Improving Practice for Urinary Continence Care on Adult Acute Medical and Rehabilitation Wards: A Multi-Site, Co-Created Implementation Study
Abstract
1. Introduction
1.1. Primary
1.2. Secondary
- 1.
- Does the implementation of our SCAMP urinary continence care intervention increase the proportion of:
- (a)
- Inpatients with UI/LUTS who have an assessment and diagnosis of type(s) of UI/LUTS?
- (b)
- Inpatients with UI/LUTS and their caregivers who are involved in the development of the management plan?
- 2.
- Does the implementation of our SCAMP urinary continence care intervention reduce rates of complications that can be associated with UI/LUTS?
- 3.
- What is the change in the above outcomes at 12 months after the implementation commenced?
- 4.
- Is the practice-change package feasible for wards to adopt, with good fidelity to the implementation strategies?
2. Materials and Methods
2.1. Design
Frameworks
- Knowledge to Action Framework as the process framework that guided development of the intervention (“knowledge creation” phase) and implementation (“action cycle” phase) [18].
- Theoretical Domains Framework to identify potential influencers on implementation (barriers and facilitators) and the accompanying COM-B model to identify strategies to address the key barriers [20]. The Theoretical Domains Framework is frequently used when assessing individual-level barriers and facilitators, rather than those at a systems level.
- RE-AIM Framework (reach (R), effectiveness (E), adoption (A), implementation (I), and maintenance (M)) [19] as it is a useful structure for evaluation implementation efforts. It can be used to evaluate program elements that may improve sustainable adoption and implementation.
2.2. Sample
2.2.1. Participating Wards
2.2.2. Target Population
2.2.3. Included and Excluded Medical Records
2.3. Data Collection
2.3.1. Medical Record Audit
2.3.2. Feasibility and Fidelity Evaluation
2.4. Sample Size and Power Calculation
2.5. Study Intervention—Practice-Change Package
2.5.1. SCAMP Intervention
- a.
