]
- Russell, A.P.; Foletta, V.C.; Snow, R.J.; Wadley, G.D. Skeletal muscle mitochondria: A major player in exercise, health and disease. Biochim. Biophys. Acta 2014, 1840, 1276–1284. [Google Scholar] [CrossRef]
- Barbieri, E.; Agostini, D.; Polidori, E.; Potenza, L.; Guescini, M.; Lucertini, F.; Annibalini, G.; Stocchi, L.; De Santi, M.; Stocchi, V. The pleiotropic effect of physical exercise on mitochondrial dynamics in aging skeletal muscle. Oxid. Med. Cell. Longev. 2015, 2015, 917085. [Google Scholar] [CrossRef]
- Hötting, K.; Röder, B. Beneficial effects of physical exercise on neuroplasticity and cognition. Neurosci. Biobehav. Rev. 2013, 37, 2243–2257. [Google Scholar] [CrossRef] [PubMed]
- Jäger, S.; Handschin, C.; St-Pierre, J.; Spiegelman, B.M. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc. Natl. Acad. Sci. USA 2007, 104, 12017–12022. [Google Scholar] [CrossRef] [PubMed]
- Cartee, G.D.; Hepple, R.T.; Bamman, M.M.; Zierath, J.R. Exercise Promotes Healthy Aging of Skeletal Muscle. Cell Metab. 2016, 23, 1034–1047. [Google Scholar] [CrossRef]
- Wahl, D.; Solon-Biet, S.M.; Cogger, V.C.; Fontana, L.; Simpson, S.J.; Le Couteur, D.G.; Ribeiro, R.V. Aging, lifestyle and dementia. Neurobiol. Dis. 2019, 130, 104481. [Google Scholar] [CrossRef] [PubMed]
- Zainal, T.A.; Oberley, T.D.; Allison, D.B.; Szweda, L.I.; Weindruch, R. Caloric restriction of rhesus monkeys lowers oxidative damage in skeletal muscle. FASEB J. 2000, 14, 1825–1836. [Google Scholar] [CrossRef] [PubMed]
- Barja, G. Endogenous oxidative stress: Relationship to aging, longevity and caloric restriction. Ageing Res. Rev. 2002, 1, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Hossain, S.; Beydoun, M.A.; Weiss, J.; Kuczmarski, M.F.; Evans, M.K.; Zonderman, A.B. Longitudinal associations between dietary quality and Alzheimer’s disease genetic risk on cognitive performance among African American adults. Br. J. Nutr. 2020, 124, 1264–1276. [Google Scholar] [CrossRef]
- Nutaitis, A.C.; Tharwani, S.D.; Serra, M.C.; Goldstein, F.C.; Zhao, L.; Sher, S.S.; Verble, D.D.; Wharton, W. Diet as a Risk Factor for Cognitive Decline in African Americans and Caucasians with a Parental History of Alzheimer’s Disease: A Cross-Sectional Pilot Study Dietary Patterns. J. Prev. Alzheimers Dis. 2019, 6, 50–55. [Google Scholar] [CrossRef]
- Morris, M.C.; Evans, D.A.; Tangney, C.C.; Bienias, J.L.; Wilson, R.S. Fish consumption and cognitive decline with age in a large community study. Arch. Neurol. 2005, 62, 1849–1853. [Google Scholar] [CrossRef]
- Morris, M.C.; Evans, D.A.; Tangney, C.C.; Bienias, J.L.; Wilson, R.S. Associations of vegetable and fruit consumption with age-related cognitive change. Neurology 2006, 67, 1370–1376. [Google Scholar] [CrossRef]
- Agarwal, P.; Holland, T.M.; Wang, Y.; Bennett, D.A.; Morris, M.C. Association of Strawberries and Anthocyanidin Intake with Alzheimer’s Dementia Risk. Nutrients 2019, 11, 60. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Dhana, K.; Barnes, L.L.; Tangney, C.C.; Agarwal, P.; Aggarwal, N.; Holland, T.M.; Beck, T.; Evans, D.A.; Rajan, K.B. A healthy plant-based diet was associated with slower cognitive decline in African American older adults: A biracial community-based cohort. Am. J. Clin. Nutr. 2022, 116, 875–886. [Google Scholar] [CrossRef]
