Hyoid Bone Syndrome in a Patient Undergoing Left Ventricular Assist Device Implantation
Abstract
1. Introduction
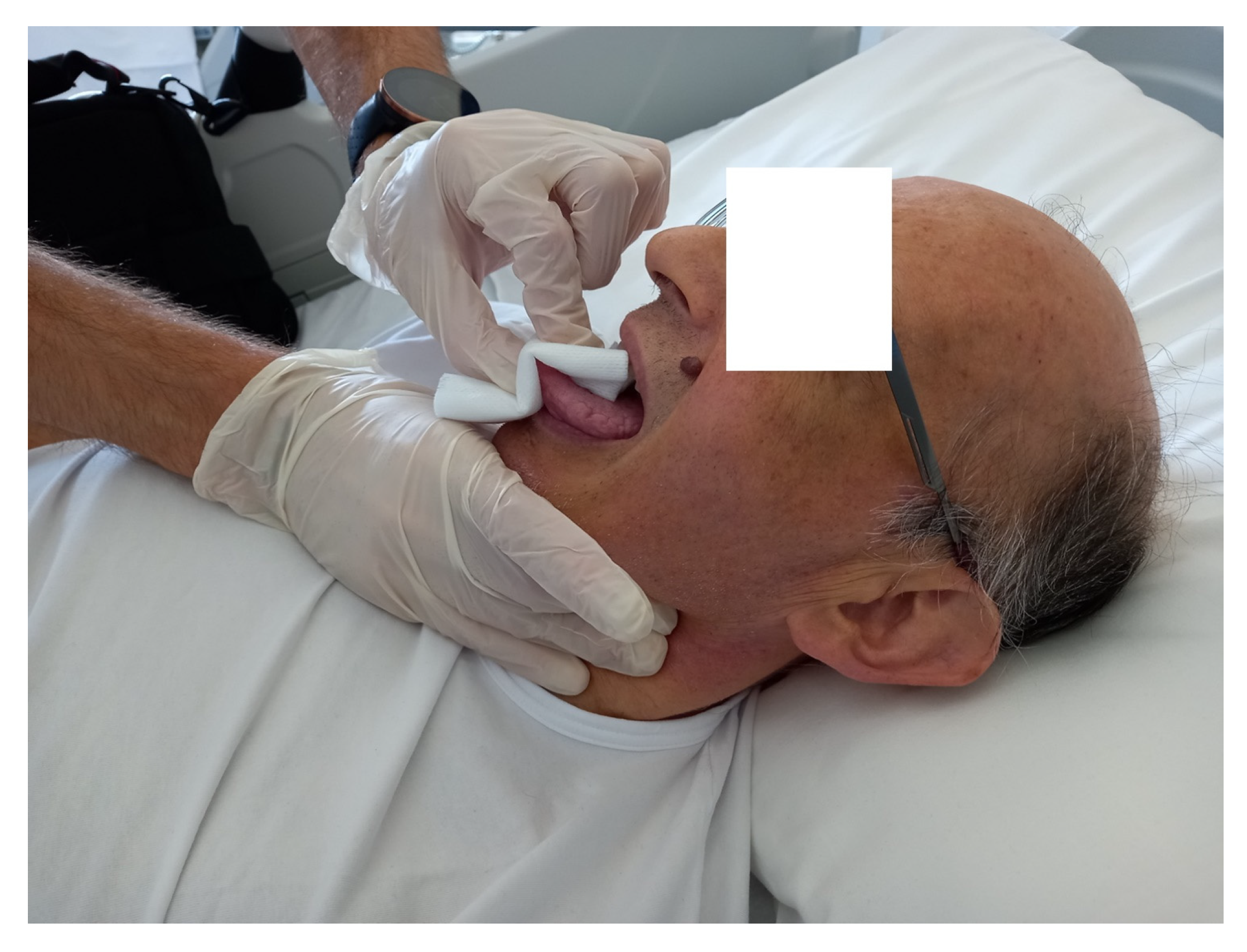
2. Case Description
3. Discussion
Future Directions and Clinical Implications
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Inyom, C.; Haese, T.; Schoenrath, F.; Potapov, E.; Knierim, J. Lived experiences of patients implanted with left ventricular assist devices. Heart Lung 2022, 55, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Giménez-Milà, M.; Sandoval, E.; Farrero, M. Let’s Reduce Bleeding Complications in Patients with Left Ventricular Assist Device. J. Cardiothorac. Vasc. Anesth. 2022, 36, 3435–3438. [Google Scholar] [CrossRef]
- Menchaca, K.; Ostos Perez, C.A.; Draguljevic, N.; Paciletti, J.; Ghumman, W.; Faber, C.; Mirza, S. Long-Term Withdrawal of Anticoagulation and Antiplatelet Therapy in a HeartMate 3 Left Ventricular Assist Device. Cureus 2022, 14, e28779. [Google Scholar] [CrossRef]
- Kerrigan, D.J.; Cowger, J.A.; Keteyian, S.J. Exercise in patients with left ventricular devices: The interaction between the device and the patient. Prog. Cardiovasc. Dis. 2022, 70, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Xia, W.; Greenberg, P.; Okwuosa, I.; Setoguchi, S.; Akhabue, E. Relation of Sociodemographic Factors with Primary Cause of Hospitalization among Patients with Left Ventricular Assist Devices (from the National Inpatient Sample 2012 to 2017). Am. J. Cardiol. 2022, 180, 81–90. [Google Scholar] [CrossRef]
- Mihalj, M.; Heinisch, P.P.; Schober, P.; Wieser, M.; Martinelli, M.; de By, T.M.M.H.; Schefold, J.C.; Luedi, M.M.; Kadner, A.; Carrel, T.; et al. Third-generation continuous-flow left ventricular assist devices: A comparative outcome analysis by device type. ESC Heart Fail. 2022. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Vidula, H.; Takeda, K.; Estep, J.D.; Silvestry, S.C.; Milano, C.; Cleveland, J.C., Jr.; Goldstein, D.J.; Uriel, N.; Kormos, R.L.; Dirckx, N.; et al. Hospitalization Patterns and Impact of a Magnetically-Levitated Left Ventricular Assist Device in the MOMENTUM 3 Trial. JACC Heart Fail. 2022, 10, 470–481. [Google Scholar] [CrossRef]
- Ibeh, C.; Tirschwell, D.L.; Mahr, C.; Creutzfeldt, C.J. Medical and Surgical Management of Left Ventricular Assist Device-Associated Intracranial Hemorrhage. J. Stroke Cerebrovasc. Dis. 2021, 30, 106053. [Google Scholar] [CrossRef]
