Economic Evaluation of Oral Cancer Screening Programs: Review of Outcomes and Study Designs
Abstract
1. Introduction
2. Materials and Methods
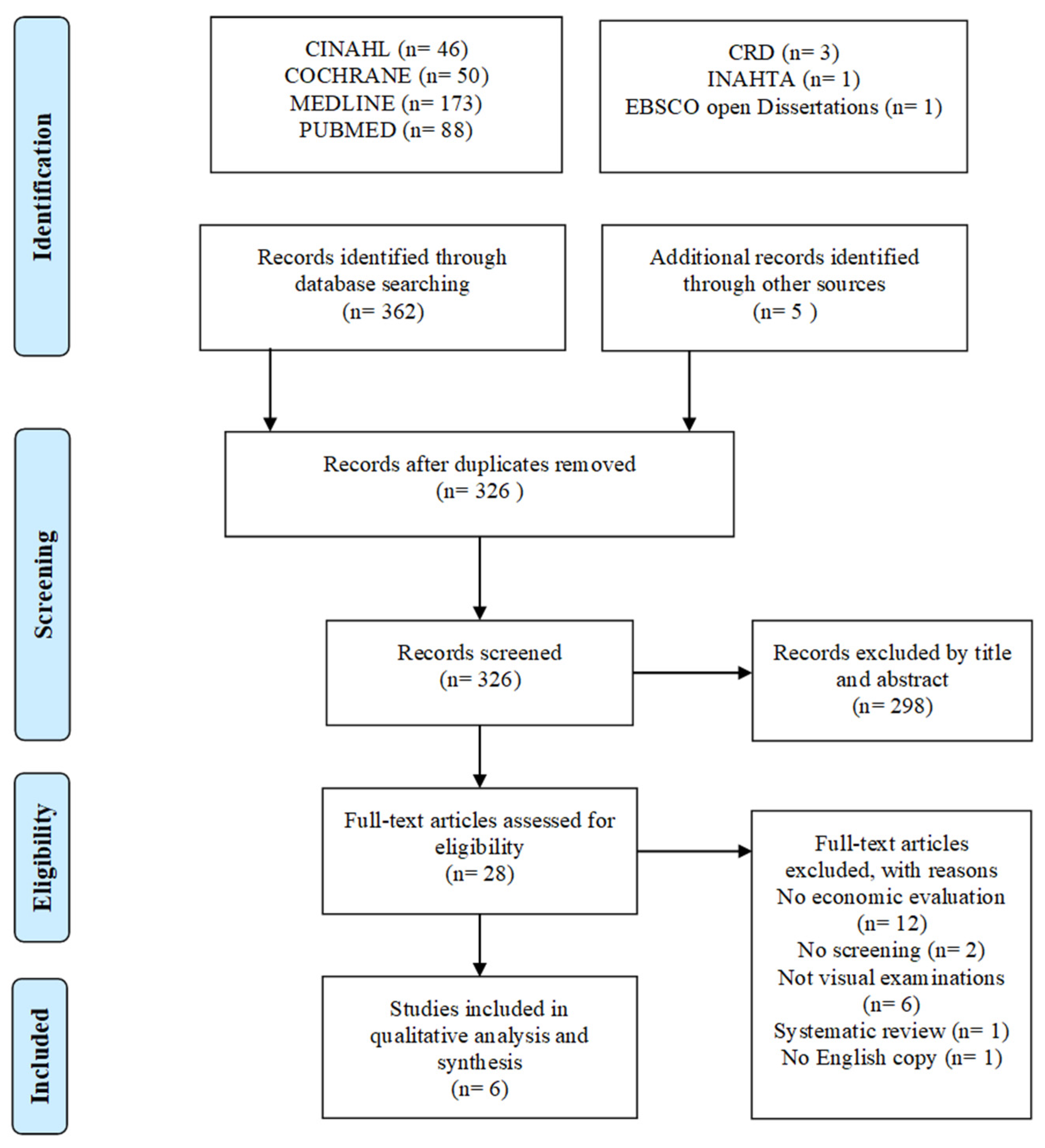
2.1. Source and Search Strategy
2.2. Study Selection
2.3. Quality Assessments
2.4. Data Extraction and Analysis
3. Results
3.1. Study Type and Population
3.2. Screening Strategies
3.3. Cost Perspectives
3.4. Screening Outcomes
3.5. Incremental Cost-effectiveness Ratio
3.6. Modeling Approaches to Economic Evaluation
3.7. Quality and Validity of Models
3.8. Sensitivity Analysis
4. Discussion
4.1. Cost-Effectiveness of Screening Strategies
4.2. Guidelines for Future Models
4.3. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Cheong, S.C.; Vatanasapt, P.; Yi-Hsin, Y.; Zain, R.B.; Kerr, A.R.; Johnson, N.W. Oral cancer in South East Asia:Current status and future directions. Transl. Res. Oral Oncol. 2017, 2, 2057178X17702921. [Google Scholar] [CrossRef]
- Wang, H.; Naghavi, M.; Allen, C.; Barber, R.M.; Bhutta, Z.A.; Carter, A.; Casey, D.C.; Charlson, F.J.; Chen, A.Z.; Coates, M.M. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1459–1544. [Google Scholar] [CrossRef] [PubMed]
- Max Roser, H.R. Cancer. Available online: https://ourworldindata.org/cancer (accessed on 6 June 2022).
- Gupta, N.; Gupta, R.; Acharya, A.K.; Patthi, B.; Goud, V.; Reddy, S.; Garg, A.; Singla, A. Changing Trends in oral cancer-a global scenario. Nepal J. Epidemiol. 2016, 6, 613. [Google Scholar] [CrossRef]
- Warnakulasuriya, S.; Johnson, N.; Van der Waal, I. Nomenclature and classification of potentially malignant disorders of the oral mucosa. J. Oral Pathol. Med. 2007, 36, 575–580. [Google Scholar] [CrossRef]
- Brocklehurst, P.; Kujan, O.; O’Malley, L.A.; Ogden, G.; Shepherd, S.; Glenny, A.M. Screening programmes for the early detection and prevention of oral cancer. Cochrane Database Syst. Rev. 2013, 11. [Google Scholar] [CrossRef]
- Sankaranarayanan, R.; Ramadas, K.; Amarasinghe, H. Oral Cancer: Prevention, Early Detection, and Treatment; The International Bank for Reconstruction and Development/The World Bank: Washington, DC, USA, 2015; Volume 12, pp. 1313–1320. [Google Scholar]
- Chuang, S.L.; Su, W.W.Y.; Chen, S.L.S.; Yen, A.M.F.; Wang, C.P.; Fann, J.C.Y.; Chiu, S.Y.H.; Lee, Y.C.; Chiu, H.M.; Chang, D.C. Population-based screening program for reducing oral cancer mortality in 2,334,299 Taiwanese cigarette smokers and/or betel quid chewers. Cancer 2017, 123, 1597–1609. [Google Scholar] [CrossRef]
- Petersen, P.E. Oral cancer prevention and control–the approach of the World Health Organization. Oral Oncol. 2009, 45, 454–460. [Google Scholar] [CrossRef]
- Ofman, J.J.; Sullivan, S.D.; Neumann, P.J.; Chiou, C.-F.; Henning, J.M.; Wade, S.W.; Hay, J.W. Examining the value and quality of health economic analyses: Implications of utilizing the QHES. J. Manag. Care Pharm. 2003, 9, 53–61. [Google Scholar] [CrossRef]