- Dhana, K.; Barnes, L.L.; Liu, X.; Agarwal, P.; Desai, P.; Krueger, K.R.; Holland, T.M.; Halloway, S.; Aggarwal, N.T.; Evans, D.A.; et al. Genetic risk, adherence to a healthy lifestyle, and cognitive decline in African Americans and European Americans. Alzheimers Dement. 2022, 18, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Power, M.C.; Bennett, E.E.; Turner, R.W.; Dowling, N.M.; Ciarleglio, A.; Glymour, M.M.; Gianattasio, K.Z. Trends in Relative Incidence and Prevalence of Dementia Across Non-Hispanic Black and White Individuals in the United States, 2000-2016. JAMA Neurol. 2021, 78, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Froehlich, T.E.; Bogardus, S.T., Jr.; Inouye, S.K. Dementia and race: Are there differences between African Americans and Caucasians? J. Am. Geriatr. Soc. 2001, 49, 477–484. [Google Scholar] [CrossRef]
- Lennon, J.C.; Aita, S.L.; Bene, V.A.D.; Rhoads, T.; Resch, Z.J.; Eloi, J.M.; Walker, K.A. Black and White individuals differ in dementia prevalence, risk factors, and symptomatic presentation. Alzheimers Dement. 2022, 18, 1461–1471. [Google Scholar] [CrossRef]
- Gottesman, R.F.; Albert, M.S.; Alonso, A.; Coker, L.H.; Coresh, J.; Davis, S.M.; Deal, J.A.; McKhann, G.M.; Mosley, T.H.; Sharrett, A.R.; et al. Associations Between Midlife Vascular Risk Factors and 25-Year Incident Dementia in the Atherosclerosis Risk in Communities (ARIC) Cohort. JAMA Neurol. 2017, 74, 1246–1254. [Google Scholar] [CrossRef]
- Baumgart, M.; Snyder, H.M.; Carrillo, M.C.; Fazio, S.; Kim, H.; Johns, H. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: A population-based perspective. Alzheimers Dement. 2015, 11, 718–726. [Google Scholar] [CrossRef]
- Shiekh, S.I.; Forbes, H.; Mathur, R.; Smeeth, L.; Pearce, N.; Warren-Gash, C. Ethnicity and risk of diagnosed dementia after stroke: A cohort study using the Clinical Practice Research Datalink. J. Epidemiol. Community Health 2020, 74, 114–119. [Google Scholar] [CrossRef]
- Babulal, G.M.; Quiroz, Y.T.; Albensi, B.C.; Arenaza-Urquijo, E.; Astell, A.J.; Babiloni, C.; Bahar-Fuchs, A.; Bell, J.; Bowman, G.L.; Brickman, A.M.; et al. Perspectives on ethnic and racial disparities in Alzheimer’s disease and related dementias: Update and areas of immediate need. Alzheimers Dement. 2019, 15, 292–312. [Google Scholar] [CrossRef]
- O’Bryant, S.E.; Humphreys, J.D.; Schiffer, R.B.; Sutker, P.B. Presentation of Mexican Americans to a Memory Disorder Clinic. J. Psychopathol. Behav. Assess. 2007, 29, 137–140. [Google Scholar] [CrossRef]
- Cooper, C.; Tandy, A.R.; Balamurali, T.B.; Livingston, G. A systematic review and meta-analysis of ethnic differences in use of dementia treatment, care, and research. Am. J. Geriatr. Psychiatry 2010, 18, 193–203. [Google Scholar] [CrossRef]
- O’Bryant, S.E.; Johnson, L.; Balldin, V.; Edwards, M.; Barber, R.; Williams, B.; Devous, M.; Cushings, B.; Knebl, J.; Hall, J. Characterization of Mexican Americans with mild cognitive impairment and Alzheimer’s disease. J. Alzheimers Dis. 2013, 33, 373–379. [Google Scholar] [CrossRef]
- Hargrave, R.; Stoeklin, M.; Haan, M.; Reed, B. Clinical aspects of Alzheimer’s disease in black and white patients. J. Natl. Med. Assoc. 1998, 90, 78–84. [Google Scholar] [PubMed]
- Bassiony, M.M.; Steinberg, M.S.; Warren, A.; Rosenblatt, A.; Baker, A.S.; Lyketsos, C.G. Delusions and hallucinations in Alzheimer’s disease: Prevalence and clinical correlates. Int. J. Geriatr. Psychiatry 2000, 15, 99–107. [Google Scholar] [CrossRef]
- Gottesman, R.T.; Stern, Y. Behavioral and Psychiatric Symptoms of Dementia and Rate of Decline in Alzheimer’s Disease. Front. Pharmacol. 2019, 10, 1062. [Google Scholar] [CrossRef]
- Ehrenberg, A.J.; Suemoto, C.K.; França Resende, E.P.; Petersen, C.; Leite, R.E.P.; Rodriguez, R.D.; Ferretti-Rebustini, R.E.L.; You, M.; Oh, J.; Nitrini, R.; et al. Neuropathologic Correlates of Psychiatric Symptoms in Alzheimer’s Disease. J. Alzheimers Dis. 2018, 66, 115–126. [Google Scholar] [CrossRef]
