The Use of Cardioprotective Devices and Strategies in Patients Undergoing Percutaneous Procedures and Cardiac Surgery
Abstract
:1. Introduction
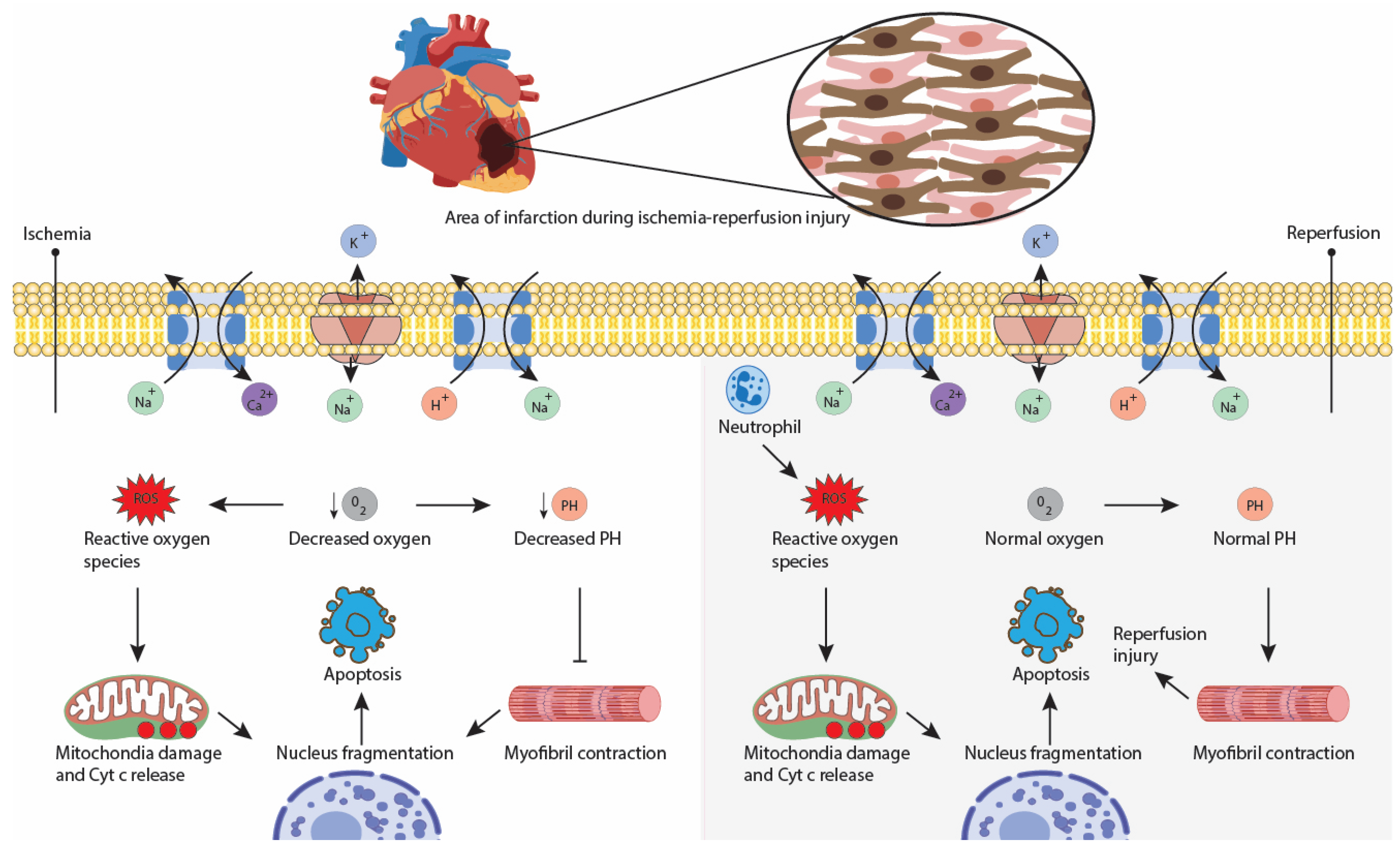
2. Cardiac Surgery/Percutaneous Procedures-Related Injuries and How They Affect Ventricular Performance
3. Principle of Ventricular Unloading
4. Benefits of Left Ventricular Unloading
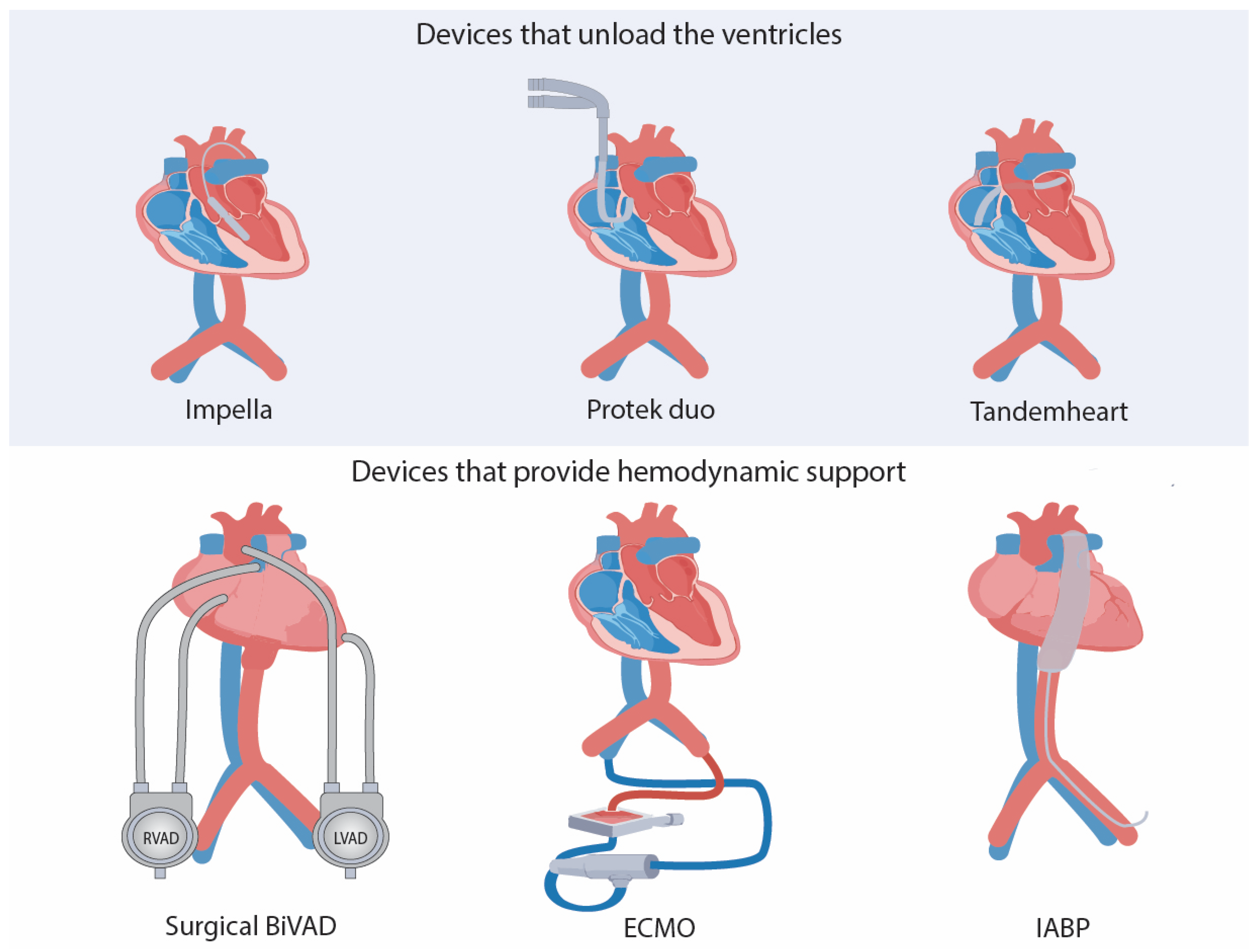
5. Cardioprotective Devices That Unload the Heart:
5.1. Ventricular Assist Devices
5.1.1. Devices That Unload the Heart
Tandemheart
Impella Family Devices
Novel Device: Protek Duo
5.1.2. Devices That Do Not Unload the Heart but Provide Hemodynamic Support
VA-ECMO
IABP
Surgical BiVAD
5.2. Myocardial Cooling Devices and Techniques
5.2.1. The Topical Myocardial Cooling Device
5.2.2. Topical Neck Cooling
5.3. Transcutaneous Vagus Stimulation
5.4. Pressure-Controlled Intermittent Coronary Sinus Occlusion
- PiCSO immediately improved microvascular function after PCI in STEMI patients;
- PiCSO positively influenced coronary microcirculatory vasodilation;
- PiCSO-assisted PCI demonstrated a smaller infarct size at 6 months;
- PiCSO showed promising results in treating inferior STEMI [102].
5.5. Supersaturated Oxygen Therapy
6. Newer Therapeutic Techniques in High-Risk Populations (Cardiogenic Shock and PCI)
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Udzik, J.; Sienkiewicz, S.; Biskupski, A.; Szylińska, A.; Kowalska, Z.; Biskupski, P. Cardiac Complications Following Cardiac Surgery Procedures. J. Clin. Med. 2020, 9, 3347. [Google Scholar] [CrossRef]
- Newman, M.F.; Mathew, J.P.; Grocott, H.P.; Mackensen, G.B.; Monk, T.; Welsh-Bohmer, K.A.; Blumenthal, J.A.; Laskowitz, D.T.; Mark, D.B. Central Nervous System Injury Associated with Cardiac Surgery. Lancet Lond. Engl. 2006, 368, 694–703. [Google Scholar] [CrossRef]
- Turer, A.T.; Hill, J.A. Pathogenesis of Myocardial Ischemia-Reperfusion Injury and Rationale for Therapy. Am. J. Cardiol. 2010, 106, 360–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laschinger, J.C.; Catinella, F.P.; Cunningham, J.N.; Knopp, E.A.; Nathan, I.M.; Spencer, F.C. Myocardial Cooling: Beneficial Effects of Topical Hypothermia. J. Thorac. Cardiovasc. Surg. 1982, 84, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Harky, A.; Joshi, M.; Gupta, S.; Teoh, W.Y.; Gatta, F.; Snosi, M. Acute Kidney Injury Associated with Cardiac Surgery: A Comprehensive Literature Review. Braz. J. Cardiovasc. Surg. 2020, 35, 211–224. [Google Scholar] [CrossRef]
- Arrowsmith, J.E.; Grocott, H.P.; Reves, J.G.; Newman, M.F. Central Nervous System Complications of Cardiac Surgery. Br. J. Anaesth. 2000, 84, 378–393. [Google Scholar] [CrossRef] [Green Version]
- Ergle, K.; Parto, P.; Krim, S.R. Percutaneous Ventricular Assist Devices: A Novel Approach in the Management of Patients With Acute Cardiogenic Shock. Ochsner J. 2016, 16, 243–249. [Google Scholar]
- Gómez-Polo, J.C.; Villablanca, P.; Ramakrishna, H. Left Ventricular Assist Devices in Acute Cardiovascular Care Patients and High-Risk Percutaneous Coronary Interventions. REC Interv. Cardiol. Engl. Ed. 2020, 2, 280–287. [Google Scholar] [CrossRef]
- Munoz Tello, C.; Jamil, D.; Tran, H.H.-V.; Mansoor, M.; Butt, S.R.; Satnarine, T.; Ratna, P.; Sarker, A.; Ramesh, A.S.; Mohammed, L. The Therapeutic Use of Impella Device in Cardiogenic Shock: A Systematic Review. Cureus 2022, 14, e30045. [Google Scholar] [CrossRef] [PubMed]
- Villamater, J.; Charlton, C.; Spector, M.; Williams, W.; Trusler, A. A Topical Myocardial Cooling Device for Paediatrics. Perfusion 1986, 1, 289–292. [Google Scholar] [CrossRef]
- Berman, M.; Coleman, J.; Bartnik, A.; Kaul, P.; Nachum, E.; Osman, M. Insertion of a Biventricular Assist Device. Multimed. Man. Cardiothorac. Surg. 2020, 2020. [Google Scholar] [CrossRef]
- Bartlett, R.H.; Gazzaniga, A.B.; Jefferies, M.R.; Huxtable, R.F.; Haiduc, N.J.; Fong, S.W. Extracorporeal Membrane Oxygenation (ECMO) Cardiopulmonary Support in Infancy. Trans.-Am. Soc. Artif. Intern. Organs 1976, 22, 80–93. [Google Scholar] [PubMed]
- Thiele, H.; Sick, P.; Boudriot, E.; Diederich, K.-W.; Hambrecht, R.; Niebauer, J.; Schuler, G. Randomized Comparison of Intra-Aortic Balloon Support with a Percutaneous Left Ventricular Assist Device in Patients with Revascularized Acute Myocardial Infarction Complicated by Cardiogenic Shock. Eur. Heart J. 2005, 26, 1276–1283. [Google Scholar] [CrossRef] [Green Version]
