Sex and Age Differences in Association between Physical Activity and Metabolic Syndrome: Results from NHANES 2003–2006
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Sample
2.3. Accelerometer-Based Physical Activity
2.4. Metabolic Syndrome
2.5. Statistical Analysis
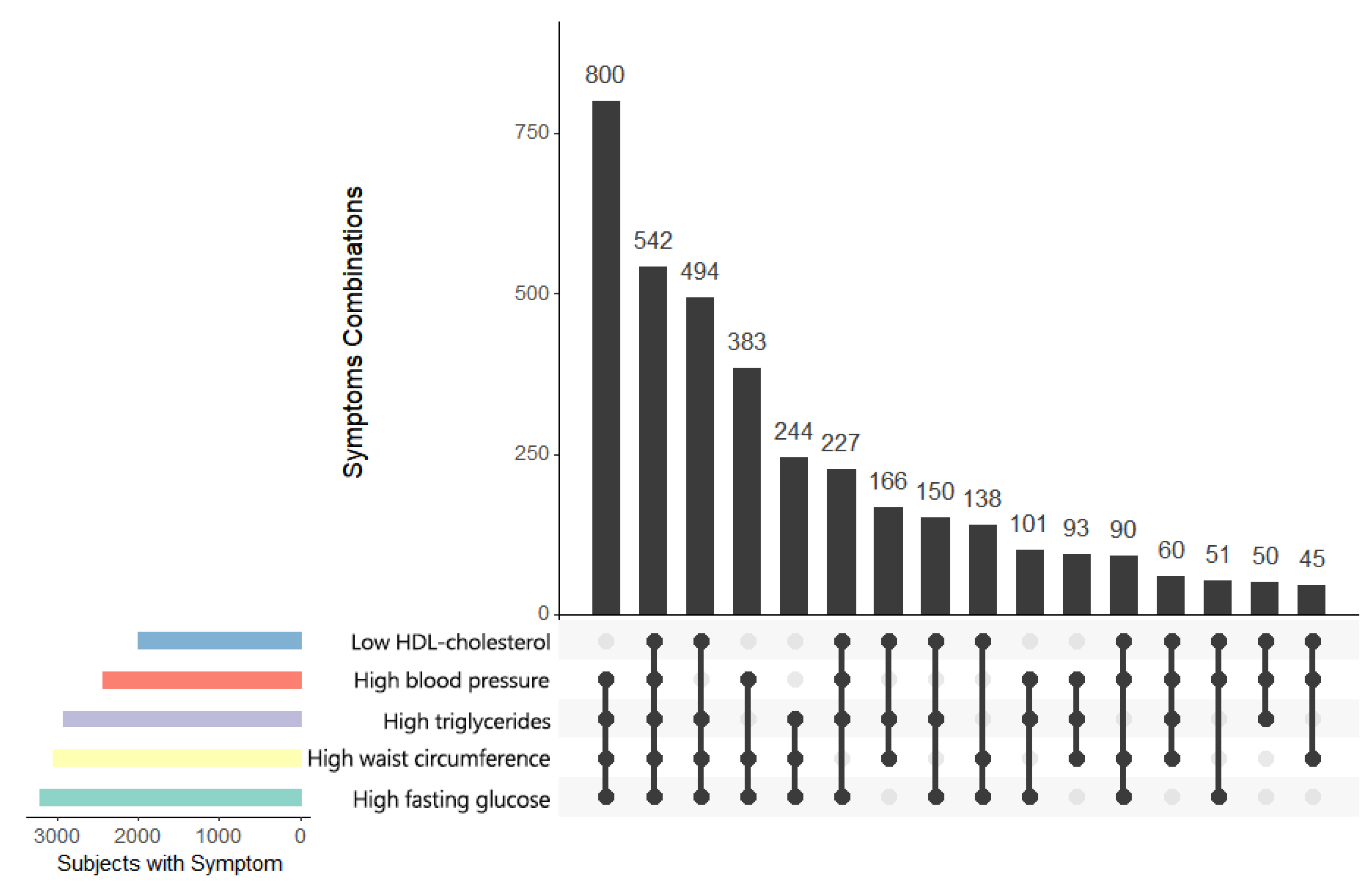
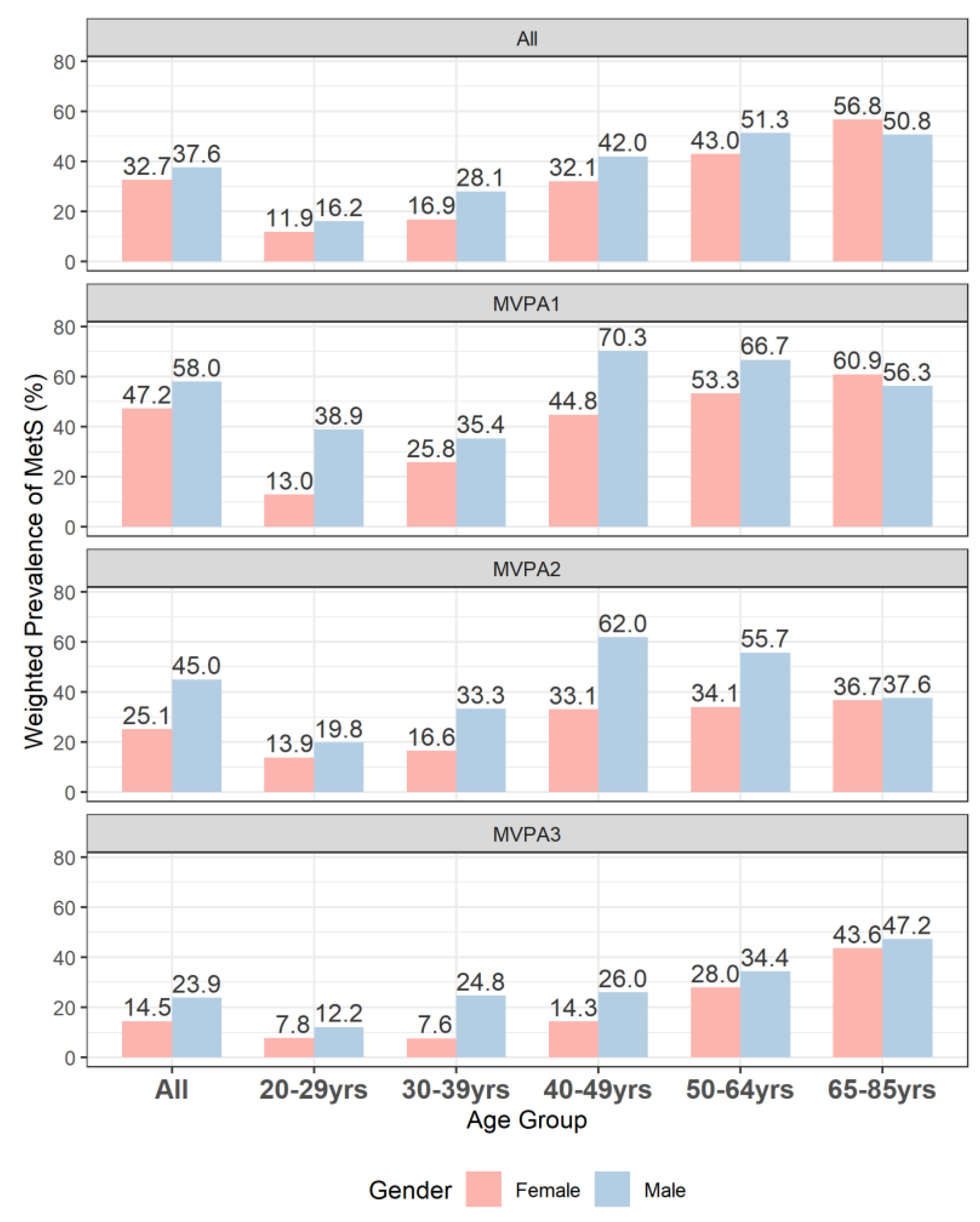
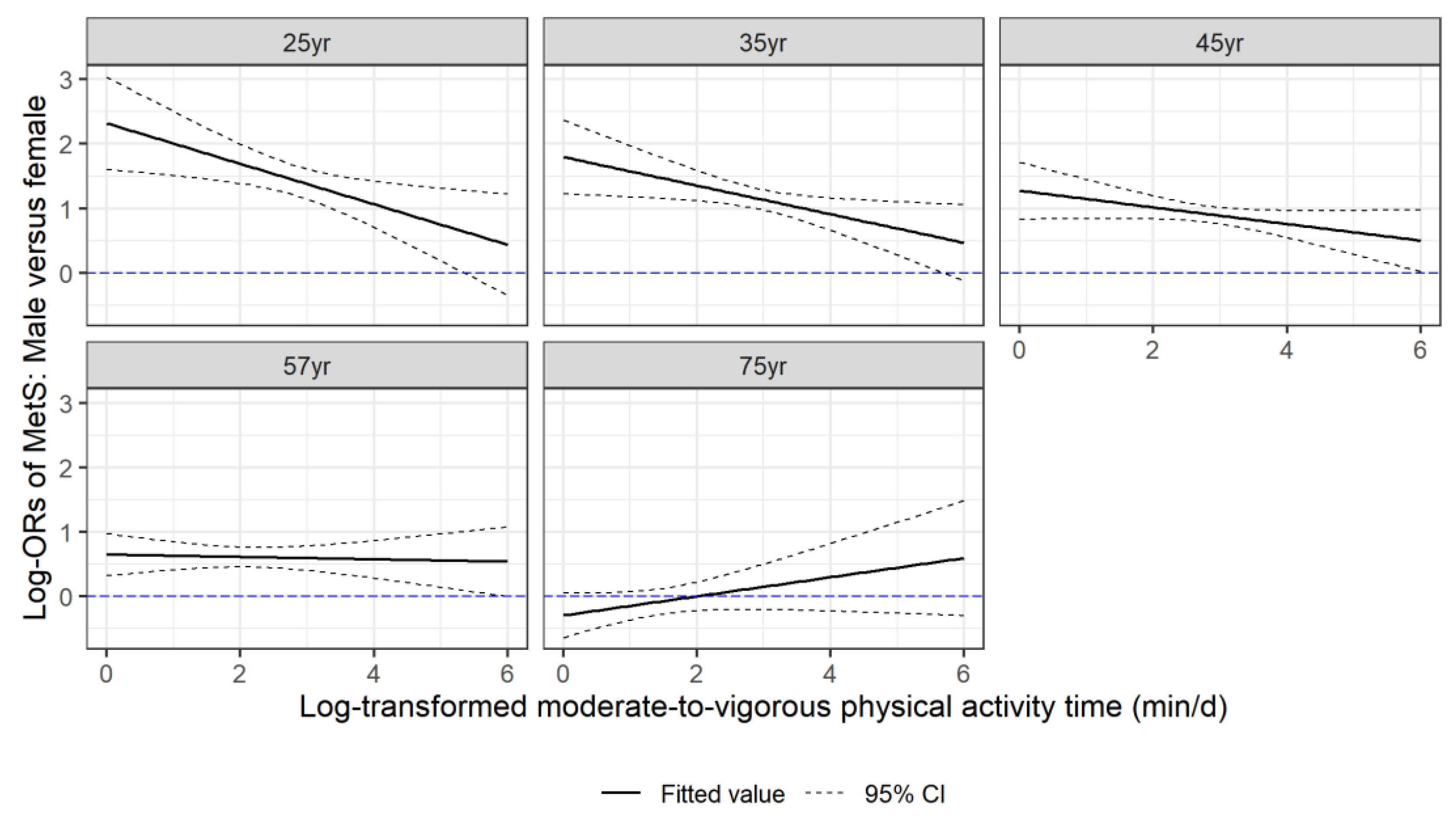
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McCracken, E.; Monaghan, M.; Sreenivasan, S. Pathophysiology of the metabolic syndrome. Clin. Dermatol. 2018, 36, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Cornier, M.-A.; Dabelea, D.; Hernandez, T.L.; Lindstrom, R.C.; Steig, A.J.; Stob, N.R.; Van Pelt, R.E.; Wang, H.; Eckel, R.H. The metabolic syndrome. Endocr. Rev. 2008, 29, 777–822. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Brewer, B.; Cleeman, J.I.; Smith, S.C.; Lenfant, C. Definition of Metabolic Syndrome. Arteriosclerosis, Thrombosis, and Vascular Biology. J. Am. Heart Assoc. 2004, 24, e13–e18. [Google Scholar]
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.T.; Loria, C.M.; Smith, S.C., Jr. Harmonizing the Metabolic Syndrome. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef] [Green Version]
- Ford, E.S. Risks for All-Cause Mortality, Cardiovascular Disease, and Diabetes Associated with the Metabolic Syndrome: A summary of the evidence. Diabetes Care 2005, 28, 1769–1778. [Google Scholar] [CrossRef] [Green Version]
- Hirode, G.; Wong, R.J. Trends in the Prevalence of Metabolic Syndrome in the United States, 2011–2016. JAMA 2020, 323, 2526–2528. [Google Scholar] [CrossRef]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [Green Version]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, H.R.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C., Jr.; et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef] [Green Version]
- Dulloo, A.G.; Montani, J.-P. Pathways from dieting to weight regain, to obesity and to the metabolic syndrome: An overview. Obes. Rev. 2015, 16 (Suppl. 1), 1–6. [Google Scholar] [CrossRef]