- Chakraborty, S.; Lennon, J.C.; Malkaram, S.A.; Zeng, Y.; Fisher, D.W.; Dong, H. Serotonergic system, cognition, and BPSD in Alzheimer’s disease. Neurosci. Lett. 2019, 704, 36–44. [Google Scholar] [CrossRef]
- Manly, J.J. Deconstructing Race and Ethnicity. Med. Care 2006, 44, S10–S16. [Google Scholar] [CrossRef]
- Chui, H.C.; Gatz, M. Cultural Diversity in Alzheimer Disease. Alzheimer Dis. Assoc. Disord. 2005, 19, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Gilligan, A.M.; Malone, D.C.; Warholak, T.L.; Armstrong, E.P. Racial and Ethnic Disparities in Alzheimer’s Disease Pharmacotherapy Exposure: An Analysis Across Four State Medicaid Populations. Am. J. Geriatr. Pharmacother. 2012, 10, 303–312. [Google Scholar] [CrossRef]
- Schwartz, B.S.; Glass, T.A.; Bolla, K.I.; Stewart, W.F.; Glass, G.; Rasmussen, M.; Bressler, J.; Shi, W.; Bandeen-Roche, K. Disparities in cognitive functioning by race/ethnicity in the Baltimore Memory Study. Environ. Health Perspect. 2004, 112, 314–320. [Google Scholar] [CrossRef]
- Barnes, L.L.; Wilson, R.S.; Li, Y.; Aggarwal, N.T.; Gilley, D.W.; McCann, J.J.; Evans, D.A. Racial Differences in the Progression of Cognitive Decline in Alzheimer Disease. Am. J. Geriatr. Psychiatry 2005, 13, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Castora-Binkley, M.; Peronto, C.L.; Edwards, J.D.; Small, B.J. A Longitudinal Analysis of the Influence of Race on Cognitive Performance. J. Gerontol. Ser. B Psychol. Sci. Soc. Sci. 2013, 70, 512–518. [Google Scholar] [CrossRef]
- Wright, R.S.; Waldstein, S.R.; Gerassimakis, C.S.; Sprung, M.R.; Moody, D.L.B.; Taylor, A.D.; Al’Najjar, E.; McNeely, J.M.; Zhang, Z.; Evans, M.K.; et al. Multiple Influences on Cognitive Function Among Urban-Dwelling African Americans. J. Racial Ethn. Health Disparities 2019, 6, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Fyffe, D.C.; Mukherjee, S.; Barnes, L.L.; Manly, J.J.; Bennett, D.A.; Crane, P.K. Explaining differences in episodic memory performance among older African Americans and Whites: The roles of factors related to cognitive reserve and test bias. J. Int. Neuropsychol. Soc. 2011, 17, 625–638. [Google Scholar] [CrossRef] [PubMed]
- Kittner, S.J.; White, L.R.; Farmer, M.E.; Wolz, M.; Kaplan, E.; Moes, E.; Brody, J.A.; Feinleib, M. Methodological issues in screening for dementia: The problem of education adjustment. J. Chronic Dis. 1986, 39, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Rivera Mindt, M.; Byrd, D.; Saez, P.; Manly, J. Increasing culturally competent neuropsychological services for ethnic minority populations: A call to action. Clin. Neuropsychol. 2010, 24, 429–453. [Google Scholar] [CrossRef] [PubMed]
- Lamar, M.; Lerner, A.J.; James, B.D.; Yu, L.; Glover, C.M.; Wilson, R.S.; Barnes, L.L. Relationship of Early-Life Residence and Educational Experience to Level and Change in Cognitive Functioning: Results of the Minority Aging Research Study. J. Gerontol. B Psychol. Sci. Soc. Sci. 2020, 75, e81–e92. [Google Scholar] [CrossRef]
- Barnes, L.L.; Lewis, T.T.; Begeny, C.T.; Yu, L.; Bennett, D.A.; Wilson, R.S. Perceived discrimination and cognition in older African Americans. J. Int. Neuropsychol. Soc. 2012, 18, 856–865. [Google Scholar] [CrossRef]
- Turner, A.D.; James, B.D.; Capuano, A.W.; Aggarwal, N.T.; Barnes, L.L. Perceived Stress and Cognitive Decline in Different Cognitive Domains in a Cohort of Older African Americans. Am. J. Geriatr. Psychiatry 2017, 25, 25–34. [Google Scholar] [CrossRef]
- Vonk, J.M.J.; Arce Rentería, M.; Avila, J.F.; Schupf, N.; Noble, J.M.; Mayeux, R.; Brickman, A.M.; Manly, J.J. Secular trends in cognitive trajectories of diverse older adults. Alzheimers Dement. 2019, 15, 1576–1587. [Google Scholar] [CrossRef]
