Effect of Load Distribution on Trunk Muscle Activity with Lunge Exercises in Amateur Athletes: Cross-Sectional Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Sample Size Calculation
2.3. Sample
2.4. Outcomes
2.5. Procedure
2.5.1. Preparatory Procedures
2.5.2. Electrode Placement
2.5.3. Normalisation of the EMG
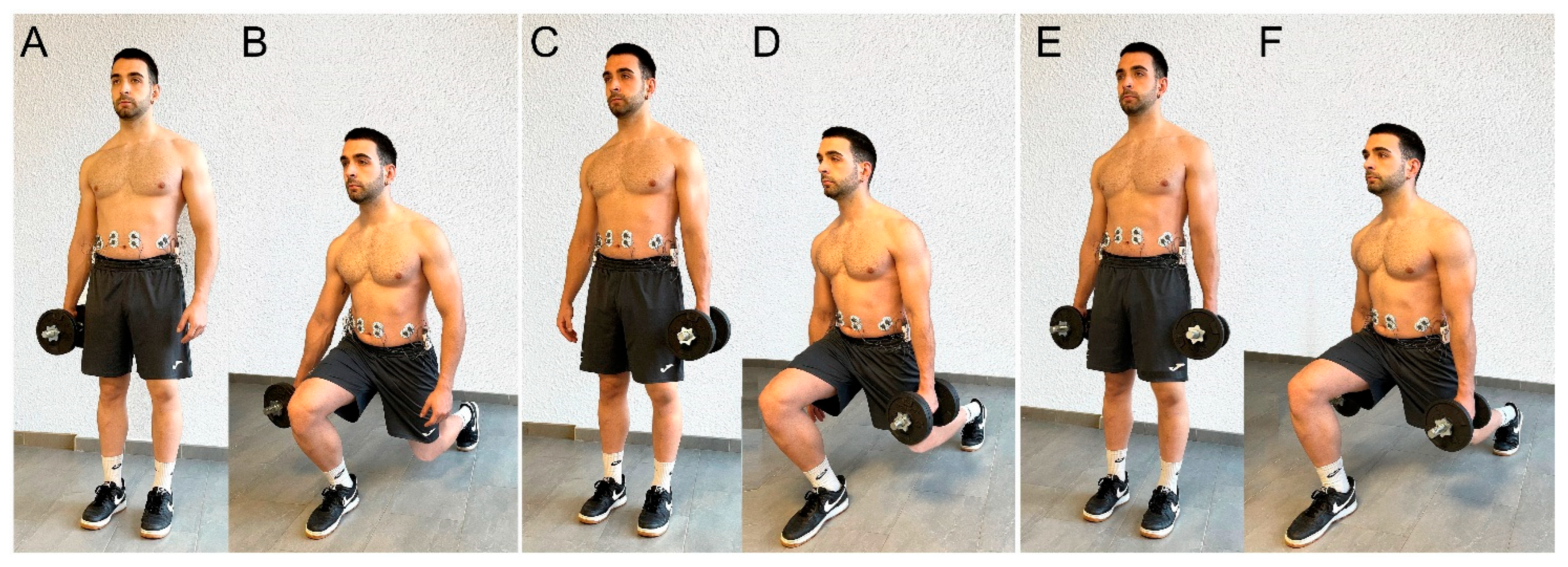
2.5.4. Lunge
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, H.-W.; Tsai, C.-F.; Liang, K.-H.; Chang, Y.-W. Effect of Loading Devices on Muscle Activation in Squat and Lunge. J. Sport Rehabil. 2020, 29, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Andersen, L.L.; Andersen, C.H.; Mortensen, O.S.; Poulsen, O.M.; Bjørnlund, I.B.T.; Zebis, M.K. Muscle Activation and Perceived Loading During Rehabilitation Exercises: Comparison of Dumbbells and Elastic Resistance. Phys. Ther. 2010, 90, 538–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGill, S.M.; Marshall, L.W. Kettlebell Swing, Snatch, and Bottoms-Up Carry: Back and Hip Muscle Activation, Motion, and Low Back Loads. J. Strength Cond. Res. 2012, 26, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Ylinen, J.; Takala, E.-P.; Nykänen, M.; Häkkinen, A.; Mälkiä, E.; Pohjolainen, T.; Karppi, S.-L.; Kautiainen, H.; Airaksinen, O. Active Neck Muscle Training in the Treatment of Chronic Neck Pain in Women. JAMA 2003, 289, 2509. [Google Scholar] [CrossRef] [PubMed]
- Nelson, N.L. Muscle Strengthening Activities and Fibromyalgia: A Review of Pain and Strength Outcomes. J. Bodyw. Mov. Ther. 2015, 19, 370–376. [Google Scholar] [CrossRef]
- Shimada, K.; Onishi, T.; Ogawa, Y.; Yamauchi, J.; Kawada, S. Effects of Motor Imagery Combined with Action Observation Training on the Lateral Specificity of Muscle Strength in Healthy Subjects. Biomed. Res. 2019, 40, 107–113. [Google Scholar] [CrossRef] [Green Version]
- Heinecke, M.L.; Mauldin, M.L.; Hunter, M.L.; Mann, J.B.; Mayhew, J.L. Relationship of Barbell and Dumbbell Repetitions with One Repetition Maximum Bench Press in College Football Players. J. Strength Cond. Res. 2021, 35, S66–S71. [Google Scholar] [CrossRef]
- Oliveira, M.; Júnior, P.L.; Imoto, A.M.; Santos, T.; Borges, J.H.S.; Nunes, P.; Barin, F.R.; Damando, M.; Peccin, M.S. Unilateral Versus Bilateral Resistance Exercise in Postoperative Rehabilitation After ACL Reconstruction with Bone–Patellar Tendon–Bone Graft: A Randomized Controlled Trial. Orthop. J. Sport. Med. 2022, 10, 232596712210888. [Google Scholar] [CrossRef]
- Nijem, R.M.; Galpin, A.J. Unilateral Versus Bilateral Exercise and the Role of the Bilateral Force Deficit. Strength Cond. J. 2014, 36, 113–118. [Google Scholar] [CrossRef]
- Graber, K.A.; Loverro, K.L.; Baldwin, M.; Nelson-Wong, E.; Tanor, J.; Lewis, C.L. Hip and Trunk Muscle Activity and Mechanics During Walking with and without Unilateral Weight. J. Appl. Biomech. 2021, 37, 351–358. [Google Scholar] [CrossRef]