- Wijnen, B.; Van Mastrigt, G.; Redekop, W.; Majoie, H.; De Kinderen, R.; Evers, S. How to prepare a systematic review of economic evaluations for informing evidence-based healthcare decisions: Data extraction, risk of bias, and transferability (part 3/3). Expert Rev. Pharm. Outcomes Res. 2016, 16, 723–732. [Google Scholar] [CrossRef]
- Philips, Z.; Bojke, L.; Sculpher, M.; Claxton, K.; Golder, S. Good practice guidelines for decision-analytic modelling in health technology assessment. Pharmacoeconomics 2006, 24, 355–371. [Google Scholar] [CrossRef] [PubMed]
- Meadows, A.; Kaambwa, B.; Novielli, N.; Huissoon, A.; Fry-Smith, A.; Meads, C.; Barton, P.; Dretzke, J. A systematic review and economic evaluation of subcutaneous and sublingual allergen immunotherapy in adults and children with seasonal allergic rhinitis. Health Technol. Assess. 2013, 17, 1–322. [Google Scholar] [CrossRef] [PubMed]
- Van der Meij, E.; Bezemer, P.; Van der Waal, I. Cost-effectiveness of screening for the possible development of cancer in patients with oral lichen planus. Community Dent. Oral Epidemiol. 2002, 30, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-C.; Lin, C.-N.; Chung, C.-H.; Hwang, J.-S.; Tsai, S.-T.; Wang, J.-D. Cost-effectiveness analysis of the oral cancer screening program in Taiwan. Oral Oncol. 2019, 89, 59–65. [Google Scholar] [CrossRef]
- George, A.; Sreenivasan, B.; Sunil, S.; Varghese, S.S.; Thomas, J.; Gopakumar, D.; Mani, V. Potentially Malignant Disorder of Oral Cavity. Oral Maxillofac. Pathol. J. 2011, 2, 95–100. [Google Scholar]
- Hamed, M.; Maryam, B.; Masoumeh, M. Oral Potentially Malignant Disorders: An Overview of More than 20 Entities. J. Dent. Res. Dent. Clin. Dent. Prospect. 2014, 8, 6. [Google Scholar]
- Subramanian, S.; Sankaranarayanan, R.; Bapat, B.; Somanathan, T.; Thomas, G.; Mathew, B.; Vinoda, J.; Ramadas, K. Cost-effectiveness of oral cancer screening: Results from a cluster randomized controlled trial in India. Bull. World Health Organ. 2009, 87, 200–206. [Google Scholar] [CrossRef]
- Speight, P.; Palmer, S.; Moles, D.; Downer, M.; Smith, D.; Henriksson, M.; Augustovski, F. The cost-effectiveness of screening for oral cancer in primary care. Health Technol. Assess. 2006, 10, 1–144. [Google Scholar] [CrossRef]
- Dedhia, R.C.; Smith, K.J.; Johnson, J.T.; Roberts, M. The cost-effectiveness of community-based screening for oral cancer in high-risk males in the United States: A Markov decision analysis approach. Laryngoscope 2011, 121, 952–960. [Google Scholar] [CrossRef]
- Kumdee, C.; Kulpeng, W.; Teerawattananon, Y. Cost-utility analysis of the screening program for early oral cancer detection in Thailand. PLoS ONE 2018, 13, e0207442. [Google Scholar] [CrossRef]
- Downer, M.; Jullien, J.; Speight, P. An interim determination of health gain from oral cancer and precancer screening: 1. Obtaining health state utilities. Community Dent. Health 1997, 14, 139–142. [Google Scholar] [PubMed]
- Sanghera, S.; Coast, J.; Martin, R.M.; Donovan, J.L.; Mohiuddin, S. Cost-effectiveness of prostate cancer screening: A systematic review of decision-analytical models. BMC Cancer 2018, 18, 84. [Google Scholar] [CrossRef] [PubMed]