- Zahodne, L.B.; Sol, K.; Kraal, Z. Psychosocial Pathways to Racial/Ethnic Inequalities in Late-Life Memory Trajectories. J. Gerontol. B Psychol. Sci. Soc. Sci. 2019, 74, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Sehar, U.; Rawat, P.; Choudhury, M.; Boles, A.; Culberson, J.; Khan, H.; Malhotra, K.; Basu, T.; Reddy, P.H. Comprehensive Understanding of Hispanic Caregivers: Focus on Innovative Methods and Validations. J. Alzheimer’s Dis. Rep. 2022, Pre-press, 1–18. [Google Scholar] [CrossRef]
- Brewster, G.S.; Bonds, K.; McLennon, S.; Moss, K.O.; Epps, F.; Lopez, R.P. Missing the Mark: The Complexity of African American Dementia Family Caregiving. J. Fam. Nurs. 2020, 26, 294–301. [Google Scholar] [CrossRef]
- Lindauer, A.; Harvath, T.A.; Berry, P.H.; Wros, P. The Meanings African American Caregivers Ascribe to Dementia-Related Changes: The Paradox of Hanging on to Loss. Gerontologist 2015, 56, 733–742. [Google Scholar] [CrossRef] [PubMed]
- den Hertog, T.N.; Gilmoor, A.R. Informal care for people with chronic psychotic symptoms: Four case studies in a San community in South Africa. Health Soc. Care Community 2017, 25, 538–547. [Google Scholar] [CrossRef]
- Roth, D.L.; Haley, W.E.; Owen, J.E.; Clay, O.J.; Goode, K.T. Latent growth models of the longitudinal effects of dementia caregiving: A comparison of African American and White family caregivers. Psychol. Aging 2001, 16, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Owen, J.E.; Goode, K.T.; Haley, W.E. End of life care and reactions to death in African-American and white family caregivers of relatives with Alzheimer’s disease. Omega 2001, 43, 349–361. [Google Scholar] [CrossRef]
- Disbrow, E.A.; Arnold, C.L.; Glassy, N.; Tilly, C.M.; Langdon, K.M.; Gungor, D.; Davis, T.C. Alzheimer Disease and Related Dementia Resources: Perspectives of African American and Caucasian Family Caregivers in Northwest Louisiana. J. Appl. Gerontol. 2021, 40, 209–219. [Google Scholar] [CrossRef]
- Spurlock, W.R. Spiritual well-being and caregiver burden in Alzheimer’s caregivers. Geriatr. Nurs. 2005, 26, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Ellison, C.G.; Boardman, J.D.; Williams, D.R.; Jackson, J.S. Religious involvement, stress, and mental health: Findings from the 1995 Detroit Area Study. Soc. Forces 2001, 80, 215–249. [Google Scholar] [CrossRef]
- Epps, F.; Heidbreder, V.; Alexander, K.; Tomlinson, A.; Freeman, V.; Williams, N. A dementia-friendly church: How can the African American church support families affected by dementia? Dementia 2021, 20, 556–569. [Google Scholar] [CrossRef] [PubMed]
- Wilks, S.E.; Spurlock, W.R.; Brown, S.C.; Teegen, B.C.; Geiger, J.R. Examining spiritual support among African American and Caucasian Alzheimer’s caregivers: A risk and resilience study. Geriatr. Nurs. 2018, 39, 663–668. [Google Scholar] [CrossRef]
- McLennon, S.M.; Anderson, J.G.; Epps, F.; Rose, K.M. “It’s just part of life”: African American daughters caring for parents with dementia. J. Women Aging 2020, 32, 168–182. [Google Scholar] [CrossRef]
- Heo, G.J.; Koeske, G. The role of religious coping and race in Alzheimer’s disease caregiving. J. Appl. Gerontol. 2013, 32, 582–604. [Google Scholar] [CrossRef]
- Epps, F.; Alexander, K.; Brewster, G.S.; Parker, L.J.; Chester, M.; Tomlinson, A.; Adkins, A.; Zingg, S.; Thornton, J. Promoting dementia awareness in African-American faith communities. Public Health Nurs. 2020, 37, 715–721. [Google Scholar] [CrossRef]
- Knight, B.G.; Silverstein, M.; McCallum, T.; Fox, L.S. A sociocultural stress and coping model for mental health outcomes among African American caregivers in Southern California. J. Gerontol. Ser. B Psychol. Sci. Soc. Sci. 2000, 55, P142–P150. [Google Scholar] [CrossRef]