- Jiritano, F.; Lo Coco, V.; Matteucci, M.; Fina, D.; Willers, A.; Lorusso, R. Temporary Mechanical Circulatory Support in Acute Heart Failure. Card. Fail. Rev. 2020, 6, e01. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuno, T.; Takagi, H.; Ando, T.; Kodaira, M.; Numasawa, Y.; Fox, J.; Bangalore, S. Safety and Efficacy of Mechanical Circulatory Support with Impella or Intra-Aortic Balloon Pump for High-Risk Percutaneous Coronary Intervention and/or Cardiogenic Shock: Insights from a Network Meta-Analysis of Randomized Trials. Catheter. Cardiovasc. Interv. Off. J. Soc. Card. Angiogr. Interv. 2021, 97, E636–E645. [Google Scholar] [CrossRef] [PubMed]
- Khorsandi, M.; Dougherty, S.; Bouamra, O.; Pai, V.; Curry, P.; Tsui, S.; Clark, S.; Westaby, S.; Al-Attar, N.; Zamvar, V. Extra-Corporeal Membrane Oxygenation for Refractory Cardiogenic Shock after Adult Cardiac Surgery: A Systematic Review and Meta-Analysis. J. Cardiothorac. Surg. 2017, 12, 55. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Han, Y.; Sun, S.; Zhang, C.; Liu, H.; Wang, B.; Wei, S. Mortality in Cardiogenic Shock Patients Receiving Mechanical Circulatory Support: A Network Meta-Analysis. BMC Cardiovasc. Disord. 2022, 22, 48. [Google Scholar] [CrossRef]
- van den Buijs, D.M.F.; Wilgenhof, A.; Knaapen, P.; Zivelonghi, C.; Meijers, T.; Vermeersch, P.; Arslan, F.; Verouden, N.; Nap, A.; Sjauw, K.; et al. Prophylactic Impella CP versus VA-ECMO in Patients Undergoing Complex High-Risk Indicated PCI. J. Intervent. Cardiol. 2022, 2022, 8167011. [Google Scholar] [CrossRef]
- Asleh, R.; Resar, J.R. Utilization of Percutaneous Mechanical Circulatory Support Devices in Cardiogenic Shock Complicating Acute Myocardial Infarction and High-Risk Percutaneous Coronary Interventions. J. Clin. Med. 2019, 8, 1209. [Google Scholar] [CrossRef] [Green Version]
- Zeng, P.; Yang, C.; Chen, J.; Fan, Z.; Cai, W.; Huang, Y.; Xiang, Z.; Yang, J.; Zhang, J.; Yang, J. Comparison of the Efficacy of ECMO with or without IABP in Patients With Cardiogenic Shock: A Meta-Analysis. Front. Cardiovasc. Med. 2022, 9, 917610. [Google Scholar] [CrossRef]
- Panoulas, V.; Fiorelli, F. Impella as Unloading Strategy during VA-ECMO: Systematic Review and Meta-Analysis. Rev. Cardiovasc. Med. 2021, 22, 1503–1511. [Google Scholar] [CrossRef]
- Udesen, N.L.J.; Helgestad, O.K.L.; Banke, A.B.S.; Frederiksen, P.H.; Josiassen, J.; Jensen, L.O.; Schmidt, H.; Edelman, E.R.; Chang, B.Y.; Ravn, H.B.; et al. Impact of Concomitant Vasoactive Treatment and Mechanical Left Ventricular Unloading in a Porcine Model of Profound Cardiogenic Shock. Crit. Care 2020, 24, 95. [Google Scholar] [CrossRef] [Green Version]
- Sun, P.; Wang, J.; Zhao, S.; Yang, Z.; Tang, Z.; Ravindra, N.; Bradley, J.; Ornato, J.P.; Peberdy, M.A.; Tang, W. Improved Outcomes of Cardiopulmonary Resuscitation in Rats Treated With Vagus Nerve Stimulation and Its Potential Mechanism. Shock Augusta Ga 2018, 49, 698–703. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Ruel, M. Prevention of Ischemia-Reperfusion Injury in Cardiac Surgery: Therapeutic Strategies Targeting Signaling Pathways. J. Thorac. Cardiovasc. Surg. 2015, 149, 910–911. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Bellomo, R. Cardiac Surgery-Associated Acute Kidney Injury: Risk Factors, Pathophysiology and Treatment. Nat. Rev. Nephrol. 2017, 13, 697–711. [Google Scholar] [CrossRef] [PubMed]
- French, J.K.; Armstrong, P.W.; Cohen, E.; Kleiman, N.S.; O’Connor, C.M.; Hellkamp, A.S.; Stebbins, A.; Holmes, D.R.; Hochman, J.S.; Granger, C.B.; et al. Cardiogenic Shock and Heart Failure Post-Percutaneous Coronary Intervention in ST-Elevation Myocardial Infarction: Observations from “Assessment of Pexelizumab in Acute Myocardial Infarction”. Am. Heart J. 2011, 162, 89–97. [Google Scholar] [CrossRef]
- Verma, S.; Fedak, P.W.M.; Weisel, R.D.; Butany, J.; Rao, V.; Maitland, A.; Li, R.-K.; Dhillon, B.; Yau, T.M. Fundamentals of Reperfusion Injury for the Clinical Cardiologist. Circulation 2002, 105, 2332–2336. [Google Scholar] [CrossRef]
- Haddad, F.; Couture, P.; Tousignant, C.; Denault, A.Y. The Right Ventricle in Cardiac Surgery, a Perioperative Perspective: II. Pathophysiology, Clinical Importance, and Management. Anesth. Analg. 2009, 108, 422–433. [Google Scholar] [CrossRef] [Green Version]
- Curran, J.; Burkhoff, D.; Kloner, R.A. Beyond Reperfusion: Acute Ventricular Unloading and Cardioprotection During Myocardial Infarction. J. Cardiovasc. Transl. Res. 2019, 12, 95–106. [Google Scholar] [CrossRef] [Green Version]
- Uriel, N.; Sayer, G.; Annamalai, S.; Kapur, N.K.; Burkhoff, D. Mechanical Unloading in Heart Failure. J. Am. Coll. Cardiol. 2018, 72, 569–580. [Google Scholar] [CrossRef]