- Kumble, S.; Strickland, A.; Cole, T.K.; Canner, J.K.; Frost, N.; Madeira, T.; Alejo, D.; Steele, A.; Schena, S. Association Between Early Speech-Language Pathology Consultation and Pneumonia After Cardiac Surgery. Am. J. Speech Lang Pathol. 2022, 31, 2123–2131. [Google Scholar] [CrossRef] [PubMed]
- Sezai, A.; Akahoshi, T.; Osaka, S.; Yaoita, H.; Arimoto, M.; Hata, H.; Tanaka, M.; Sekino, H.; Akashiba, T. Sleep disordered breathing in cardiac surgery patients: The NU-SLEEP trial. Int. J. Cardiol. 2017, 227, 342–346. [Google Scholar] [CrossRef]
- Wuschek, A.; Iqbal, S.; Estep, J.; Quigley, E.; Richards, D. Left ventricular assist device hemolysis leading to dysphagia. World J. Gastroenterol. 2015, 21, 5735–5738. [Google Scholar] [CrossRef] [PubMed]
- Mentz, R.J.; Schlendorf, K.; Hernandez, A.F.; Milano, C.A.; Felker, G.M.; Blue, L.J.; Schroder, J.N.; Rogers, J.G.; Patel, C.B. Dysphagia in the setting of left ventricular assist device hemolysis. ASAIO J. 2013, 59, 322–323. [Google Scholar] [CrossRef] [PubMed]
- Kumai, Y.; Seguchi, O.; Mochizuki, H.; Kimura, Y.; Iwasaki, K.; Kuroda, K.; Nakajima, S.; Matsumoto, Y.; Watanabe, T.; Yanase, M.; et al. Impact of sleep-disordered breathing on ventricular tachyarrhythmias after left ventricular assist device implantation. J. Artif. Organs. 2022, 25, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Milicic, D.; Ben Avraham, B.; Chioncel, O.; Barac, Y.D.; Goncalvesova, E.; Grupper, A.; Altenberger, J.; Frigeiro, M.; Ristic, A.; De Jonge, N.; et al. Heart Failure Association of the European Society of Cardiology position paper on the management of left ventricular assist device-supported patients for the non-left ventricular assist device specialist healthcare provider: Part 2: At the emergency department. ESC Heart Fail. 2021, 8, 4409–4424. [Google Scholar] [CrossRef]
- Bordoni, B.; Escher, A.R. Functional evaluation of the diaphragm with a noninvasive test. J. Osteopath. Med. 2021, 121, 835–842. [Google Scholar] [CrossRef]
- Mohamedali, B.; Bhat, G.; Yost, G.; Tatooles, A. Changes in Spirometry After Left Ventricular Assist Device Implantation. Artif. Organs. 2015, 39, 1046–1050. [Google Scholar] [CrossRef]
- Sajgalik, P.; Kim, C.H.; Stulak, J.M.; Kushwaha, S.S.; Maltais, S.; Joyce, D.L.; Joyce, L.D.; Johnson, B.D.; Schirger, J.A. Pulmonary function assessment post-left ventricular assist device implantation. ESC Heart Fail. 2019, 6, 53–61. [Google Scholar] [CrossRef]