- Stastny, P.; Lehnert, M.; Zaatar, A.M.Z.; Svoboda, Z.; Xaverova, Z. Does the Dumbbell-Carrying Position Change the Muscle Activity in Split Squats and Walking Lunges? J. Strength Cond. Res. 2015, 29, 3177–3187. [Google Scholar] [CrossRef] [Green Version]
- Gao, L.; Lu, Z.; Liang, M.; Baker, J.S.; Gu, Y. Influence of Different Load Conditions on Lower Extremity Biomechanics during the Lunge Squat in Novice Men. Bioengineering 2022, 9, 272. [Google Scholar] [CrossRef]
- Bouillon, L.E.; Wilhelm, J.; Eisel, P.; Wiesner, J.; Rachow, M.; Hatteberg, L. Electromyographic Assessment of Muscle Activity between Genders during Unilateral Weight-Bearing Tasks Using Adjusted Distances. Int. J. Sports Phys. Ther. 2012, 7, 595–605. [Google Scholar]
- Ekstrom, R.A.; Donatelli, R.A.; Carp, K.C. Electromyographic Analysis of Core Trunk, Hip, and Thigh Muscles During 9 Rehabilitation Exercises. J. Orthop. Sport. Phys. Ther. 2007, 37, 754–762. [Google Scholar] [CrossRef] [Green Version]
- Krause, D.A.; Elliott, J.J.; Fraboni, D.F.; McWilliams, T.J.; Rebhan, R.L.; Hollman, J.H. Electromyography of the hip and thigh muscles during two variations of the lunge exercise: A cross-sectional study. Int. J. Sports Phys. Ther. 2018, 13, 137–142. [Google Scholar] [CrossRef] [Green Version]
- Mausehund, L.; Skard, A.E.; Krosshaug, T. Muscle Activation in Unilateral Barbell Exercises: Implications for Strength Training and Rehabilitation. J. Strength Cond. Res. 2019, 33, S85–S94. [Google Scholar] [CrossRef]
- Flanagan, S.P.; Wang, M.-Y.; Greendale, G.A.; Azen, S.P.; Salem, G.J. Biomechanical Attributes of Lunging Activities for Older Adults. J. Strength Cond. Res. 2004, 18, 599. [Google Scholar] [CrossRef]
- Farrokhi, S.; Pollard, C.D.; Souza, R.B.; Chen, Y.-J.; Reischl, S.; Powers, C.M. Trunk Position Influences the Kinematics, Kinetics, and Muscle Activity of the Lead Lower Extremity During the Forward Lunge Exercise. J. Orthop. Sport. Phys. Ther. 2008, 38, 403–409. [Google Scholar] [CrossRef] [Green Version]
- Oliva-Lozano, J.M.; Muyor, J.M. Core Muscle Activity during Physical Fitness Exercises: A Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 4306. [Google Scholar] [CrossRef]
- Lehman, G.J.; Buchan, D.D.; Lundy, A.; Myers, N.; Nalborczyk, A. Variations in Muscle Activation Levels during Traditional Latissimus Dorsi Weight Training Exercises: An Experimental Study. Dyn. Med. 2004, 3, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Lake, J.P.; Lauder, M.A. Mechanical Demands of Kettlebell Swing Exercise. J. Strength Cond. Res. 2012, 26, 3209–3216. [Google Scholar] [CrossRef] [PubMed]