- Stone, N.J.; Schmeltz, L.R. Metabolic syndrome management. Expert Opin. Pharmacother. 2007, 8, 2059–2075. [Google Scholar] [CrossRef]
- Myers, J.; Kokkinos, P.; Nyelin, E. Physical Activity, Cardiorespiratory Fitness, and the Metabolic Syndrome. Nutrients 2019, 11, 1652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Füzéki, E.; Engeroff, T.; Banzer, W. Health Benefits of Light-Intensity Physical Activity: A Systematic Review of Accelerometer Data of the National Health and Nutrition Examination Survey (NHANES). Sports Med. 2017, 47, 1769–1793. [Google Scholar] [CrossRef] [PubMed]
- Steeves, J.A.; Murphy, R.A.; Crainiceanu, C.M.; Zipunnikov, V.; Van Domelen, D.R.; Harris, T.B. Daily patterns of physical activity by type 2 diabetes definition: Comparing diabetes, prediabetes, and participants with normal glucose levels in NHANES 2003–2006. Prev. Med. Rep. 2015, 2, 152–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostman, C.; Jewiss, D.; King, N.; Smart, N. Clinical outcomes to exercise training in type 1 diabetes: A systematic review and meta-analysis. Diabetes Res. Clin. Pract. 2018, 139, 380–391. [Google Scholar] [CrossRef]
- VanKim, N.A.; Nelson, T.F. Vigorous Physical Activity, Mental Health, Perceived Stress, and Socializing among College Students. Am. J. Health Promot. 2013, 28, 7–15. [Google Scholar] [CrossRef] [Green Version]
- Young, D.R.; Haskell, W.L. Accumulation of Moderate-to-Vigorous Physical Activity and All-Cause Mortality. J. Am. Heart Assoc. 2018, 7, e008929. [Google Scholar] [CrossRef]
- Rennie, K.; McCarthy, N.; Yazdgerdi, S.; Marmot, M.; Brunner, E. Association of the metabolic syndrome with both vigorous and moderate physical activity. Int. J. Epidemiol. 2003, 32, 600–606. [Google Scholar] [CrossRef] [Green Version]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.-P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef]