- Schiller-Frühwirth, I.C.; Jahn, B.; Arvandi, M.; Siebert, U. Cost-effectiveness models in breast cancer screening in the general population: A systematic review. Appl. Health Econ. Health Policy 2017, 15, 333–351. [Google Scholar] [CrossRef] [PubMed]
- Warnakulasuriya, S.; Kerr, A. Oral cancer screening: Past, present, and future. J. Dent. Res. 2021, 100, 1313–1320. [Google Scholar] [CrossRef]
- Thankappan, K.; Subramanian, S.; Balasubramanian, D.; Kuriakose, M.A.; Sankaranarayanan, R.; Iyer, S. Cost-effectiveness of oral cancer screening approaches by visual examination: Systematic review. Head Neck 2021, 43, 3646–3661. [Google Scholar] [CrossRef]
- Carta, A.; Conversano, C. On the use of Markov models in pharmacoeconomics: Pros and cons and implications for policy makers. Front. Public Health 2020, 703, 569500. [Google Scholar] [CrossRef]
- Payakachat, N.; Murawski, M.M.; Summers, K.H. Health utility and economic analysis: Theoretical and practical issues. Expert Rev. Pharm. Outcomes Res. 2009, 9, 289–292. [Google Scholar] [CrossRef]
- Brazier, J.; Akehurst, R.; Brennan, A.; Dolan, P.; Claxton, K.; McCabe, C.; Sculpher, M.; Tsuchyia, A. Should patients have a greater role in valuing health states? Appl. Health Econ. Health Policy 2005, 4, 201–208. [Google Scholar] [CrossRef]
- Briggs, A.H.; Weinstein, M.C.; Fenwick, E.A.; Karnon, J.; Sculpher, M.J.; Paltiel, A.D. Model parameter estimation and uncertainty analysis: A report of the ISPOR-SMDM Modeling Good Research Practices Task Force Working Group–6. Med. Decis. Mak. 2012, 32, 722–732. [Google Scholar] [CrossRef]
- Moyer, V.A. Screening for oral cancer: US Preventive Services Task Force recommendation statement. Ann. Intern. Med. 2014, 160, 55–60. [Google Scholar] [CrossRef]
- Vokó, Z.; Túri, G.; Zsólyom, A. A szájüregi szűrés költséghatékonysága Magyarországon. Orv. Hetil. 2016, 157, 1161–1170. [Google Scholar] [CrossRef] [PubMed]
- Birur, P.; Patrick, S.; Bajaj, S.; Raghavan, S.; Suresh, A.; Sunny, S.P.; Chigurupati, R.; Wilder-Smith, P.; Gurushanth, K.; Gurudath, S.; et al. A Novel Mobile Health Approach to Early Diagnosis of Oral Cancer. J. Contemp. Dent. Pract. 2018, 19, 1122–1128. [Google Scholar] [PubMed]
- Balevi, B. Assessing the usefulness of three adjunctive diagnostic devices for oral cancer screening: A probabilistic approach. Community Dent. Oral Epidemiol. 2011, 39, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Cariati, P.; Cabello-Serrano, A.; Perez-de Perceval-Tara, M.; Monsalve-Iglesias, F.; Martínez-Lara, I. Oral and oropharyngeal squamous cell carcinoma in young adults: A retrospective study in Granada University Hospital. Med. Oral Patol. Oral Y Cir. Bucal 2017, 22, e679–e685. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, R.; Gupta, B.; Bose, S. Oral Screening for Pre-cancerous Lesions Among Areca-nut Chewing Population from Rural India. Oral Health Prev. Dent. 2015, 13, 509–514. [Google Scholar]