- Harrison, A.L.; Thorp, J.N.; Ritzline, P.D. A Proposed Diagnostic Classification of Patients with Temporomandibular Disorders: Implications for Physical Therapists. J. Orthop. Sport. Phys. Ther. 2014, 44, 182–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brumitt, J.; En Gilpin, H.; Brunette, M.; Meira, E.P. Incorporating Kettlebells into a Lower Extremity Sports Rehabilitation Program. N. Am. J. Sports Phys. Ther. 2010, 5, 257–265. [Google Scholar] [PubMed]
- Lanza, M.B. The Lack of Electromyography Normalization May Limit the Conclusions in: Traditional vs. Suspended Push-up Muscle Activation in Athletes and Sedentary Women. J. Strength Cond. Res. 2018, 32, e58. [Google Scholar] [CrossRef]
- Vigotsky, A.D.; Halperin, I.; Lehman, G.J.; Trajano, G.S.; Vieira, T.M. Interpreting Signal Amplitudes in Surface Electromyography Studies in Sport and Rehabilitation Sciences. Front. Physiol. 2018, 8, 985. [Google Scholar] [CrossRef] [Green Version]
- French, H.P.; Dunleavy, M.; Cusack, T. Activation Levels of Gluteus Medius during Therapeutic Exercise as Measured with Electromyography: A Structured Review. Phys. Ther. Rev. 2010, 15, 92–105. [Google Scholar] [CrossRef]
- Marcolin, G.; Panizzolo, F.A.; Petrone, N.; Moro, T.; Grigoletto, D.; Piccolo, D.; Paoli, A. Differences in Electromyographic Activity of Biceps Brachii and Brachioradialis While Performing Three Variants of Curl. PeerJ 2018, 6, e5165. [Google Scholar] [CrossRef]
- Komi, P.V.; Linnamo, V.; Silventoinen, P.; Sillanpää, M. Force and EMG Power Spectrum during Eccentric and Concentric Actions. Med. Sci. Sports Exerc. 2000, 32, 1757–1762. [Google Scholar] [CrossRef]
- Marchetti, P.H.; Guiselini, M.A.; da Silva, J.J.; Tucker, R.; Behm, D.G.; Brown, L.E. Balance and Lower Limb Muscle Activation Between In-Line and Traditional Lunge Exercises. J. Hum. Kinet. 2018, 62, 15–22. [Google Scholar] [CrossRef] [Green Version]
- Muyor, J.M.; Martín-Fuentes, I.; Rodríguez-Ridao, D.; Antequera-Vique, J.A. Electromyographic Activity in the Gluteus Medius, Gluteus Maximus, Biceps Femoris, Vastus Lateralis, Vastus Medialis and Rectus Femoris during the Monopodal Squat, Forward Lunge and Lateral Step-Up Exercises. PLoS ONE 2020, 15, e0230841. [Google Scholar] [CrossRef]
- Dwyer, M.K.; Boudreau, S.N.; Mattacola, C.G.; Uhl, T.L.; Lattermann, C. Comparison of Lower Extremity Kinematics and Hip Muscle Activation During Rehabilitation Tasks Between Sexes. J. Athl. Train. 2010, 45, 181–190. [Google Scholar] [CrossRef] [Green Version]
- Jönhagen, S.; Halvorsen, K.; Benoit, D.L. Muscle Activation and Length Changes during Two Lunge Exercises: Implications for Rehabilitation. Scand. J. Med. Sci. Sports 2009, 19, 561–568. [Google Scholar] [CrossRef]