- Kobayashi, J.; Nishimura, K.; Matoba, M.; Maekawa, N.; Mabuchi, H. Generation and Gender Differences in the Components Contributing to the Diagnosis of the Metabolic Syndrome According to the Japanese Criteria. Circ. J. 2007, 71, 1734–1737. [Google Scholar] [CrossRef] [Green Version]
- Beigh, S.H.; Jain, S. Prevalence of metabolic syndrome and gender differences. Bioinformation 2012, 8, 613–616. [Google Scholar] [CrossRef]
- Mauvais-Jarvis, F. Gender differences in glucose homeostasis and diabetes. Physiol. Behav. 2018, 187, 20–23. [Google Scholar] [CrossRef] [PubMed]
- Reckelhoff, J.F. Gender Differences in the Regulation of Blood Pressure. Hypertension 2001, 37, 1199–1208. [Google Scholar] [CrossRef] [Green Version]
- Knopp, R.H.; Paramsothy, P.; Retzlaff, B.M.; Fish, B.; Walden, C.; Dowdy, A.; Tsunehara, C.; Aikawa, K.; Cheung, M.C. Gender differences in lipoprotein metabolism and dietary response: Basis in hormonal differences and implications for cardiovascular disease. Curr. Atheroscler. Rep. 2005, 7, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.; Katz, E.G.; Huxley, R.R. Associations between gender, age and waist circumference. Eur. J. Clin. Nutr. 2010, 64, 6–15. [Google Scholar] [CrossRef] [Green Version]
- Regitz-Zagrosek, V.; Lehmkuhl, E.; Weickert, M.O. Gender differences in the metabolic syndrome and their role for cardiovascular disease. Clin. Res. Cardiol. 2006, 95, 147. [Google Scholar] [CrossRef] [Green Version]
- Lazzer, S.; Bedogni, G.; Lafortuna, C.; Marazzi, N.; Busti, C.; Galli, R.; De Col, A.; Agosti, F.; Sartorio, A. Relationship Between Basal Metabolic Rate, Gender, Age, and Body Composition in 8,780 White Obese Subjects. Obesity 2010, 18, 71–78. [Google Scholar] [CrossRef]
- Fukagawa, N.K.; Bandini, L.G.; Young, J.B. Effect of age on body composition and resting metabolic rate. Am. J. Physiol. 1990, 259, E233–E238. [Google Scholar] [CrossRef] [PubMed]