- The 4-page Structured urinary Continence Assessment and Management Plan (SCAMP) decision support tool, which can be downloaded from within each of the web-based modules below. This tool guides clinicians through conducting a urinary continence assessment, determining the type of UI/LUTS, and developing an individualised management plan for those with or at risk of symptoms in conjunction with the patient or carer.
- b.
- The associated Clinical Practice Guideline.
- c.
- Eight web-based education modules and a local module on how to use the SCAMP decision support tool (PowerPoint presentation with voice-over). The web-based modules cover information on normal bladder function, why continence is an issue after stroke, and six common inpatient UI and LUTS types. They are hosted on the Stroke Foundation website https://informme.org.au/modules/urinary-continence-and-stroke (accessed on 17 April 2023).
2.5.2. Implementation Strategies
2.6. Data Analysis
3. Results
3.1. Ward Participation
3.2. Characteristics of the Inpatients Whose Medical Records Were Observed
3.2.1. Screening
3.2.2. Audits
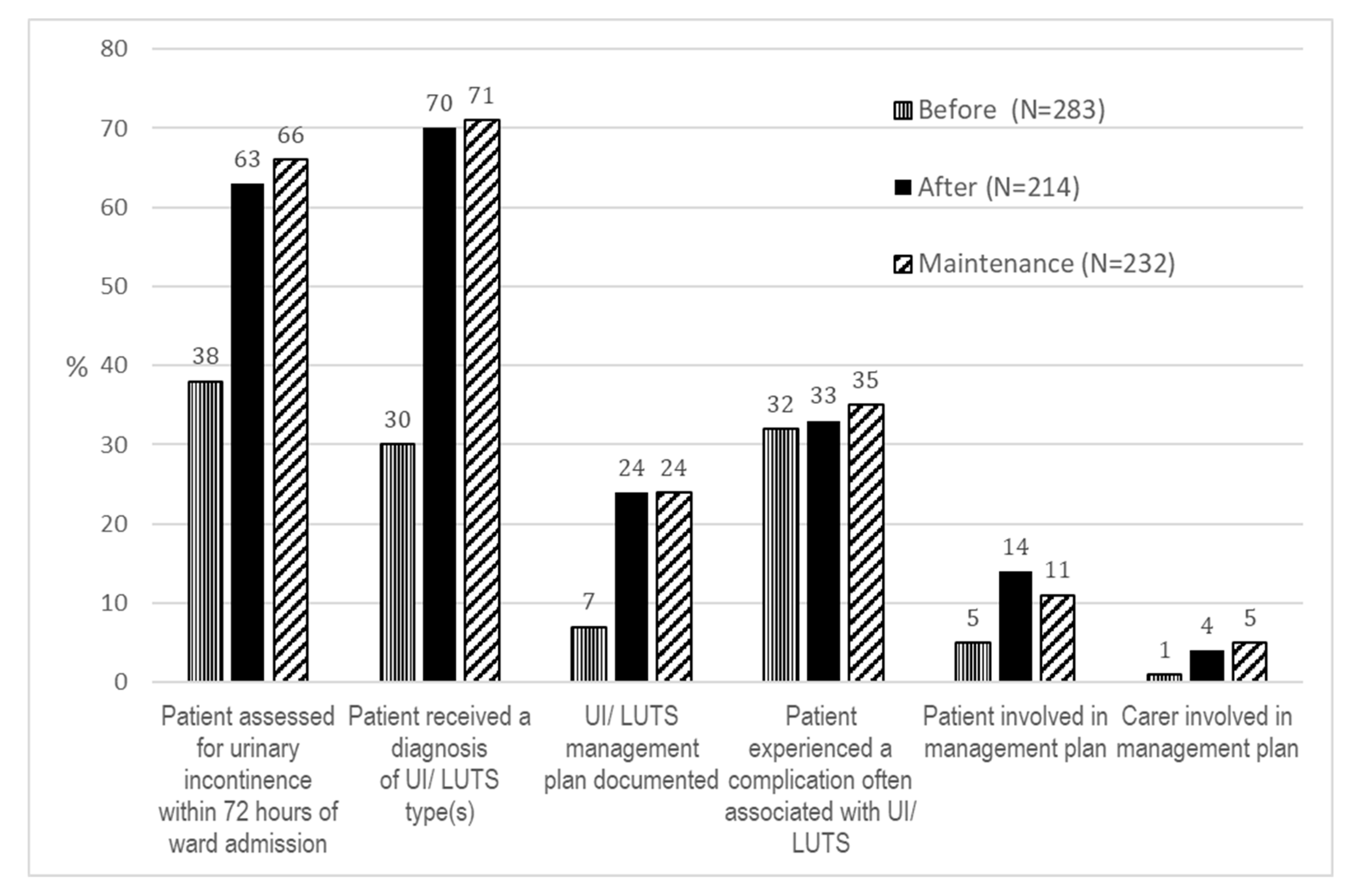
3.3. Clinical Care Delivery Outcomes
3.4. Patient Complication Outcomes
3.5. Feasibility and Fidelity Evaluation
4. Discussion
4.1. Strengths and Limitations
4.2. Recommendations for Further Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abrams, P.; Cardozo, L.; Wagg, A.; Wein, A. Incontinence, 6th ed.; International Continence Society (ICS) & International Consultation on Urological Diseases (ICUD): Tokyo, Japan, 2017. [Google Scholar]
- Ostaszkiewicz, J.; O’Connell, B.; Millar, L. Incontinence: Managed or mismanaged in hospital settings? Int. J. Nurs. Pract. 2008, 14, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Nambiar, A.K.; Bosch, R.; Cruz, F.; Lemack, G.E.; Thiruchelvam, N.; Tubaro, A.; Bedretdinova, D.A.; Ambuhl, D.; Farag, F.; Lombardo, R.; et al. EAU Guidelines on Assessment and Nonsurgical Management of Urinary Incontinence. Eur. Urol. 2018, 73, 596–609. [Google Scholar] [CrossRef] [PubMed]
- Australian Commission on Safety and Quality in Health Care. Hospital-Acquired Complications Information Kit; ACSQHC: Sydney, Austrilia, 2018. [Google Scholar]
- Stroke Foundation. Clinical Guidelines for Stroke Management. 2022. Available online: https://informme.org.au/en/Guidelines/Clinical-Guidelines-for-Stroke-Management (accessed on 12 December 2022).