- Bezerra, E.D.S.; Diefenthaeler, F.; Nunes, J.P.; Sakugawa, R.L.; Heberle, I.; Moura, B.M.; Moro, A.R.P.; Marcolin, G.; Paoli, A. Influence of Trunk Position during Three Lunge Exercises on Muscular Activation in Trained Women. Int. J. Exerc. Sci. 2021, 14, 202–210. [Google Scholar]
- Cholewicki, J.; Simons, A.P.D.; Radebold, A. Effects of External Trunk Loads on Lumbar Spine Stability. J. Biomech. 2000, 33, 1377–1385. [Google Scholar] [CrossRef]
- Radebold, A.; Cholewicki, J.; Panjabi, M.M.; Patel, T.C. Muscle Response Pattern to Sudden Trunk Loading in Healthy Individuals and in Patients with Chronic Low Back Pain. Spine 2000, 25, 947–954. [Google Scholar] [CrossRef]
- Ferguson, S.A.; Marras, W.S.; Burr, D.L.; Davis, K.G.; Gupta, P. Differences in Motor Recruitment and Resulting Kinematics between Low Back Pain Patients and Asymptomatic Participants during Lifting Exertions. Clin. Biomech. 2004, 19, 992–999. [Google Scholar] [CrossRef]
- Hanada, E.Y.; Johnson, M.; Hubley-Kozey, C. A Comparison of Trunk Muscle Activation Amplitudes During Gait in Older Adults with and without Chronic Low Back Pain. PM&R 2011, 3, 920–928. [Google Scholar] [CrossRef]
- Hodges, P.W.; Moseley, G.L. Pain and Motor Control of the Lumbopelvic Region: Effect and Possible Mechanisms. J. Electromyogr. Kinesiol. 2003, 13, 361–370. [Google Scholar] [CrossRef]
- Nelson-Wong, E.; Callaghan, J.P. Is Muscle Co-Activation a Predisposing Factor for Low Back Pain Development during Standing? A Multifactorial Approach for Early Identification of at-Risk Individuals. J. Electromyogr. Kinesiol. 2010, 20, 256–263. [Google Scholar] [CrossRef]
- Nelson-Wong, E.; Alex, B.; Csepe, D.; Lancaster, D.; Callaghan, J.P. Altered Muscle Recruitment during Extension from Trunk Flexion in Low Back Pain Developers. Clin. Biomech. 2012, 27, 994–998. [Google Scholar] [CrossRef]
- Molina-Molina, A.; Ruiz-Malagón, E.J.; Carrillo-Pérez, F.; Roche-Seruendo, L.E.; Damas, M.; Banos, O.; García-Pinillos, F. Validation of MDurance, A Wearable Surface Electromyography System for Muscle Activity Assessment. Front. Physiol. 2020, 11, 606287. [Google Scholar] [CrossRef] [PubMed]
- Hermens, H.J.; Freriks, B.; Merletti, R.; Stegeman, D.; Blok, J.; Rau, G.; Disselhorst-Klug, C.; Hägg, G. European Recommendations for Surface ElectroMyoGraphy; Roessingh Research and Development: Enschede, The Netherlands, 1999; pp. 8–11. [Google Scholar]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of Recommendations for SEMG Sensors and Sensor Placement Procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Murofushi, K.; Oshikawa, T.; Kaneoka, K.; Akuzawa, H.; Yamaguchi, D.; Mitomo, S.; Furuya, H.; Hirohata, K.; Yagishita, K. Differences in Trunk and Lower Extremity Muscle Activity during Squatting Exercise with and without Hammer Swing. Sci. Rep. 2022, 12, 13387. [Google Scholar] [CrossRef] [PubMed]