- Westerterp, K.R.; Meijer, E.P. Physical activity and parameters of aging: A physiological perspective. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, 7–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tudor-Locke, C.; Camhi, S.M.; Troiano, R. A Catalog of Rules, Variables, and Definitions Applied to Accelerometer Data in the National Health and Nutrition Examination Survey, 2003–2006. Prev. Chronic Dis. 2012, 9, E113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Troiano, R.P.; Berrigan, D.; Dodd, K.W.; Mâsse, L.C.; Tilert, T.; Mcdowell, M. Physical Activity in the United States Measured by Accelerometer. Med. Sci. Sports Exerc. 2008, 40, 181–188. [Google Scholar] [CrossRef]
- Stekhoven, D.J.; Buhlmann, P. MissForest—Non-parametric missing value imputation for mixed-type data. Bioinformatics 2012, 28, 112–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ford, E.S.; Li, C.; Zhao, G. Prevalence and correlates of metabolic syndrome based on a harmonious definition among adults in the US*. J. Diabetes 2010, 2, 180–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, X.; Xu, X.; Zhang, K.; Wang, H. Gender difference of metabolic syndrome and its association with dietary diversity at different ages. Oncotarget 2017, 8, 73568–73578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janiszewski, P.; Ross, R. The Utility of Physical Activity in the Management of Global Cardiometabolic Risk. Obesity 2009, 17, S3–S14. [Google Scholar] [CrossRef] [PubMed]
- Leskinen, T.; Stenholm, S.; Heinonen, O.J.; Pulakka, A.; Aalto, V.; Kivimäki, M.; Vahtera, J. Change in physical activity and accumulation of cardiometabolic risk factors. Prev. Med. 2018, 112, 31–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Infurna, F.J.; Gerstorf, D. Perceived control relates to better functional health and lower cardio-metabolic risk: The mediating role of physical activity. Health Psychol. 2014, 33, 85–94. [Google Scholar] [CrossRef] [Green Version]
- Ekelund, U.; Luan, J.; Sherar, L.B.; Esliger, D.W.; Griew, P.; Cooper, A. Moderate to Vigorous Physical Activity and Sedentary Time and Cardiometabolic Risk Factors in Children and Adolescents. JAMA 2012, 307, 704–712. [Google Scholar] [CrossRef] [Green Version]