- Cromwell, I.; Regier, D.A.; Peacock, S.J.; Poh, C.F. Cost-Effectiveness Analysis of Using Loss of Heterozygosity to Manage Premalignant Oral Dysplasia in British Columbia, Canada. Oncologist 2016, 21, 1099–1106. [Google Scholar] [CrossRef]
- Eadie, D.; MacKintosh, A.M.; MacAskill, S.; Brown, A. Development and evaluation of an early detection intervention for mouth cancer using a mass media approach. Br. J. Cancer 2009, 101 (Suppl. 2), S73–S79. [Google Scholar] [CrossRef]
- Gandhi, S.; Lata, J.; Gandhi, N. Fine needle aspiration cytology: A diagnostic aid for oral lesions. J. Oral Maxillofac. Surg. 2011, 69, 1668–1677. [Google Scholar] [CrossRef]
- González Segura, I.; Secchi, D.; Carrica, A.; Barello, R.; Arbelo, D.; Burgos, A.; Brunotto, M.; Zarate, A.M. Exfoliative cytology as a tool for monitoring pre-malignant and malignant lesions based on combined stains and morphometry techniques. J. Oral Pathol. Med. Off. Publ. Int. Assoc. Oral Pathol. Am. Acad. Oral Pathol. 2015, 44, 178–184. [Google Scholar] [CrossRef]
- Hur, C.; Choi, S.E.; Kong, C.Y.; Wang, G.Q.; Xu, H.; Polydorides, A.D.; Xue, L.Y.; Perzan, K.E.; Tramontano, A.C.; Richards-Kortum, R.R.; et al. High-resolution microendoscopy for esophageal cancer screening in China: A cost-effectiveness analysis. World J. Gastroenterol. 2015, 21, 5513–5523. [Google Scholar] [CrossRef]
- Jacobson, J.J.; Epstein, J.B.; Eichmiller, F.C.; Gibson, T.B.; Carls, G.S.; Vogtmann, E.; Wang, S.; Murphy, B. The cost burden of oral, oral pharyngeal, and salivary gland cancers in three groups: Commercial insurance, Medicare, and Medicaid. Head Neck Oncol. 2012, 4, 15. [Google Scholar] [CrossRef] [PubMed]
- Junaid, M.; Suhail, A.; Umer, B.; Ikram, M.; Sobani, Z.U.A.; Ali, N.S.; Nawaz, A. Toluidine blue: Yet another low cost method for screening oral cavity tumour margins in third world countries. JPMA J. Pak. Med. Assoc. 2013, 63, 835. [Google Scholar] [PubMed]
- Lee, M.K.; Dodson, T.B.; Nalliah, R.P.; Karimbux, N.Y.; Allareddy, V. Nine-year trend analysis of hospitalizations attributed to oral and oropharyngeal cancers in the United States. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2014, 118, 47–67. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.; Moles, D.R.; Downer, M.C.; Speight, P.M. Opportunistic screening for oral cancer and precancer in general dental practice: Results of a demonstration study. Br. Dent. J. 2003, 194, 497–502. [Google Scholar] [CrossRef]
- Nunn, H.; Lalli, A.; Fortune, F.; Croucher, R. Oral cancer screening in the Bangladeshi community of Tower Hamlets: A social model. Br. J. Cancer 2009, 101 (Suppl. 2), S68–S72. [Google Scholar] [CrossRef]
- Rahman, M.S.; Ingole, N.; Roblyer, D.; Stepanek, V.; Richards-Kortum, R.; Gillenwater, A.; Shastri, S.; Chaturvedi, P. Evaluation of a low-cost, portable imaging system for early detection of oral cancer. Head Neck Oncol. 2010, 2, 10. [Google Scholar] [CrossRef]
- Sweeny, L.; Dean, N.R.; Magnuson, J.S.; Carroll, W.R.; Clemons, L.; Rosenthal, E.L. Assessment of tissue autofluorescence and reflectance for oral cavity cancer screening. Otolaryngol. Head Neck Surg. 2011, 145, 956–960. [Google Scholar] [CrossRef]