- Escamilla, R.F.; Babb, E.; DeWitt, R.; Jew, P.; Kelleher, P.P.; Burnham, T.; Busch, J.; D’Anna, K.; Mowbray, R.; Imamura, R.T. Electromyographic Analysis of Traditional and Nontraditional Abdominal Exercises: Implications for Rehabilitation and Training. Phys. Ther. 2006, 86, 656–671. [Google Scholar] [CrossRef] [PubMed]
- Martinez, R.; Assila, N.; Goubault, E.; Begon, M. Sex Differences in Upper Limb Musculoskeletal Biomechanics during a Lifting Task. Appl. Ergon. 2020, 86, 103106. [Google Scholar] [CrossRef]
- Bolgla, L.A.; Cruz, M.F.; Roberts, L.H.; Buice, A.M.; Pou, T.S. Relative Electromyographic Activity in Trunk, Hip, and Knee Muscles during Unilateral Weight Bearing Exercises: Implications for Rehabilitation. Physiother. Theory Pract. 2016, 3985, 130–138. [Google Scholar] [CrossRef]
- Lauder, M.A.; Lake, J.P. Biomechanical Comparison of Unilateral and Bilateral Power Snatch Lifts. J. Strength Cond. Res. 2008, 22, 653–660. [Google Scholar] [CrossRef]
- Arokoski, J.P.; Valta, T.; Airaksinen, O.; Kankaanpää, M. Back and Abdominal Muscle Function during Stabilization Exercises. Arch. Phys. Med. Rehabil. 2001, 82, 1089–1098. [Google Scholar] [CrossRef]
- Calatayud, J.; Colado, J.C.; Martin, F.; Casaña, J.; Jakobsen, M.D.; Andersen, L.L. Core muscle activity during the clean and jerk lift with barbell versus sandbags and water bags. Int. J. Sports Phys. Ther. 2015, 10, 803–810. [Google Scholar]

| Men (n = 21) | Women (n = 21) | |
|---|---|---|
| Age (years) | 23.71 ± 4.71 | 24.33 ± 9.43 |
| Height (meters) | 1.78 ± 0.04 | 1.67 ± 0.06 |
| Weight (kilograms) | 72.52 ± 8.68 | 57.29 ± 8.56 |
| BMI (kg/m2) | 22.87 ± 2.64 | 20.56 ± 2.69 |
| Dominance | ||
| Right | 15 (71.4%) | 19 (90.5%) |
| Left | 6 (28.6%) | 2 (9.5%) |
| Type of Sport | ||
| Fitness | 12 (57.1%) | 10 (47.6%) |
| Track sport | 3 (14.3%) | 3 (14.3%) |
| Field Sport | 1 (4.8%) | 3 (14.3%) |
| Others | 5 (23.8%) | 5 (23.8%) |
| Weekly hours of sport | 6.71 ± 3.54 | 5.98 ± 3.88 |
| Anterior Side | Posterior Side | Difference between Anterior–Posterior Side | ||||
|---|---|---|---|---|---|---|
| RMS (%MVIC) | RMS (%MVIC) | Mean | 95% CI | p | ŋ2 | |
| MEN | ||||||
| Lunge Ipsilateral Load | ||||||
| Latissimus dorsi | 10.00 ± 7.37 | 11.02 ± 9.74 | −1.02 | −5.73; 3.69 | 0.655 | 0.00 |
| Erector spinae | 9.87 ± 4.69 | 16.24 ± 8.42 | −6.37 | −10.65; −2.09 | 0.006 | 0.18 |
| Rectus abdominis | 6.32 ± 5.12 | 8.44 ± 8.56 | −2.12 | −6.61; 2.37 | 0.336 | 0.02 |
| External oblique | 17.69 ± 13.07 | 26.83 ± 17.81 | −9.14 | −19.43; 1.16 | 0.079 | 0.08 |
| Lunge Contralateral Load | ||||||
| Latissimus dorsi | 8.69 ± 4.50 | 14.00 ± 14.53 | −5.31 | −11.44; 0.83 | 0.086 | 0.06 |
| Erector spinae | 15.05 ± 13.07 | 10.36 ± 6.20 | 4.69 | 0.15; 9.23 | 0.044 | 0.05 |
| Rectus abdominis | 6.86 ± 3.95 | 5.35 ± 3.78 | 1.51 | −0.62; 3.64 | 0.155 | 0.04 |