- Dilworth-Anderson, P.; Brummett, B.H.; Goodwin, P.; Williams, S.W.; Williams, R.B.; Siegler, I.C. Effect of race on cultural justifications for caregiving. J. Gerontol. Ser. B Psychol. Sci. Soc. Sci. 2005, 60, S257–S262. [Google Scholar] [CrossRef]
- Samson, Z.B.; Parker, M.; Dye, C.; Hepburn, K. Experiences and learning needs of African American family dementia caregivers. Am. J. Alzheimer’s Dis. Other Dement.® 2016, 31, 492–501. [Google Scholar] [CrossRef]
- Dilworth-Anderson, P.; Boswell, G.; Cohen, M.D. Spiritual and religious coping values and beliefs among African American caregivers: A qualitative study. J. Appl. Gerontol. 2007, 26, 355–369. [Google Scholar] [CrossRef]
- Turner, W.L.; Wallace, B.R.; Anderson, J.R.; Bird, C. The last mile of the way: Understanding caregiving in African American families at the end-of-life. J. Marital Fam. Ther. 2004, 30, 427–438. [Google Scholar] [CrossRef]
- Cothran, F.A.; Paun, O.; Strayhorn, S.; Barnes, L.L. ‘Walk a mile in my shoes:’ African American caregiver perceptions of caregiving and self-care. Ethn. Health 2022, 27, 435–452. [Google Scholar] [CrossRef] [PubMed]
- Guest, M.A.; Smith, M.P. In Our Community, Dementia Speaks: Pilot of a person-centered training targeting African-American caregivers of persons-living with dementia (innovative practice). Dementia 2021, 20, 391–397. [Google Scholar] [CrossRef]
- Czaja, S.J.; Loewenstein, D.; Schulz, R.; Nair, S.N.; Perdomo, D. A videophone psychosocial intervention for dementia caregivers. Am. J. Geriatr. Psychiatry 2013, 21, 1071–1081. [Google Scholar] [CrossRef] [PubMed]
- Brewster, G.S.; Epps, F.; Dye, C.E.; Hepburn, K.; Higgins, M.K.; Parker, M.L. The effect of the “Great Village” on psychological outcomes, burden, and mastery in African American caregivers of persons living with dementia. J. Appl. Gerontol. 2020, 39, 1059–1068. [Google Scholar] [CrossRef] [PubMed]
- Wells, B.A.; Glueckauf, R.L.; Bernabe, D.; Kazmer, M.M.; Schettini, G.; Springer, J.; Sharma, D.; Meng, H.; Willis, F.B.; Graff-Radford, N. African American Dementia Caregiver Problem Inventory: Descriptive analysis and initial psychometric evaluation. Rehabil. Psychol. 2017, 62, 25–35. [Google Scholar] [CrossRef]
- Bisht, J.; Rawat, P.; Sehar, U.; Reddy, P.H. Caregivers with Cancer Patients: Focus on Hispanics. Cancers 2023, 15, 626. [Google Scholar] [CrossRef]
- Liu, C.; Badana, A.N.; Burgdorf, J.; Fabius, C.D.; Roth, D.L.; Haley, W.E. Systematic review and meta-analysis of racial and ethnic differences in dementia caregivers’ well-being. Gerontologist 2021, 61, e228–e243. [Google Scholar] [CrossRef]
- Mars, D.; Davis, B.L.; Montgomery, A.J.; Gregoski, M.J.; Burns, D.P.; Coffey, D. The lived experience of African-American informal caregivers of family members with Alzheimer’s disease and related dementias. J. Natl. Black Nurses Assoc. 2017, 28, 19–25. [Google Scholar]
- Musa, D.; Schulz, R.; Harris, R.; Silverman, M.; Thomas, S.B. Trust in the health care system and the use of preventive health services by older black and white adults. Am. J. Public Health 2009, 99, 1293–1299. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, D.D.; Manheim, L.M.; Song, J.; Chang, R.W. Gender and ethnic/racial disparities in health care utilization among older adults. J. Gerontol. Ser. B Psychol. Sci. Soc. Sci. 2002, 57, S221–S233. [Google Scholar] [CrossRef]
- Alliance, F.C.; National Center on Caregiving. Definitions. 2018. Available online: https://eastonad.ucla.edu/sites/g/files/oketem336/files/media/documents/Depression_and_Caregiving-FCA-2021.pdf (accessed on 6 March 2022).