- Uthoff, R.D.; Song, B.; Sunny, S.; Patrick, S.; Suresh, A.; Kolur, T.; Keerthi, G.; Spires, O.; Anbarani, A.; Wilder-Smith, P.; et al. Point-of-care, smartphone-based, dual-modality, dual-view, oral cancer screening device with neural network classification for low-resource communities. PLoS ONE 2018, 13, e0207493. [Google Scholar] [CrossRef]
- Yan, S.K.; Wei, B.J.; Lin, Z.Y.; Yang, Y.; Zhou, Z.T.; Zhang, W.D. A metabonomic approach to the diagnosis of oral squamous cell carcinoma, oral lichen planus and oral leukoplakia. Oral Oncol. 2008, 44, 477–483. [Google Scholar] [CrossRef]
- Younis, R.T.; Hesse, S.V.; Anand, V.K. Evaluation of the utility and cost-effectiveness of obtaining histopathologic diagnosis on all routine tonsillectomy specimens. Laryngoscope 2001, 111, 2166–2169. [Google Scholar] [CrossRef]
- Meregaglia, M.; Cairns, J.; Licitra, L.; Bossi, P. The use of intensive radiological assessments in routine surveillance after treatment for head and neck cancer: An economic evaluation. Eur. J. Cancer 2018, 93, 89–98. [Google Scholar] [CrossRef] [PubMed]
| Concept | Examples of Similar Phrases |
|---|---|
| OPMD | Oral leukoplakia, pre-malignant, and oral lesion/dysplasia. |
| Oral cancer | Oral cancer/neoplasm/carcinoma, and squamous cell carcinoma. |
| Screening | Screening, diagnosis, oral examination, and early detection. |
| Economic evaluation | Economic evaluations, cost-effectiveness, cost-benefit, and cost-utility. |
| Authors (Year), Country | Study Design | Perspective | Population | Time Horizon | Strategies (Visual Examination) | Outcomes Measured |
|---|---|---|---|---|---|---|
| Subramaniam, et al. (2009) [19], India | Randomized controlled trial | Societal | Population in 13 municipal units, over 35 years | 9 years | No screening; 3-yearly screening (THW) | Cancers detected, life-years saved, costs, ICER |
| Huang et al. (2019) [16], Taiwan | Retrospective observational study | Healthcare system | All populations between 2010 and 2013 | Lifetime | No screening; Biannual, free (MP) 3 | Life expectancy, EYLL, lifetime medical cost, and ICER. |
| Meij et al. (2002) [15], Netherland | Decision-analytic model | Healthcare system 1 | Hypothetical population with OLP over 55 years old | 25 years | No screening (standard care and educational messages); Population screening by OS; Population screening (DP) | QALYs, equivalent lives saved, and costs. |
| Speigh et al. (2006) [20], UK | Markov state-transition model | Healthcare system | Hypothetical healthy population over 40 years old | 60 years | No screening; Invitational screening (MP); Invitational screening (DP); Opportunistic screening (MP); Opportunistic screening (DP); Opportunistic ‘high-risk’ screening (MP); Opportunistic ‘high-risk’ screening (DP); Invitational screening (OS) | QALYs, lifetime costs, and ICER. |
| Dedhia et al. (2011) [21], USA | Markov state-transition model | Societal | Hypothetical population of high-risk males 2 over 40 years old | 40 years | No screening; Annual, community-based screening by trained health workers | Annual cancer deaths, QALYs, costs, and ICER. |