- Morrato, E.H.; Hill, J.O.; Wyatt, H.R.; Ghushchyan, V.; Sullivan, P.W. Physical Activity in U.S. Adults with Diabetes and At Risk for Developing Diabetes, 2003. Diabetes Care 2007, 30, 203–209. [Google Scholar] [CrossRef] [Green Version]
- Hu, F.B.; Sigal, R.J.; Rich-Edwards, J.W.; Colditz, G.A.; Solomon, C.G.; Willett, W.C.; Speitzer, F.E.; Manson, J.E. Walking compared with vigorous physical activity and risk of type 2 diabetes in women: A prospective study. JAMA 1999, 282, 1433–1439. [Google Scholar] [CrossRef] [Green Version]
- Lesniak, K.T.; Dubbert, P.M. Exercise and hypertension. Curr. Opin. Cardiol. 2001, 16, 356–359. [Google Scholar] [CrossRef]
- Chen, S.-P.; Chang, H.-C.; Hsiao, T.-M.; Yeh, C.-J.; Yang, H.-J. Gender Differences in the Effects of the Frequency of Physical Activity on the Incidence of Metabolic Syndrome: Results from a Middle-Aged Community Cohort in Taiwan. Metab. Syndr. Relat. Disord. 2018, 16, 224–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donahoo, W.T.; Levine, J.A.; Melanson, E.L. Variability in energy expenditure and its components. Curr. Opin. Clin. Nutr. Metab. Care 2004, 7, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Kravitz, L.; Robergs, R.A.; Heyward, V.H.; Wagner, D.R.; Powers, K. Exercise mode and gender comparisons of energy expenditure at self-selected intensities. Med. Sci. Sports Exerc. 1997, 29, 1028–1035. [Google Scholar] [CrossRef]
- Keim, N.L.; Belko, A.Z.; Barbieri, T.F. Body Fat Percentage and Gender: Associations with Exercise Energy Expenditure, Substrate Utilization, and Mechanical Work Efficiency. Int. J. Sport Nutr. Exerc. Metab. 1996, 6, 356–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Uffelen, J.G.Z.; Khan, A.; Burton, N.W. Gender differences in physical activity motivators and context preferences: A population-based study in people in their sixties. BMC Public Health 2017, 17, 624. [Google Scholar] [CrossRef] [Green Version]