- Bordoni, B.; Escher, A. Non-Instrumental Test for the Evaluation of Tongue Function. Cureus 2021, 13, e18333. [Google Scholar] [CrossRef]
- Li, C.X.; Hu, L.; Gong, Z.C. Reconsideration of hyoid bone syndrome-A case series with a review of the literature. J. Stomatol. Oral Maxillofac. Surg. 2022, 124, 101263. [Google Scholar] [CrossRef]
- Jose, A.; Nagori, S.A.; Arya, S.; Roychoudhury, A. Hyoid bone syndrome masquerading as temporomandibular joint dysfunction. Br. J. Oral Maxillofac. Surg. 2019, 57, 477–478. [Google Scholar] [CrossRef]
- Aydil, U.; Ekinci, O.; Köybaşioğlu, A.; Kizil, Y. Hyoid bone insertion tendinitis: Clinicopathologic correlation. Eur. Arch. Otorhinolaryngol. 2007, 264, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Rubin, A.D.; Codino, J.; Bottalico, P.; Parrish, S.; Jackson-Menaldi, C. Hyoid Bone Syndrome and Dysphonia: Can Throat Pain Affect the Voice? Laryngoscope 2021, 131, E2303–E2308. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, K.; Palmer, J.B. Kinematic linkage of the tongue, jaw, and hyoid during eating and speech. Arch. Oral Biol. 2010, 55, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Neelapu, B.C.; Kharbanda, O.P.; Sardana, H.K.; Balachandran, R.; Sardana, V.; Kapoor, P.; Gupta, A.; Vasamsetti, S. Craniofacial and upper airway morphology in adult obstructive sleep apnea patients: A systematic review and meta-analysis of cephalometric studies. Sleep Med. Rev. 2017, 31, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Vest, A.R.; Wong, W.W.; Chery, J.; Coston, A.; Telfer, L.; Lawrence, M.; Celkupa, D.; Kiernan, M.S.; Couper, G.; Kawabori, M.; et al. Skeletal Muscle Mass Recovery Early after Left Ventricular Assist Device Implantation in Patients with Advanced Systolic Heart Failure. Circ. Heart Fail. 2022, 15, e009012. [Google Scholar] [CrossRef]
- Bordoni, B.; Morabito, B.; Simonelli, M.; Nicoletti, L.; Rinaldi, R.; Tobbi, F.; Caiazzo, P. Osteopathic approach with a patient undergoing cardiac transplantation: The five diaphragms. Int. Med. Case Rep. J. 2019, 12, 303–308. [Google Scholar] [CrossRef]
- Racca, V.; Bordoni, B.; Castiglioni, P.; Modica, M.; Ferratini, M. Osteopathic manipulative treatment improves heart surgery outcomes: A randomized controlled trial. Ann. Thorac. Surg. 2017, 104, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Bordoni, B.; Marelli, F.; Morabito, B.; Sacconi, B.; Severino, P. Post-sternotomy pain syndrome following cardiac surgery: Case report. J. Pain Res. 2017, 10, 1163–1169. [Google Scholar] [CrossRef]