- Swain, L.; Reyelt, L.; Bhave, S.; Qiao, X.; Thomas, C.J.; Zweck, E.; Crowley, P.; Boggins, C.; Esposito, M.; Chin, M.; et al. Transvalvular Ventricular Unloading Before Reperfusion in Acute Myocardial Infarction. J. Am. Coll. Cardiol. 2020, 76, 684–699. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.L.; Zhang, Y.; Qiao, X.; Reyelt, L.; Paruchuri, V.; Schnitzler, G.R.; Morine, K.J.; Annamalai, S.K.; Bogins, C.; Natov, P.S.; et al. Left Ventricular Unloading before Reperfusion Promotes Functional Recovery After Acute Myocardial Infarction. J. Am. Coll. Cardiol. 2018, 72, 501–514. [Google Scholar] [CrossRef]
- Miyashita, S.; Banlengchit, R.; Marbach, J.A.; Chweich, H.; Kawabori, M.; Kimmelstiel, C.D.; Kapur, N.K. Left Ventricular Unloading Before Percutaneous Coronary Intervention Is Associated With Improved Survival in Patients With Acute Myocardial Infarction Complicated by Cardiogenic Shock: A Systematic Review and Meta-Analysis. Cardiovasc. Revasc. Med. 2022, 39, 28–35. [Google Scholar] [CrossRef]
- Huang, C.; Gu, H.; Zhang, W.; Manukyan, M.C.; Shou, W.; Wang, M. SDF-1/CXCR4 Mediates Acute Protection of Cardiac Function through Myocardial STAT3 Signaling Following Global Ischemia/Reperfusion Injury. Am. J. Physiol.-Heart Circ. Physiol. 2011, 301, H1496–H1505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sieweke, J.-T.; Pfeffer, T.J.; Berliner, D.; König, T.; Hallbaum, M.; Napp, L.C.; Tongers, J.; Kühn, C.; Schmitto, J.D.; Hilfiker-Kleiner, D.; et al. Cardiogenic Shock Complicating Peripartum Cardiomyopathy: Importance of Early Left Ventricular Unloading and Bromocriptine Therapy. Eur. Heart J. Acute Cardiovasc. Care 2020, 9, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Kapur, N.K.; Paruchuri, V.; Urbano-Morales, J.A.; Mackey, E.E.; Daly, G.H.; Qiao, X.; Pandian, N.; Perides, G.; Karas, R.H. Mechanically Unloading the Left Ventricle before Coronary Reperfusion Reduces Left Ventricular Wall Stress and Myocardial Infarct Size. Circulation 2013, 128, 328–336. [Google Scholar] [CrossRef] [Green Version]
- Afzal, A.; Hall, S.A. Percutaneous Temporary Circulatory Support Devices and Their Use as a Bridge to Decision during Acute Decompensation of Advanced Heart Failure. Bayl. Univ. Med. Cent. Proc. 2018, 31, 453–456. [Google Scholar] [CrossRef]
- Stretch, R.; Sauer, C.M.; Yuh, D.D.; Bonde, P. National Trends in the Utilization of Short-Term Mechanical Circulatory Support: Incidence, Outcomes, and Cost Analysis. J. Am. Coll. Cardiol. 2014, 64, 1407–1415. [Google Scholar] [CrossRef] [Green Version]
- Goldstein, D.J.; Soltesz, E. High-Risk Cardiac Surgery: Time to Explore a New Paradigm. JTCVS Open 2021, 8, 10–15. [Google Scholar] [CrossRef]
- Hette, A.N.; Sobral, M.L.P. Mechanical Circulatory Assist Devices: Which Is the Best Device as Bridge to Heart Transplantation? Braz. J. Cardiovasc. Surg. 2022, 37, 737–743. [Google Scholar] [CrossRef]
- Chen, Q.; Pollet, M.; Mehta, A.; Wang, S.; Dean, J.; Parenti, J.; Rojas-Delgado, F.; Simpson, L.; Cheng, J.; Mathuria, N. Delayed Removal of a Percutaneous Left Ventricular Assist Device for Patients Undergoing Catheter Ablation of Ventricular Tachycardia Is Associated with Increased 90-Day Mortality. J. Interv. Card. Electrophysiol. 2021, 62, 49–56. [Google Scholar] [CrossRef]
- Gomez-Abraham, J.A.; Brann, S.; Aggarwal, V.; O’Neill, B.; Alvarez, R.; Hamad, E.; Toyoda, Y. (239)-Use of Protek Duo Cannula (RVAD) for Percutaneous Support in Various Clinical Settings. A Safe and Effective Option. J. Heart Lung Transplant. 2018, 37 (Suppl. 4), S102. [Google Scholar] [CrossRef]
- Fernando, S.M.; Price, S.; Mathew, R.; Slutsky, A.S.; Combes, A.; Brodie, D. Mechanical Circulatory Support in the Treatment of Cardiogenic Shock. Curr. Opin. Crit. Care 2022, 28, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Griffioen, A.M.; Van Den Oord, S.C.H.; Van Wely, M.H.; Swart, G.C.; Van Wetten, H.B.; Danse, P.W.; Damman, P.; Van Royen, N.; Van Geuns, R.J.M. Short-Term Outcomes of Elective High-Risk PCI with Extracorporeal Membrane Oxygenation Support: A Single-Centre Registry. J. Intervent. Cardiol. 2022, 2022, 7245384. [Google Scholar] [CrossRef] [PubMed]
- Telukuntla, K.S.; Estep, J.D. Acute Mechanical Circulatory Support for Cardiogenic Shock. Methodist DeBakey Cardiovasc. J. 2020, 16, 27–35. [Google Scholar] [CrossRef]
- Harano, T.; Chan, E.G.; Furukawa, M.; Reck Dos Santos, P.; Morrell, M.R.; Sappington, P.L.; Sanchez, P.G. Oxygenated Right Ventricular Assist Device with a Percutaneous Dual-Lumen Cannula as a Bridge to Lung Transplantation. J. Thorac. Dis. 2022, 14, 832–840. [Google Scholar] [CrossRef]
- Ivins-O’Keefe, K.M.; Cahill, M.S.; Mielke, A.R.; Sobieszczyk, M.J.; Sams, V.G.; Mason, P.E.; Read, M.D. Percutaneous Pulmonary Artery Cannulation to Treat Acute Secondary Right Heart Failure While on Veno-Venous Extracorporeal Membrane Oxygenation. ASAIO J. Am. Soc. Artif. Intern. Organs 1992 2022, 68, 1483–1489. [Google Scholar] [CrossRef]