| Kumdee et al. (2018) [22], Thailand | Markov state-transition model | Societal | Hypothetical healthy population over 40 years old | 60 years | No screening; MSE + screening by TDN, DP, and OS; Screening by TDN, DP, and OS; Screening by DP and OS; MSE + screening by DP and OS | QALYs, lifetime costs, and ICER. |
| Study (Country) | Strategy | Screener | ICER | Threshold |
|---|---|---|---|---|
| Subramaniam et al. [19] (India) | 3-yearly screening in municipal units | THW | USD 835/LYS (all) USD 156/LYS (high risk) | USD 2900 |
| Huang et al. [16] (Taiwan) | Biannual, national, screening program | MP | USD 28,516/LYS (all) USD 2515/LYS (Stage 0) 1,2 USD 5579/LYS (Stage 1) 1 | USD 25,873 3 |
| Meij et al. [15] (Netherland) | OLP population screening | OS | USD 2137/QALY USD 53,430/LYS | USD 53,430 per LYS |
| DP | USD 1339/QALY | |||
| Speigh et al. [20] (UK) | Invitational population screening | MP | GBP 26,586/QALY | GBP 20,000–30,000 per QALY |
| DP | GBP 28,160/QALY | |||
| OS | GBP 39,300/QALY | |||
| Opportunistic population screening | MP | GBP 24,149/QALY | ||
| DP | GBP 23,367/QALY | |||
| Opportunistic ‘high-risk’ screening | MP | GBP 23,118/QALY | ||
| DP | GBP 23,147/QALY | |||
| Dedhia et al. [21] (USA) | Community-based ‘high-risk’ screening | THW | −USD 6232/QALY 2 | USD 75,000 per QALY |
| Kumdee et al. [22] (Thailand) | Population screening | MSE + TDN + DP + OS | THB 320,618/QALY | THB 160,000 per QALY |
| TDN + DP + OS | THB 174,621/QALY | |||
| DP + OS | THB 100,016/QALY | |||
| MSE + DP + OS | THB 82,292/QALY |
| Design | Strengths | Weaknesses | Considerations |
|---|---|---|---|
| Randomised controlled trial | Allows for an accurate and complete estimation of programmatic costs incurred. Effectiveness is measured in real-time. A valid control prevents overestimation or underestimation of outcomes. Able to obtain demographic and clinical information for management. Accurate information on inputs such as MTR, incidence, and detection rate. | Needs a high capacity of human and financial resources. Effectiveness is limited to the study period. Long-term consequences and costs, such as the extension of life expectancy and productivity, are not able to be captured. It is cost-intensive to explore multiple possible strategies. Findings might be limited to the population investigated, and extrapolation is still needed for a wider national policy. | Ensure the availability of sufficient resources and support. The screening strategy should be well investigated and feasible for implementation. Ensure the length of the study period is comparable to effectiveness outcomes, such as 5-year survival. A diverse population and subgroup analysis may assist in identifying specific targets for screening. Additional projections of outcomes and costs over the total life span of the population (with appropriate discounting). |