- Slark, J.; Stewart, L. Advances in nursing assessment and management of urinary incontinence for stroke survivors. Br. J. Neurosci. Nurs. 2017, 13, S16–S23. [Google Scholar] [CrossRef]
- Moon, S.; Chung, H.S.; Kim, Y.J.; Kim, S.J.; Kwon, O.; Lee, Y.G.; Yu, J.M.; Cho, S.T. The impact of urinary incontinence on falls: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0251711. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Storme, O.; Tiran Saucedo, J.; Garcia-Mora, A.; Dehesa-Davila, M.; Naber, K.G. Risk factors and predisposing conditions for urinary tract infection. Ther. Adv. Urol. 2019, 11, 1756287218814382. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lachenbruch, C.; Ribble, D.; Emmons, K.; VanGilder, C. Pressure Ulcer Risk in the Incontinent Patient: Analysis of Incontinence and Hospital-Acquired Pressure Ulcers From the International Pressure Ulcer Prevalence Survey. J. Wound Ostomy Cont. Nurs. 2016, 43, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Barakat-Johnson, M.; Barnett, C.; Lai, M.; Wand, T.; White, K. Incontinence, Incontinence-Associated Dermatitis, and Pressure Injuries in a Health District in Australia: A Mixed-Methods Study. J. Wound Ostomy Cont. Nurs. 2018, 45, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Avery, J.C.; Stocks, N.P.; Duggan, P.; Braunack-Mayer, A.J.; Taylor, A.W.; Goldney, R.D.; MacLennan, A.H. Identifying the quality of life effects of urinary incontinence with depression in an Australian population. BMC Urol. 2013, 13, 11. [Google Scholar] [CrossRef]
- Ersoz, M.; Tunc, H.; Akyuz, M.; Ozel, S. Bladder storage and emptying disorder frequencies in hemorrhagic and ischemic stroke patients with bladder dysfunction. Cereb. Dis. 2005, 20, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Jordan, L.-A.; Quain, D.; Marsden, D.; White, J.; Bullen, K.; Wright, S.; Galvin, R.; Dunne, J.; Baines, H. Managing urinary incontinence following stroke: Can we do better? HNE Handover for Nurses and Midwives. 2011, 4, 4–8. Available online: https://pdfs.semanticscholar.org/3987/b000f0aae5bdfbbc613ab71639b4e821f4c1.pdf?_ga=2.48050855.1878501815.591848991-288409287.571190931 (accessed on 28 March 2018).
- Stroke Foundation. National Stroke Audit Acute Services Report 2017. 2017. Available online: https://informme.org.au/en/stroke-data/Acute-audits (accessed on 4 April 2018).
- Stroke Foundation. National Stroke Audit Rehabiliation Services Report 2018. 2018. Available online: https://informme.org.au/stroke-data/Rehabilitation-audits (accessed on 12 March 2019).