- Brewer, J.M.; Capoccia, M.; Maybauer, D.M.; Lorusso, R.; Swol, J.; Maybauer, M.O. The ProtekDuo Dual-Lumen Cannula for Temporary Acute Mechanical Circulatory Support in Right Heart Failure: A Systematic Review. Perfusion 2023, 2676591221149859. [Google Scholar] [CrossRef]
- Salna, M.; Garan, A.R.; Kirtane, A.J.; Karmpaliotis, D.; Green, P.; Takayama, H.; Sanchez, J.; Kurlansky, P.; Yuzefpolskaya, M.; Colombo, P.C.; et al. Novel Percutaneous Dual-Lumen Cannula-Based Right Ventricular Assist Device Provides Effective Support for Refractory Right Ventricular Failure after Left Ventricular Assist Device Implantation. Interact. Cardiovasc. Thorac. Surg. 2020, 30, 499–506. [Google Scholar] [CrossRef]
- Khan, T.M.; Siddiqui, A.H. Intra-Aortic Balloon Pump. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Naqvi, S.Y.; Salama, I.G.; Yoruk, A.; Chen, L. Ambulatory Intra Aortic Balloon Pump in Advanced Heart Failure. Card. Fail. Rev. 2018, 4, 43–45. [Google Scholar] [CrossRef] [Green Version]
- Mishra, S. BVS, RDN, IABP: The Afghanistan of Interventional Cardiology Trials. Indian Heart J. 2018, 70, 1–3. [Google Scholar] [CrossRef]
- Wang, Y.; Koenig, S.C.; Wu, Z.; Slaughter, M.S.; Giridharan, G.A. Sensor-Based Physiologic Control Strategy for Biventricular Support with Rotary Blood Pumps. ASAIO J. Am. Soc. Artif. Intern. Organs 1992 2018, 64, 338–350. [Google Scholar] [CrossRef]
- Hernandez, N.B.; Kirk, R.; Sutcliffe, D.; Davies, R.; Jaquiss, R.; Gao, A.; Zhang, S.; Butts, R.J. Utilization and Outcomes in Biventricular Assist Device Support in Pediatrics. J. Thorac. Cardiovasc. Surg. 2020, 160, 1301–1308.e2. [Google Scholar] [CrossRef] [PubMed]
- El Farissi, M.; Mast, T.P.; van de Kar, M.R.D.; Dillen, D.M.M.; Demandt, J.P.A.; Vervaat, F.E.; Eerdekens, R.; Dello, S.A.G.; Keulards, D.C.; Zelis, J.M.; et al. Hypothermia for Cardioprotection in Patients with St-Elevation Myocardial Infarction: Do Not Give It the Cold Shoulder Yet! J. Clin. Med. 2022, 11, 1082. [Google Scholar] [CrossRef]
- Naggar, I.; Nakase, K.; Lazar, J.; Salciccioli, L.; Selesnick, I.; Stewart, M. Vagal Control of Cardiac Electrical Activity and Wall Motion during Ventricular Fibrillation in Large Animals. Auton. Neurosci. Basic Clin. 2014, 183, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Capilupi, M.J.; Kerath, S.M.; Becker, L.B. Vagus Nerve Stimulation and the Cardiovascular System. Cold Spring Harb. Perspect. Med. 2020, 10, a034173. [Google Scholar] [CrossRef]
- Gibson, C.M.; Ajmi, I.; von Koenig, C.L.; Turco, M.A.; Stone, G.W. Pressure-Controlled Intermittent Coronary Sinus Occlusion: A Novel Approach to Improve Microvascular Flow and Reduce Infarct Size in STEMI. Cardiovasc. Revasculariz. Med. Mol. Interv. 2022, 45, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Egred, M.; Bagnall, A.; Spyridopoulos, I.; Purcell, I.F.; Das, R.; Palmer, N.; Grech, E.D.; Jain, A.; Stone, G.W.; Nijveldt, R.; et al. Effect of Pressure-Controlled Intermittent Coronary Sinus Occlusion (PiCSO) on Infarct Size in Anterior STEMI: PiCSO in ACS Study. Int. J. Cardiol. Heart Vasc. 2020, 28, 100526. [Google Scholar] [CrossRef]
- Mohl, W.; Spitzer, E.; Mader, R.M.; Wagh, V.; Nguemo, F.; Milasinovic, D.; Jusić, A.; Khazen, C.; Szodorai, E.; Birkenberg, B.; et al. Acute Molecular Effects of Pressure-controlled Intermittent Coronary Sinus Occlusion in Patients with Advanced Heart Failure. ESC Heart Fail. 2018, 5, 1176–1183. [Google Scholar] [CrossRef]
- Schäfer, A.; Akin, M.; Diekmann, J.; König, T. Intracoronary Application of Super-Saturated Oxygen to Reduce Infarct Size Following Myocardial Infarction. J. Clin. Med. 2022, 11, 1509. [Google Scholar] [CrossRef]
- Kloner, R.A.; Creech, J.L.; Stone, G.W.; O’Neill William, W.; Burkhoff, D.; Spears, J.R. Update on Cardioprotective Strategies for STEMI. JACC Basic Transl. Sci. 2021, 6, 1021–1033. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, K.; Abbott, J.D. Supersaturated Oxygen Therapy in Acute Anterior Myocardial Infarction: Going Small Is the next Big Thing. Catheter. Cardiovasc. Interv. Off. J. Soc. Card. Angiogr. Interv. 2021, 97, 1127–1128. [Google Scholar] [CrossRef]
- Wong, A.S.K.; Sin, S.W.C. Short-Term Mechanical Circulatory Support (Intra-Aortic Balloon Pump, Impella, Extracorporeal Membrane Oxygenation, TandemHeart): A Review. Ann. Transl. Med. 2020, 8, 829. [Google Scholar] [CrossRef] [PubMed]
- Burkhoff, D.; Naidu, S.S. The Science behind Percutaneous Hemodynamic Support: A Review and Comparison of Support Strategies. Catheter. Cardiovasc. Interv. Off. J. Soc. Card. Angiogr. Interv. 2012, 80, 816–829. [Google Scholar] [CrossRef]