| Retrospective observational study | Allows for the precise estimation of clinical outcomes. Controlled trials are easier to conduct and consume fewer resources compared to uncontrolled trials. Effectiveness is measured in real-time. Able to obtain demographic and clinical information for sub-group analysis. Accurate patient-specific direct medical cost. | Highly dependent on the availability and quality of data. Needs an extended period of observation to establish long-term consequences. Effectiveness is limited to the study period. Long-term consequences and costs, such as the extension of life expectancy and productivity, cannot be captured. Unable to obtain societal or indirect medical costs. Programmatic costs may be underestimated if they cannot be distinguished from medical records. | A well-established and interlinked registry, national databases, and clinical records. Availability of medical cost/expenditure data, reimbursement data, or universal coverage. Additional estimation and discounting of outcomes and costs over the total life span of the population (if the observation period is short). Subgroup analysis (comorbidities or risk factors) may assist in identifying specific targets for screening. |
| Decision analysis | Fastest and least resource-consuming. Outcomes can be simulated easily for a range of variables or strategies. Crude long-term outcomes can also be estimated via the incorporation of sufficient parameters and assumptions. | Requires a good and validated decision structure to simulate the disease and the screening progress. Outcomes are highly dependent on the quality of the information and the intuitiveness of the decision structure. | The model needs to be able to reflect the long-term outcomes well and be validated. Sufficient efforts should be focused to ensure the accuracy and robustness of the information applied. Capitalize on the approach by exploring various implementational strategies. Conduct an extensive sensitivity analysis. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raman, S.; Shafie, A.A.; Tan, B.Y.; Abraham, M.T.; Chen Kiong, S.; Cheong, S.C. Economic Evaluation of Oral Cancer Screening Programs: Review of Outcomes and Study Designs. Healthcare 2023, 11, 1198. https://doi.org/10.3390/healthcare11081198
Raman S, Shafie AA, Tan BY, Abraham MT, Chen Kiong S, Cheong SC. Economic Evaluation of Oral Cancer Screening Programs: Review of Outcomes and Study Designs. Healthcare. 2023; 11(8):1198. https://doi.org/10.3390/healthcare11081198
Chicago/Turabian StyleRaman, Sivaraj, Asrul Akmal Shafie, Bee Ying Tan, Mannil Thomas Abraham, Shim Chen Kiong, and Sok Ching Cheong. 2023. "Economic Evaluation of Oral Cancer Screening Programs: Review of Outcomes and Study Designs" Healthcare 11, no. 8: 1198. https://doi.org/10.3390/healthcare11081198
APA StyleRaman, S., Shafie, A. A., Tan, B. Y., Abraham, M. T., Chen Kiong, S., & Cheong, S. C. (2023). Economic Evaluation of Oral Cancer Screening Programs: Review of Outcomes and Study Designs. Healthcare, 11(8), 1198. https://doi.org/10.3390/healthcare11081198