| External oblique | 35.16 ± 26.44 | 15.36 ± 9.90 | 19.80 | 7.61; 31.99 | 0.003 | 0.20 |
| Lunge Bilateral Load | ||||||
| Latissimus dorsi | 8.12 ± 4.38 | 10.16 ± 8.29 | −2.04 | −1.31; 5.40 | 0.219 | 0.02 |
| Erector spinae | 11.40 ± 4.94 | 14.07 ± 10.99 | −2.68 | −7.66; 2.31 | 0.277 | 0.02 |
| Rectus abdominis | 5.39 ± 3.48 | 5.37 ± 3.52 | 0.17 | −2.01; 2.05 | 0.987 | 0.00 |
| External oblique | 18.39 ± 13.27 | 15.51 ± 9.28 | 2.89 | −1.59; 7.36 | 0.193 | 0.01 |
| WOMEN | ||||||
| Lunge Ipsilateral Load | ||||||
| Latissimus dorsi | 23.48 ± 26.96 | 17.03 ± 9.98 | 6.46 | −5.40; 18.31 | 0.269 | 0.02 |
| Erector spinae | 11.51 ± 7.22 | 23.89 ± 19.51 | −12.38 | −18.79; −5.96 | 0.001 | 0.15 |
| Rectus abdominis | 7.57 ± 4.91 | 10.01 ± 5.75 | −2.43 | −4.91; 0.04 | 0.053 | 0.05 |
| External oblique | 22.43 ± 15.97 | 35.31 ± 20.60 | −2.88 | −26.43; 0.68 | 0.061 | 0.11 |
| Lunge Contralateral Load | ||||||
| Latissimus dorsi | 16.27 ± 21.05 | 20.23 ± 24.64 | −3.96 | −12.15; 4.23 | 0.325 | 0.01 |
| Erector spinae | 21.74 ± 22.03 | 12.26 ± 8.91 | 9.48 | 1.26; 17.70 | 0.026 | 0.07 |
| Rectus abdominis | 10.58 ± 9.41 | 9.14 ± 8.06 | 1.45 | −3.93; 6.83 | 0.581 | 0.01 |
| External oblique | 39.19 ± 26.96 | 22.25 ± 14.32 | 16.94 | 4.15; 29.74 | 0.012 | 0.14 |
| Lunge Bilateral Load | ||||||
| Latissimus dorsi | 11.44 ± 8.17 | 16.61 ± 15.88 | −5.17 | −12.18; 1.84 | 0.140 | 0.04 |
| Erector spinae | 15.69 ± 15.33 | 16.01 ± 10.06 | −0.32 | −3.88; 3.24 | 0.853 | 0.00 |
| Rectus abdominis | 6.65 ± 3.36 | 8.62 ± 6.77 | −1.97 | −4.65; 0.72 | 0.142 | 0.03 |
| External oblique | 26.10 ± 19.39 | 21.64 ± 13.73 | 4.48 | −1.57; 10.50 | 0.139 | 0.01 |
| Men | Women | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Lunge vs. Lunge | Mean | 95% CI | p | ŋ2 | Mean | 95% CI | p | ŋ2 | ||
| Anterior Side | ||||||||||
| LIL | LCL | 1.30 | −2.38; 4.98 | 1.000 | 0.01 | 7.21 | −4.60; 19.03 | 0.379 | 0.02 | |
| Latissimus dorsi | LIL | LBL | 1.88 | −1.05; 4.81 | 0.328 | 0.00 | 12.05 | −2.01; 26.10 | 0.110 | 0.08 |
| LCL | LBL | 0.58 | −0.98; 2.13 | 1.000 | 0.00 | 4.83 | −7.09; 16.75 | 0.907 | 0.02 | |
| LIL | LCL | −5.18 | −9.19; −1.16 | 0.009 | 0.07 | −10.22 | −19.72; −0.73 | 0.032 | 0.09 | |
| Erector spinae | LIL | LBL | −1.53 | −3.88; 0.83 | 0.319 | 0.03 | −4.17 | −9.70; 1.36 | 0.189 | 0.03 |
| LCL | LBL | 3.65 | 0.92; 6.39 | 0.007 | 0.03 | 6.05 | 1.84; 10.26 | 0.004 | 0.02 | |
| LIL | LCL | −0.55 | −2.70; 1.60 | 1.000 | 0.00 | −3.01 | −8.00; 1.99 | 0.393 | 0.04 | |
| Rectus abdominis | LIL | LBL | 0.93 | −0.38; 2.24 | 0.236 | 0.01 | 0.92 | −0.73; 2.58 | 0.484 | 0.01 |
| LCL | LBL | 1.48 | 0.30; 2.65 | 0.011 | 0.04 | 3.93 | −1.23; 9.09 | 0.181 | 0.07 | |
| LIL | LCL | −17.47 | −29.21; −5.73 | 0.003 | 0.18 | −16.76 | −35.13; 1.62 | 0.082 | 0.13 | |