- Rihal, C.S.; Naidu, S.S.; Givertz, M.M.; Szeto, W.Y.; Burke, J.A.; Kapur, N.K.; Kern, M.; Garratt, K.N.; Goldstein, J.A.; Dimas, V.; et al. 2015 SCAI/ACC/HFSA/STS Clinical Expert Consensus Statement on the Use of Percutaneous Mechanical Circulatory Support Devices in Cardiovascular Care (Endorsed by the American Heart Association, the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencion; Affirmation of Value by the Canadian Association of Interventional Cardiology-Association Canadienne de Cardiologie d’intervention). J. Card. Fail. 2015, 21, 499–518. [Google Scholar] [CrossRef]
- Mandawat, A.; Rao, S.V. Percutaneous Mechanical Circulatory Support Devices in Cardiogenic Shock. Circ. Cardiovasc. Interv. 2017, 10, e004337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ni hIci, T.; Boardman, H.M.; Baig, K.; Stafford, J.L.; Cernei, C.; Bodger, O.; Westaby, S. Mechanical Assist Devices for Acute Cardiogenic Shock. Cochrane Database Syst. Rev. 2020, 2020, CD013002. [Google Scholar] [CrossRef]
- Alli, O.O.; Singh, I.M.; Holmes, D.R.; Pulido, J.N.; Park, S.J.; Rihal, C.S. Percutaneous Left Ventricular Assist Device with TandemHeart for High-Risk Percutaneous Coronary Intervention: The Mayo Clinic Experience. Catheter. Cardiovasc. Interv. Off. J. Soc. Card. Angiogr. Interv. 2012, 80, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Neupane, S.; Basir, M.; Alqarqaz, M.; O’Neill, W.; Alaswad, K. High-Risk Chronic Total Occlusion Percutaneous Coronary Interventions Assisted With TandemHeart. J. Invasive Cardiol. 2020, 32, 94–97. [Google Scholar]
- Mariani, S.; Napp, L.C.; Kraaier, K.; Li, T.; Bounader, K.; Hanke, J.S.; Dogan, G.; Schmitto, J.D.; Lorusso, R. Prophylactic Mechanical Circulatory Support for Protected Ventricular Tachycardia Ablation: A Meta-Analysis of the Literature. Artif. Organs 2021, 45, 987–997. [Google Scholar] [CrossRef]
- Virk, S.A.; Keren, A.; John, R.M.; Santageli, P.; Eslick, A.; Kumar, S. Mechanical Circulatory Support During Catheter Ablation of Ventricular Tachycardia: Indications and Options. Heart Lung Circ. 2019, 28, 134–145. [Google Scholar] [CrossRef]
- Pieri, M.; Sorrentino, T.; Oppizzi, M.; Melisurgo, G.; Lembo, R.; Colombo, A.; Zangrillo, A.; Pappalardo, F. The Role of Different Mechanical Circulatory Support Devices and Their Timing of Implantation on Myocardial Damage and Mid-Term Recovery in Acute Myocardial Infarction Related Cardiogenic Shock. J. Intervent. Cardiol. 2018, 31, 717–724. [Google Scholar] [CrossRef] [Green Version]
- Algin, A.; Tonino, P.A. TCT-187 30-Day Survival in Patients with Cardiogenic Shock: Impella 2.5 versus Impella 4.0. J. Am. Coll. Cardiol. 2015, 66 (Suppl. 15), B70. [Google Scholar] [CrossRef] [Green Version]
- Markus, B.; Patsalis, N.; Chatzis, G.; Luesebrink, U.; Ahrens, H.; Schieffer, B.; Karatolios, K. Impact of Microaxillar Mechanical Left Ventricular Support on Renal Resistive Index in Patients with Cardiogenic Shock after Myocardial Infarction: A Pilot Trial to Predict Renal Organ Dysfunction in Cardiogenic Shock. Eur. Heart J. Acute Cardiovasc. Care 2020, 9, 158–163. [Google Scholar] [CrossRef] [Green Version]
- Russo, G.; Trani, C.; Burzotta, F. Chapter 3-Percutaneous Left and Right Ventricular Support Devices. In Emerging Technologies for Heart Diseases; Nussinovitch, U., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 41–54. [Google Scholar] [CrossRef]
- Elia, E.; Iannaccone, M.; D’Ascenzo, F.; Gallone, G.; Colombo, F.; Albani, S.; Attisani, M.; Rinaldi, M.; Boccuzzi, G.; Conrotto, F.; et al. Short Term Outcomes of Impella Circulatory Support for High-Risk Percutaneous Coronary Intervention a Systematic Review and Meta-Analysis. Catheter. Cardiovasc. Interv. Off. J. Soc. Card. Angiogr. Interv. 2022, 99, 27–36. [Google Scholar] [CrossRef]
- Schmack, B.; Weymann, A.; Popov, A.-F.; Patil, N.P.; Sabashnikov, A.; Kremer, J.; Farag, M.; Brcic, A.; Lichtenstern, C.; Karck, M.; et al. Concurrent Left Ventricular Assist Device (LVAD) Implantation and Percutaneous Temporary RVAD Support via CardiacAssist Protek-Duo TandemHeart to Preempt Right Heart Failure. Med. Sci. Monit. Basic Res. 2016, 22, 53–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalpey, Z.; Smith, R.; Echeverria, A.; le Tran, P.; Kazui, T. A Novel Minimally Invasive Off-Pump Biventricular Assist Device Insertion Technique. J. Thorac. Cardiovasc. Surg. 2016, 151, e5–e7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartlett, R.H.; Andrews, A.F.; Toomasian, J.M.; Haiduc, N.J.; Gazzaniga, A.B. Extracorporeal Membrane Oxygenation for Newborn Respiratory Failure: Forty-Five Cases. Surgery 1982, 92, 425–433. [Google Scholar] [CrossRef]