- Bordoni, B.; Marelli, F.; Morabito, B. The tongue after whiplash: Case report and osteopathic treatment. Int. Med. Case Rep. J. 2016, 9, 179–182. [Google Scholar] [CrossRef]
- Bordoni, B.; Escher, A.; Toccafondi, A.; Mapelli, L.; Banfi, P. Obstructive Sleep Apnea and Role of the Diaphragm. Cureus 2022, 14, e29004. [Google Scholar] [CrossRef]
- Sakamoto, Y. Morphological Features of the Glossopharyngeal Nerve in the Peripharyngeal Space, the Oropharynx, and the Tongue. Anat. Rec. 2019, 302, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Vittersø, A.D.; Halicka, M.; Buckingham, G.; Proulx, M.J.; Bultitude, J.H. The sensorimotor theory of pathological pain revisited. Neurosci. Biobehav. Rev. 2022, 139, 104735. [Google Scholar] [CrossRef] [PubMed]
- Ruffini, N.; D’Alessandro, G.; Mariani, N.; Pollastrelli, A.; Cardinali, L.; Cerritelli, F. Variations of high frequency parameter of heart rate variability following osteopathic manipulative treatment in healthy subjects compared to control group and sham therapy: Randomized controlled trial. Front. Neurosci. 2015, 9, 272. [Google Scholar] [CrossRef]
- Caravaca, A.S.; Gallina, A.L.; Tarnawski, L.; Shavva, V.S.; Colas, R.A.; Dalli, J.; Malin, S.G.; Hult, H.; Arnardottir, H.; Olofsson, P.S. Vagus nerve stimulation promotes resolution of inflammation by a mechanism that involves Alox15 and requires the α7nAChR subunit. Proc. Natl. Acad. Sci. USA 2022, 119, e2023285119. [Google Scholar] [CrossRef]
- Minasny, B. Understanding the process of fascial unwinding. Int. J. Ther. Massage Bodyw. 2009, 2, 10–17. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bordoni, B.; Escher, A.R. Hyoid Bone Syndrome in a Patient Undergoing Left Ventricular Assist Device Implantation. Healthcare 2023, 11, 1130. https://doi.org/10.3390/healthcare11081130
Bordoni B, Escher AR. Hyoid Bone Syndrome in a Patient Undergoing Left Ventricular Assist Device Implantation. Healthcare. 2023; 11(8):1130. https://doi.org/10.3390/healthcare11081130
Chicago/Turabian StyleBordoni, Bruno, and Allan R. Escher. 2023. "Hyoid Bone Syndrome in a Patient Undergoing Left Ventricular Assist Device Implantation" Healthcare 11, no. 8: 1130. https://doi.org/10.3390/healthcare11081130
APA StyleBordoni, B., & Escher, A. R. (2023). Hyoid Bone Syndrome in a Patient Undergoing Left Ventricular Assist Device Implantation. Healthcare, 11(8), 1130. https://doi.org/10.3390/healthcare11081130