| External oblique | LIL | LBL | −0.70 | −4.42; 3.02 | 1.000 | 0.00 | −3.67 | −16.22; 8.89 | 1.000 | 0.01 |
| LCL | LBL | 16.77 | 6.69; 26.84 | 0.001 | 0.14 | 13.09 | 3.96; 22.22 | 0.004 | 0.07 | |
| Posterior Side | ||||||||||
| LIL | LCL | −2.98 | −8.31; 2.35 | 0.480 | 0.01 | −3.20 | −15.31; 8.90 | 1.000 | 0.01 | |
| Latissimus dorsi | LIL | LBL | 0.86 | −2.15; 3.87 | 1.000 | 0.00 | 0.42 | −7.95; 8.78 | 1.000 | 0.00 |
| LCL | LBL | 3.84 | −0.15; 7.83 | 0.062 | 0.03 | 3.62 | −2.99; 10.23 | 0.504 | 0.01 | |
| LIL | LCL | 5.88 | 0.75; 11.02 | 0.022 | 0.14 | 11.63 | 3.11; 20.15 | 0.006 | 0.23 | |
| Erector spinae | LIL | LBL | 2.17 | −2.97; 7.31 | 0.849 | 0.01 | 7.88 | 1.77; 14.00 | 0.009 | 0.11 |
| LCL | LBL | −3.71 | −8.20; 0.77 | 0.129 | 0.04 | −3.75 | −6.95; −0.55 | 0.019 | 0.04 | |
| LIL | LCL | 3.08 | −0.23; 6.40 | 0.074 | 0.05 | 0.87 | −3.87; 5.61 | 1.000 | 0.00 | |
| Rectus abdominis | LIL | LBL | 3.07 | −0.22; 6.36 | 0.073 | 0.05 | 1.39 | −0.45; 3.23 | 0.186 | 0.01 |
| LCL | LBL | −0.02 | −0.82; 0.79 | 1.000 | 0.00 | 0.52 | −3.78; 4.81 | 1.000 | 0.00 | |
| LIL | LCL | 11.47 | 1.45; 21.50 | 0.022 | 0.14 | 13.06 | −1.38; 27.51 | 0.085 | 0.12 | |
| External oblique | LIL | LBL | 11.33 | 2.39; 20.26 | 0.010 | 0.14 | 13.68 | 3.21; 24.15 | 0.008 | 0.13 |
| LCL | LBL | −0.15 | −4.03; 3.74 | 1.000 | 0.00 | 0.61 | −7.56; 8.79 | 1.000 | 0.00 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-de-Celis, C.; Labata-Lezaun, N.; Romaní-Sánchez, S.; Gassó-Villarejo, S.; Garcia-Ribell, E.; Rodríguez-Sanz, J.; Pérez-Bellmunt, A. Effect of Load Distribution on Trunk Muscle Activity with Lunge Exercises in Amateur Athletes: Cross-Sectional Study. Healthcare 2023, 11, 916. https://doi.org/10.3390/healthcare11060916
López-de-Celis C, Labata-Lezaun N, Romaní-Sánchez S, Gassó-Villarejo S, Garcia-Ribell E, Rodríguez-Sanz J, Pérez-Bellmunt A. Effect of Load Distribution on Trunk Muscle Activity with Lunge Exercises in Amateur Athletes: Cross-Sectional Study. Healthcare. 2023; 11(6):916. https://doi.org/10.3390/healthcare11060916
Chicago/Turabian StyleLópez-de-Celis, Carlos, Noé Labata-Lezaun, Sergi Romaní-Sánchez, Sergi Gassó-Villarejo, Erik Garcia-Ribell, Jacobo Rodríguez-Sanz, and Albert Pérez-Bellmunt. 2023. "Effect of Load Distribution on Trunk Muscle Activity with Lunge Exercises in Amateur Athletes: Cross-Sectional Study" Healthcare 11, no. 6: 916. https://doi.org/10.3390/healthcare11060916
APA StyleLópez-de-Celis, C., Labata-Lezaun, N., Romaní-Sánchez, S., Gassó-Villarejo, S., Garcia-Ribell, E., Rodríguez-Sanz, J., & Pérez-Bellmunt, A. (2023). Effect of Load Distribution on Trunk Muscle Activity with Lunge Exercises in Amateur Athletes: Cross-Sectional Study. Healthcare, 11(6), 916. https://doi.org/10.3390/healthcare11060916