- Chakaramakkil, M.J.; Sivathasan, C. ECMO and Short-Term Support for Cardiogenic Shock in Heart Failure. Curr. Cardiol. Rep. 2018, 20, 87. [Google Scholar] [CrossRef] [PubMed]
- Alba, A.C.; Foroutan, F.; Buchan, T.A.; Alvarez, J.; Kinsella, A.; Clark, K.; Zhu, A.; Lau, K.; McGuinty, C.; Aleksova, N.; et al. Mortality in Patients with Cardiogenic Shock Supported with VA ECMO: A Systematic Review and Meta-Analysis Evaluating the Impact of Etiology on 29,289 Patients. J. Heart Lung Transplant. Off. Publ. Int. Soc. Heart Transplant. 2021, 40, 260–268. [Google Scholar] [CrossRef]
- Sheu, J.-J.; Tsai, T.-H.; Lee, F.-Y.; Fang, H.-Y.; Sun, C.-K.; Leu, S.; Yang, C.-H.; Chen, S.-M.; Hang, C.-L.; Hsieh, Y.-K.; et al. Early Extracorporeal Membrane Oxygenator-Assisted Primary Percutaneous Coronary Intervention Improved 30-Day Clinical Outcomes in Patients with ST-Segment Elevation Myocardial Infarction Complicated with Profound Cardiogenic Shock. Crit. Care Med. 2010, 38, 1810–1817. [Google Scholar] [CrossRef]
- Wood, K.L.; Ayers, B.; Gosev, I.; Kumar, N.; Melvin, A.L.; Barrus, B.; Prasad, S. Venoarterial-Extracorporeal Membrane Oxygenation Without Routine Systemic Anticoagulation Decreases Adverse Events. Ann. Thorac. Surg. 2020, 109, 1458–1466. [Google Scholar] [CrossRef] [PubMed]
- Vallabhajosyula, S.; Vallabhajosyula, S.; Vaidya, V.R.; Patlolla, S.H.; Desai, V.; Mulpuru, S.K.; Noseworthy, P.A.; Kapa, S.; Egbe, A.C.; Gersh, B.J.; et al. Venoarterial Extracorporeal Membrane Oxygenation Support for Ventricular Tachycardia Ablation: A Systematic Review. ASAIO J. Am. Soc. Artif. Intern. Organs 1992 2020, 66, 980–985. [Google Scholar] [CrossRef]
- Alushi, B.; Douedari, A.; Froehlig, G.; Knie, W.; Wurster, T.H.; Leistner, D.M.; Staehli, B.-E.; Mochmann, H.-C.; Pieske, B.; Landmesser, U.; et al. Impella versus IABP in Acute Myocardial Infarction Complicated by Cardiogenic Shock. Open Heart 2019, 6, e000987. [Google Scholar] [CrossRef] [Green Version]
- Benenati, S.; Toma, M.; Canale, C.; Vergallo, R.; Bona, R.D.; Ricci, D.; Canepa, M.; Crimi, G.; Santini, F.; Ameri, P.; et al. Mechanical Circulatory Support in Patients with Cardiogenic Shock Not Secondary to Cardiotomy: A Network Meta-Analysis. Heart Fail. Rev. 2022, 27, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-L.; Tsai, Y.-T.; Lin, C.-Y.; Ke, H.-Y.; Lin, Y.-C.; Yang, H.-Y.; Liu, C.-T.; Sung, S.-Y.; Chang, J.-T.; Wang, Y.-H.; et al. Extracorporeal Life Support and Temporary CentriMag Ventricular Assist Device to Salvage Cardiogenic-Shock Patients Suffering from Prolonged Cardiopulmonary Resuscitation. J. Clin. Med. 2022, 11, 3773. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.Y.; Tran, H.; Brambatti, M.; Adler, E.; Pretorius, V.; Pollema, T.; Hsu, J.C.; Feld, G.K.; Hoffmayer, K.; Han, F.; et al. Ventricular Arrhythmias in Patients with Biventricular Assist Devices. J. Interv. Card. Electrophysiol. 2020, 58, 243–252. [Google Scholar] [CrossRef]
- Ruhparwar, A.; Zubarevich, A.; Osswald, A.; Raake, P.W.; Kreusser, M.M.; Grossekettler, L.; Karck, M.; Schmack, B. ECPELLA 2.0—Minimally Invasive Biventricular Groin-Free Full Mechanical Circulatory Support with Impella 5.0/5.5 Pump and ProtekDuo Cannula as a Bridge-to-Bridge Concept: A First-in-Man Method Description. J. Card. Surg. 2020, 35, 195–199. [Google Scholar] [CrossRef] [Green Version]
- Gocoł, R.; Hudziak, D.; Bis, J.; Mendrala, K.; Morkisz, Ł.; Podsiadło, P.; Kosiński, S.; Piątek, J.; Darocha, T. The Role of Deep Hypothermia in Cardiac Surgery. Int. J. Environ. Res. Public Health 2021, 18, 7061. [Google Scholar] [CrossRef]
- McCullough, J.N.; Zhang, N.; Reich, D.L.; Juvonen, T.S.; Klein, J.J.; Spielvogel, D.; Ergin, M.A.; Griepp, R.B. Cerebral Metabolic Suppression during Hypothermic Circulatory Arrest in Humans. Ann. Thorac. Surg. 1999, 67, 1895–1899. [Google Scholar] [CrossRef]
- Garcia-Rinaldi, R.; Gallagher, M.W.; Rea, J.E.; Byrum, M.S.; Donovan, C.G. The Topical Myocardial Cooling Device: Correlation of Its Effectiveness with Method of Cannulation and Core Temperature of the Patient. Cardiovasc. Dis. 1981, 8, 394–404. [Google Scholar] [PubMed]
- Nikas, D.J.; Ramadan, F.M.; Elefteriades, J.A. Topical Hypothermia: Ineffective and Deleterious as Adjunct to Cardioplegia for Myocardial Protection. Ann. Thorac. Surg. 1998, 65, 28–31. [Google Scholar] [CrossRef]
- Whittaker, A.; Aboughdir, M.; Mahbub, S.; Ahmed, A.; Harky, A. Myocardial Protection in Cardiac Surgery: How Limited Are the Options? A Comprehensive Literature Review. Perfusion 2021, 36, 338–351. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeldt, F.L.; Arnold, M. Topical Cardiac Cooling by Recirculation: Comparison of a Closed System Using a Cooling Pad with an Open System Using a Topical Spray. Ann. Thorac. Surg. 1982, 34, 138–145. [Google Scholar] [CrossRef]