- Oyibo, K.; Vassileva, J. Gender Preference and Difference in Behavior Modeling in Fitness Applications: A Mixed-Method Approach. Multimodal Technol. Interact. 2020, 4, 21. [Google Scholar] [CrossRef]
- Ethun, K. Sex and Gender Differences in Body Composition, Lipid Metabolism, and Glucose Regulation. In Sex Differences in Physiology; Elsevier: Amsterdam, The Netherlands, 2016; pp. 145–165. [Google Scholar] [CrossRef]
- Bassareo, P.P.; Crisafulli, A. Gender differences in hemodynamic regulation and cardiovascular adaptations to dynamic exercise. Curr. Cardiol. Rev. 2020, 16, 65–72. [Google Scholar] [CrossRef]
- O’Toole, M.L. Gender differences in the cardiovascular response to exercise. Cardiovasc. Clin. 1989, 19, 17–33. [Google Scholar] [PubMed]
- Schindler, C. The metabolic syndrome as an endocrine disease: Is there an effective pharmacotherapeutic strategy optimally targeting the pathogenesis? Ther. Adv. Cardiovasc. Dis. 2007, 1, 7–26. [Google Scholar] [CrossRef]
- Dušková, M.; Pospíšilová, H. The Role of Non-Aromatizable Testosterone Metabolite in Metabolic Pathways. Physiol. Res. 2011, 60, 253–261. [Google Scholar] [CrossRef]
- Iyer, A.; Kauter, K.; Brown, L. Gender differences in metabolic syndrome—A Key research issue. Endocr. Metab. Immune Disord.-Drug Targets (Former. Curr. Drug Targets-Immune Endocr. Metab. Disord.) 2011, 11, 182–188. [Google Scholar] [CrossRef]
- Lapauw, B.; Taes, Y.; Simoens, S.; Van Caenegem, E.; Weyers, S.; Goemaere, S.; Toye, K.; Kaufman, J.-M.; T’Sjoen, G.G. Body composition, volumetric and areal bone parameters in male-to-female transsexual persons. Bone 2008, 43, 1016–1021. [Google Scholar] [CrossRef] [PubMed]
- Björntorp, P. The origins and consequences of obesity. Diabetes. Ciba Found. Symp. 1996, 201, 68–80. [Google Scholar]
- Basiotis, P.P.; Welsh, S.O.; Cronin, F.J.; Kelsay, J.L.; Mertz, W. Number of Days of Food Intake Records Required to Estimate Individual and Group Nutrient Intakes with Defined Confidence. J. Nutr. 1987, 117, 1638–1641. [Google Scholar] [CrossRef] [PubMed]