- Newlin, K.; Knafl, K.; Melkus, G.D.E. African-American spirituality: A concept analysis. Adv. Nurs. Sci. 2002, 25, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Goosby, B.J.; Caldwell, C.H.; Bellatorre, A.; Jackson, J.S. Ethnic differences in family stress processes among African-Americans and Black Caribbeans. J. Afr. Am. Stud. 2012, 16, 406–422. [Google Scholar] [CrossRef]
- Seaton, E.K.; Caldwell, C.H.; Sellers, R.M.; Jackson, J.S. The prevalence of perceived discrimination among African American and Caribbean Black youth. Dev. Psychol. 2008, 44, 1288. [Google Scholar] [CrossRef] [PubMed]
- Cross, C.J.; Taylor, R.J.; Chatters, L.M. Family social support networks of African American and Black Caribbean adolescents. J. Child Fam. Stud. 2018, 27, 2757–2771. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A. Promoting culturally affirming parenting in African-American parents: Positive parenting in African American families. CYF News, 1 April 2017. [Google Scholar]
- Wilson, M.N.; Greene-Bates, C.; McKim, L.; Simmons, F.; Askew, T.; Curry-El, J.; Hinton, I.D. African American family life: The dynamics of interactions, relationships, and roles. New Dir. Child Adolesc. Dev. 1995, 1995, 5–21. [Google Scholar] [CrossRef]
- Wilson, M.N.; Tolson, T.F. A social interaction analysis of two-and three-generational Black families. In Praise of Fifty Years: Groves Conference on the Conservation of Marriage and the Family; Graphic Publishing Co.: Huntsville, AL, USA, 1986; pp. 43–53. [Google Scholar]
- Wilson, M.N. The Black extended family: An analytical consideration. Dev. Psychol. 1986, 22, 246. [Google Scholar] [CrossRef]
- Foster, H.J. African patterns in the Afro-American family. J. Black Stud. 1983, 14, 201–232. [Google Scholar] [CrossRef]
- Moss, K.O.; Deutsch, N.L.; Hollen, P.J.; Rovnyak, V.G.; Williams, I.C.; Rose, K.M. Understanding end-of-life decision-making terminology among African American older adults. J. Gerontol. Nurs. 2018, 44, 33–40. [Google Scholar] [CrossRef]
- Hill, S.A. Ethnicity and the ethic of caring in African American families. J. Pers. Interpers. Loss 1997, 2, 109–128. [Google Scholar] [CrossRef]
- Sabo, K.; Chin, E. Self-care needs and practices for the older adult caregiver: An integrative review. Geriatr. Nurs. 2021, 42, 570–581. [Google Scholar] [CrossRef] [PubMed]
- Spector, W.D.; Cohen, J.W.; Pesis-Katz, I. Home care before and after the Balanced Budget Act of 1997: Shifts in financing and services. Gerontologist 2004, 44, 39–47. [Google Scholar] [CrossRef]
- Molloy, G.J.; Johnston, D.W.; Witham, M.D. Family caregiving and congestive heart failure. Review and analysis. Eur. J. Heart Fail. 2005, 7, 592–603. [Google Scholar] [CrossRef] [PubMed]
- Napoles, A.M.; Chadiha, L.; Eversley, R.; Moreno-John, G. Reviews: Developing culturally sensitive dementia caregiver interventions: Are we there yet? Am. J. Alzheimer’s Dis. Other Dement.® 2010, 25, 389–406. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.A.; Thompson, H.S.; Watkins, D.C.; Shires, D.; Modlin, C.S., Jr. Peer Reviewed: Disparities in Health-Related Internet Use Among African American Men, 2010. Prev. Chronic Dis. 2014, 11, E43. [Google Scholar] [CrossRef][Green Version]
- Hostetter, M.; Klein, S. Focus: Reducing Racial Disparities in Health Care by Confronting Racism; The Commonwealth Fund: New York, NY, USA, 27 September 2018. [Google Scholar]