- Hochman, J.S.; Sleeper, L.A.; Webb, J.G.; Dzavik, V.; Buller, C.E.; Aylward, P.; Col, J.; White, H.D. Early Revascularization Improves Long-Term Survival for Cardiogenic Shock Complicating Acute Myocardial Infarction. JAMA J. Am. Med. Assoc. 2006, 295, 2511–2515. [Google Scholar] [CrossRef] [Green Version]
- Zhang, A.; Rastogi, R.; Marsh, K.M.; Yang, B.; Wu, D.; Kron, I.L.; Yang, Z. Topical Neck Cooling Without Systemic Hypothermia Attenuates Myocardial Ischemic Injury and Post-Ischemic Reperfusion Injury. Front. Cardiovasc. Med. 2022, 9, 893837. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.Y.; Marsh, K.M.; Rastogi, R.; Wu, D.; Charles, E.J.; Kron, I.L.; Sawyer, R.G.; Yang, Z. Topical Neck Cooling Prolongs Survival of Rats with Intra-Abdominal Feculent Sepsis by Activation of the Vagus Nerve. Int. J. Mol. Sci. 2021, 22, 9828. [Google Scholar] [CrossRef]
- Kenny, B.J.; Bordoni, B. Neuroanatomy, Cranial Nerve 10 (Vagus Nerve). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Sharon, O.; Fahoum, F.; Nir, Y. Transcutaneous Vagus Nerve Stimulation in Humans Induces Pupil Dilation and Attenuates Alpha Oscillations. J. Neurosci. 2021, 41, 320–330. [Google Scholar] [CrossRef]
- Scarsini, R.; Terentes-Printzios, D.; Shanmuganathan, M.; Kotronias, R.A.; Borlotti, A.; Marin, F.; Langrish, J.; Lucking, A.; Ribichini, F.; Oxford Acute Myocardial Infarction (OxAMI) Study; et al. Pressure-Controlled Intermittent Coronary Sinus Occlusion Improves the Vasodilatory Microvascular Capacity and Reduces Myocardial Injury in Patients with STEMI. Catheter. Cardiovasc. Interv. Off. J. Soc. Card. Angiogr. Interv. 2022, 99, 329–339. [Google Scholar] [CrossRef]
- Hanson, I.D.; David, S.W.; Dixon, S.R.; Metzger, D.C.; Généreux, P.; Maehara, A.; Xu, K.; Stone, G.W. “Optimized” Delivery of Intracoronary Supersaturated Oxygen in Acute Anterior Myocardial Infarction: A Feasibility and Safety Study. Catheter. Cardiovasc. Interv. Off. J. Soc. Card. Angiogr. Interv. 2015, 86 (Suppl. 1), S51–S57. [Google Scholar] [CrossRef]
- Chen, S.; David, S.W.; Khan, Z.A.; Metzger, D.C.; Wasserman, H.S.; Lotfi, A.S.; Hanson, I.D.; Dixon, S.R.; LaLonde, T.A.; Généreux, P.; et al. One-Year Outcomes of Supersaturated Oxygen Therapy in Acute Anterior Myocardial Infarction: The IC-HOT Study. Catheter. Cardiovasc. Interv. Off. J. Soc. Card. Angiogr. Interv. 2021, 97, 1120–1126. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.R.; Bartorelli, A.L.; Marcovitz, P.A.; Spears, R.; David, S.; Grinberg, I.; Qureshi, M.A.; Pepi, M.; Trabattoni, D.; Fabbiocchi, F.; et al. Initial experience with hyperoxemic reperfusion after primary angioplasty for acute myocardial infarction: Results of a pilot study utilizing intracoronary aqueous oxygen therapy. J. Am. Coll. Cardiol. 2002, 39, 387–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choy, J.S.; Berwick, Z.C.; Kalasho, B.D.; Fu, L.; Bhatt, D.L.; Navia, J.A.; Kassab, G.S. Selective Autoretroperfusion Provides Substantial Cardioprotection in Swine. JACC Basic Transl. Sci. 2020, 5, 267–278. [Google Scholar] [CrossRef] [PubMed]
| Uses in PCI and Cardiac Surgery | ||||
|---|---|---|---|---|
| Ventricular Support | Advantages | Disadvantages/Limitations | ||
| Devices that provide cardioprotection by improving hemodynamics or providing circulatory support | TandemHeart [39,40,41,42] | Left ventricular support | Hemodynamics improvement before and during PCI | No significant improvement in mortality Data limited to observational studies Need of anticoagulant therapy before placement Invasive device: need of interatrial communication |
| Impella family devices [14,42,43] | Left ventricular support Impella RP: right ventricular support | Hemodynamics improvement before and during PCI Small size cannula Approved by the US Food and Drug Administration for high-risk PCI | No significant improvement in mortality Significant major bleeding complications Need of anticoagulant therapy before placement May induce right heart failure | |
| VA-ECMO [19,32,44,45] | Biventricular support | Provides circulatory and respiratory support, ideal for patients undergoing biventricular failure Some studies show procedural success and no difference in outcomes compared to Impella family devices when used in high-risk PCI | More research is needed to conclude its efficacy in high-risk PCI | |