- Levine, E.; Guthrie, J.F. Nutrient intakes and eating patterns of teenagers. Fam. Econ. Nutr. Rev. 1997, 10, 20. [Google Scholar]
- Luke, A.; Dugas, L.R.; Durazo-Arvizu, A.R.; Cao, G.; Cooper, R.S. Assessing Physical Activity and its Relationship to Cardiovascular Risk Factors: NHANES 2003–2006. BMC Public Health 2011, 11, 387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tucker, J.M.; Welk, G.J.; Beyler, N.K.; Kim, Y. Associations between Physical Activity and Metabolic Syndrome: Comparison between Self-Report and Accelerometry. Am. J. Health Promot. 2016, 30, 155–162. [Google Scholar] [CrossRef] [PubMed]

| Total | Non-MetS | MetS | ||||
|---|---|---|---|---|---|---|
| Sample 1 (n = 9515) | Population 2 (N = 207,718,631) | Sample (n = 6018) | Population (N = 134,893,945) | Sample (n = 3497) | Population (N = 72,824,686) | |
| Age (year) | 49.3 + 19.3 | 46.4 + 17.0 | 44.7 + 18.9 | 42.7 + 16.4 | 57.4 + 17.3 | 53.3 + 16.1 |
| Sex | ||||||
| Female | 4956 (52.1) | 52.0 | 3224 (53.6) | 53.9 | 1732 (49.5) | 48.5 |
| Male | 4559 (47.9) | 48.0 | 2794 (46.4) | 46.1 | 1765 (50.5) | 51.5 |
| Race/Ethnicity | ||||||
| White | 4894 (51.4) | 71.9 | 3025 (50.3) | 70.5 | 1869 (53.4) | 75.1 |
| Mexican American | 1910 (20.1) | 7.9 | 1158 (19.2) | 8.1 | 752 (21.5) | 7.3 |
| Other Hispanic | 291 (3.1) | 3.5 | 200 (3.3) | 3.7 | 91 (2.6) | 3.1 |
| Black | 2024 (21.3) | 11.4 | 1360 (22.6) | 12.1 | 664 (19.0) | 9.8 |
| Other | 396 (4.2) | 5.4 | 275 (4.6) | 5.7 | 121 (3.5) | 4.7 |
| Drinking status | ||||||
| Non-Drinker | 3430 (36.1) | 28.9 | 1912 (31.8) | 24.6 | 1518 (43.4) | 36.8 |
| Moderate Drinker | 5492 (57.7) | 63.4 | 3696 (61.4) | 66.9 | 1796 (51.4) | 56.9 |
| Heavy Drinker | 593 (6.2) | 7.7 | 410 (6.8) | 8.5 | 183 (5.2) | 6.3 |
| Smoking status | ||||||
| Never | 4909 (51.6) | 50.3 | 3224 (53.6) | 51.5 | 1685 (48.2) | 48.0 |
| Former | 2494 (26.2) | 25.0 | 1389 (23.1) | 22.5 | 1105 (31.6) | 29.8 |
| Current | 2112 (22.2) | 24.7 | 1405 (23.3) | 26.0 | 707 (20.2) | 22.3 |