- Flanagin, A.; Frey, T.; Christiansen, S.L.; Committee, A.M.o.S. Updated Guidance on the Reporting of Race and Ethnicity in Medical and Science Journals. JAMA 2021, 326, 621–627. [Google Scholar] [CrossRef]
- Williams, D.R.; Cooper, L.A. Reducing Racial Inequities in Health: Using What We Already Know to Take Action. Int. J. Environ. Res. Public Health 2019, 16, 606. [Google Scholar] [CrossRef]


| Dementia | Features |
|---|---|
| Frontotemporal Dementia (FTD) |
|
| Lewy-body Dementia (LBD) |
|
| Multiple Etiology Dementia (MED) |
|
| Vascular Dementia (VD) |
|
| Race or Minority Group | Definition |
|---|---|
| White | A person with origins from any of the original peoples of Europe, the Middle East, or North Africa |
| Black or African American | A person with origins from any of the Black racial groups of Africa. |
| American Indian or Alaska Native | A person with origins from any of the original peoples of North and South America (including Central America) and who maintains tribal affiliation or community attachment. |
| Asian | A person with origins from any of the original peoples of the Far East, Southeast Asia, or the Indian subcontinent including, for example, Cambodia, China, India, Japan, Korea, Malaysia, Pakistan, the Philippine Islands, Thailand, and Vietnam. |
| Native Hawaiian or Other Pacific Islander | A person with origins from any of the original peoples of Hawaii, Guam, Samoa, or other Pacific Islands. |
| Multi-racial | Identifying as one of the above five major groups according to the U.S. Census Bureau |
| White | African American |
|---|---|
| CR1 (complement activation) | CR1 (complement activation) |
| BIN1 (endocytosis/apoptosis) | BIN1 (endocytosis/apoptosis) |
| EPHA1 (nervous system development) | EPHA1 (nervous system development) |
| CD33 (immune system) | CD33 (immune system) |
| ABCA7 (lipid metabolism) | ABCA7 (lipid metabolism) |
| APOE (lipid metabolism) | APOE (lipid metabolism) |
| CLU (chaperone protein) | HMHA1 (cytoskeletal remodeling and cell spreading) |
| MS4A6A/MS4E4 (Induces anti-inflammatory neuroprotective phenotype) | GRIN3B (Encodes a subunit of the N-methyl-D-aspartate (NMDA) receptor) |
| CD2AP (Stabilizes the interaction between T cells to antigen-presenting cells) | |
| PICALM (Regulates APP internalization and subsequent Aβ generation) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kopel, J.; Sehar, U.; Choudhury, M.; Reddy, P.H. Alzheimer’s Disease and Alzheimer’s Disease-Related Dementias in African Americans: Focus on Caregivers. Healthcare 2023, 11, 868. https://doi.org/10.3390/healthcare11060868
Kopel J, Sehar U, Choudhury M, Reddy PH. Alzheimer’s Disease and Alzheimer’s Disease-Related Dementias in African Americans: Focus on Caregivers. Healthcare. 2023; 11(6):868. https://doi.org/10.3390/healthcare11060868
Chicago/Turabian StyleKopel, Jonathan, Ujala Sehar, Moumita Choudhury, and P. Hemachandra Reddy. 2023. "Alzheimer’s Disease and Alzheimer’s Disease-Related Dementias in African Americans: Focus on Caregivers" Healthcare 11, no. 6: 868. https://doi.org/10.3390/healthcare11060868
APA StyleKopel, J., Sehar, U., Choudhury, M., & Reddy, P. H. (2023). Alzheimer’s Disease and Alzheimer’s Disease-Related Dementias in African Americans: Focus on Caregivers. Healthcare, 11(6), 868. https://doi.org/10.3390/healthcare11060868