| Protek Duo [45,46,47,48,49] | Right ventricular support | Safe and feasible treatment in patients with acute right heart failure resulting from implementing a left ventricular assist device. In conjunction with TandemHeart, may offer up to a month of circulatory support. Minimal invasive percutaneous full right heart support ProtekDuo as a bridge to lung transplant and heart-lung transplant | Efficacy and safety data on this device are limited. Drains only from the superior vena cava, making it harder to place it correctly in shorter patients. More expensive than a standard ECMO cannula (> USD 20,000) | |
| IABP [50,51,52] | Left ventricular support | Cost-effective method No need for anticoagulant therapy before placement | Poor performance in patients with poor left ventricular function undergoing artery bypass surgery and cardiogenic shock | |
| BiVAD [11,53,54] | Biventricular support | Good outcomes when used in patients with chronic or acute biventricular failure as a bridge to transplant or recovery Beneficial in patients undergoing right-sided heart failure | Need of sternotomy Ventricular arrhythmias after device placement More research needed to assess its efficacy in high-risk PCI | |
| IABP+ ECMO [20] | Biventricular support | May reduce mortality when treating profound cardiogenic shock (CS) Hemodynamics improvement before and during PCI | Only small observational studies available, not enough for concluding efficacy. Poor data concerning IABP+ECMO in PCI | |
| Impella + VA-ECMO [21] | Biventricular support | May reduce mortality when treating profound CS Hemodynamics improvement before and during PCI | Only small observational studies are available, which is not enough to conclude efficacy. Poor data concerning Impella+ECMO in PCI | |
| Devices that provide cardioprotection by the preservation of myocardial properties | Myocardial cooling devices [4,10,55] | NA | Used in people after induced cardiac arrest following surgery. May minimize ischemia–reperfusion injury, thereby improving cardiac surgery outcomes after cardiac arrest. Efficacious and easy to use in all pediatric cardiac surgeries. Key therapy in patients undergoing cardiopulmonary bypass surgery requiring cardiac arrest | Risk of widespread intravascular crumpling Although it has been shown to have good results in clinical trials, more research is needed to show the same results in human trials |
| Other approaches | Transcutaneous vagus stimulation [56,57] | NA | Non-invasive therapy Can induce intermittent cardiac asystole and can be used as an “on-off” switch for performing cardiac surgeries | More research is needed to assess all the advantages and risks for its use in cardiac surgery [57] |
| Pressure controlled intermittent coronary sinus occlusion [58,59,60] | NA | Increases the mean coronary sinus pressure and coronary sinus pulse pressure after a PCI PiCSO-assisted PCI has demonstrated smaller infarct size after 6 months | Limited to treating anterior ST-elevated myocardial infarction More research needed | |
| Supersaturated oxygen therapy [61,62,63] | NA | Reduces infarct size. Improves reperfusion injury. Reduces endothelial edema and capillary vasodilation. Can be started 5 min after successful revascularization, without delaying primary PC | Relatively new therapy with unknown long-term outcomes | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdul-Rahman, T.; Lizano-Jubert, I.; Garg, N.; Tejerina-Marion, E.; Awais Bukhari, S.M.; Luisa Ek, A.; Wireko, A.A.; Mares, A.C.; Sikora, V.; Gupta, R. The Use of Cardioprotective Devices and Strategies in Patients Undergoing Percutaneous Procedures and Cardiac Surgery. Healthcare 2023, 11, 1094. https://doi.org/10.3390/healthcare11081094
Abdul-Rahman T, Lizano-Jubert I, Garg N, Tejerina-Marion E, Awais Bukhari SM, Luisa Ek A, Wireko AA, Mares AC, Sikora V, Gupta R. The Use of Cardioprotective Devices and Strategies in Patients Undergoing Percutaneous Procedures and Cardiac Surgery. Healthcare. 2023; 11(8):1094. https://doi.org/10.3390/healthcare11081094
Chicago/Turabian StyleAbdul-Rahman, Toufik, Ileana Lizano-Jubert, Neil Garg, Emilio Tejerina-Marion, Syed Muhammad Awais Bukhari, Ana Luisa Ek, Andrew Awuah Wireko, Adriana C. Mares, Vladyslav Sikora, and Rahul Gupta. 2023. "The Use of Cardioprotective Devices and Strategies in Patients Undergoing Percutaneous Procedures and Cardiac Surgery" Healthcare 11, no. 8: 1094. https://doi.org/10.3390/healthcare11081094
APA StyleAbdul-Rahman, T., Lizano-Jubert, I., Garg, N., Tejerina-Marion, E., Awais Bukhari, S. M., Luisa Ek, A., Wireko, A. A., Mares, A. C., Sikora, V., & Gupta, R. (2023). The Use of Cardioprotective Devices and Strategies in Patients Undergoing Percutaneous Procedures and Cardiac Surgery. Healthcare, 11(8), 1094. https://doi.org/10.3390/healthcare11081094