| BMI (kg/m2) | 28.6 + 6.4 | 28.4 + 6.4 | 26.6 + 5.6 | 26.3 + 5.4 | 32.0 + 6.2 | 32.3 + 6.3 |
| MVPA (min/d) | 20.6 + 21.5 | 22.8 + 21.0 | 24.0 + 22.7 | 26.3 + 21.8 | 14.6 + 17.6 | 16.4 + 17.8 |
| Energy intake (kcal/d) | 2147.0 + 861.9 | 2225.3 + 885.1 | 2223.4 + 867.3 | 2272.3 + 886.6 | 2015.5 + 836.6 | 2138.1 + 875.7 |
| Waist circumference (cm) | 98.3 + 15.3 | 97.6 + 15.7 | 92.7 + 13.5 | 91.5 + 13.3 | 108.0 + 13.3 | 108.8 + 13.6 |
| Triglycerides (mg/dL) | 149.5 + 92.1 | 147.7 + 92.9 | 116.3 + 58.3 | 114.0 + 58.4 | 206.8 + 109.7 | 210.0 + 111.0 |
| HDL cholesterol (mg/dL) | 55.1 + 16.2 | 54.5 + 15.9 | 59.7 + 15.7 | 59.0 + 15.2 | 47.1 + 13.9 | 46.2 + 13.7 |
| Diastolic blood pressure (mm Hg) | 69.0 + 13.5 | 70.4 + 12.6 | 67.3 + 12.1 | 68.5 + 11.1 | 72.1 + 15.3 | 74.1 + 14.4 |
| Systolic blood pressure (mm Hg) | 125.1 + 20.3 | 122.9 + 18.2 | 118.5 + 17.0 | 117.1 + 14.9 | 136.5 + 20.4 | 133.6 + 18.7 |
| Fasting glucose (mg/dL) | 105.1 + 28.1 | 102.7 + 23.9 | 96.7 + 19.3 | 95.8 + 17.2 | 119.6 + 34.3 | 115.6 + 28.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Lynn, H.S.; Zipunnikov, V. Sex and Age Differences in Association between Physical Activity and Metabolic Syndrome: Results from NHANES 2003–2006. Healthcare 2023, 11, 1059. https://doi.org/10.3390/healthcare11081059
Li H, Lynn HS, Zipunnikov V. Sex and Age Differences in Association between Physical Activity and Metabolic Syndrome: Results from NHANES 2003–2006. Healthcare. 2023; 11(8):1059. https://doi.org/10.3390/healthcare11081059
Chicago/Turabian StyleLi, Hanying, Henry S Lynn, and Vadim Zipunnikov. 2023. "Sex and Age Differences in Association between Physical Activity and Metabolic Syndrome: Results from NHANES 2003–2006" Healthcare 11, no. 8: 1059. https://doi.org/10.3390/healthcare11081059
APA StyleLi, H., Lynn, H. S., & Zipunnikov, V. (2023). Sex and Age Differences in Association between Physical Activity and Metabolic Syndrome: Results from NHANES 2003–2006. Healthcare, 11(8), 1059. https://doi.org/10.3390/healthcare11081059