- Damschroder, L.J. Clarity out of chaos: Use of theory in implementation research. Psychiatry Res. 2019, 283, 112461. [Google Scholar] [CrossRef] [PubMed]
- Hassett, L.; Wolfenden, L. Research Note: Designing implementation trials in physiotherapy. J. Physiother. 2022, 68, 210–212. [Google Scholar] [CrossRef] [PubMed]
- Graham, I.D.; Logan, J.; Harrison, M.B.; Straus, S.E.; Tetroe, J.; Caswell, W.; Robinson, N. Lost in knowledge translation: Time for a map? J. Contin. Educ. Health Prof. 2006, 26, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Glasgow, R.E.; Vogt, T.M.; Boles, S.M. Evaluating the public health impact of health promotion interventions: The RE-AIM framework. Am. J. Public Health 1999, 89, 1322–1327. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Atkins, L.; Francis, J.; Islam, R.; O’Connor, D.; Patey, A.; Ivers, N.; Foy, R.; Duncan, E.M.; Colquhoun, H.; Grimshaw, J.M.; et al. A guide to using the Theoretical Domains Framework of behaviour change to investigate implementation problems. Implement Sci. 2017, 12, 77. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marsden, D.L.; Boyle, K.; Jordan, L.-A.; Dunne, J.; Shipp, J.; Minett, F.; Styles, A.; Birnie, J.; Ormond, S.; Parrey, K.; et al. Improving assessment, diagnosis, and management of urinary incontinence and lower urinary tract symptoms on acute and rehabilitation wards that admit adult patients: A protocol for a before-and-after implementation study. JMIR Res. Protoc. 2021, 10, e22902. [Google Scholar] [CrossRef] [PubMed Central]
- Pinnock, H.; Barwick, M.; Carpenter, C.R.; Eldridge, S.; Grandes, G.; Griffiths, C.J.; Rycroft-Malone, J.; Meissner, P.; Murray, E.; Patel, A.; et al. Standards for Reporting Implementation Studies (StaRI) Statement. BMJ 2017, 356, i6795. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Harris, D.; Cadilhac, D.; Hankey, G.; Hillier, S.; Kilkenny, M.; Lalor, E. National stroke audit: The Australasian experience. Clin. Audit. 2010, 2, 25–31. [Google Scholar]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jordan, L.A.; Mackey, E.; Coughlan, K.; Wyer, M.; Allnutt, N.; Middleton, S. Continence management in acute stroke: A survey of current practices in Australia. J. Adv. Nurs. 2011, 67, 94–104. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gurses, A.P.; Murphy, D.J.; Martinez, E.A.; Berenholtz, S.M.; Pronovost, P.J. A practical tool to identify and eliminate barriers to compliance with evidence-based guidelines. Jt. Comm. J. Qual. Patient Saf. 2009, 35, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Zurcher, S.; Saxer, S.; Schwendimann, R. Urinary incontinence in hospitalised elderly patients: Do nurses recognise and manage the problem? Nurs. Res. Pract. 2011, 2011, 671302. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Trad, W.; Flowers, K.; Caldwell, J.; Sousa, M.S.; Vigh, G.; Lizarondo, L.; Gaudin, J.; Hooper, D.; Parker, D. Nursing assessment and management of incontinence among medical and surgical adult patients in a tertiary hospital: A best practice implementation project. JBI Database Syst. Rev Implement Rep. 2019, 17, 2578–2590. [Google Scholar] [CrossRef] [PubMed]
- Grimshaw, J.M.; Eccles, M.P.; Lavis, J.N.; Hill, S.J.; Squires, J.E. Knowledge translation of research findings. Implement Sci. 2012, 7, 50. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ostaszkiewicz, J.; Tomlinson, E.; Hunter, K. The Effects of Education About Urinary Incontinence on Nurses’ and Nursing Assistants’ Knowledge, Attitudes, Continence Care Practices, and Patient Outcomes: A Systematic Review. J. Wound Ostomy Cont. Nurs. 2020, 47, 365–380. [Google Scholar] [CrossRef] [PubMed]
- Spoon, D.; Rietbergen, T.; Huis, A.; Heinen, M.; van Dijk, M.; van Bodegom-Vos, L.; Ista, E. Implementation strategies used to implement nursing guidelines in daily practice: A systematic review. Int. J. Nurs. Stud. 2020, 111, 103748. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, C.E.; Harrison, M.B.; Godfrey, C.; Nincic, V.; Khan, P.A.; Oakley, P.; Ross-White, A.; Grantmyre, H.; Graham, I.D. Use and effects of implementation strategies for practice guidelines in nursing: A systematic review. Implement. Sci. 2021, 16, 1–29. [Google Scholar] [CrossRef]
- Australian Commission on Safety and Quality in Health Care. The National Safety and Quality Health Service (NSQHS) Standards—Partnering with Consumers Standard; ACSQHC: Sydney, Austrilia, 2022. [Google Scholar]
- Mitchell, B.G.; Ferguson, J.K.; Anderson, M.; Sear, J.; Barnett, A. Length of stay and mortality associated with healthcare-associated urinary tract infections: A multi-state model. J. Hosp. Infect. 2016, 93, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Forster, A.J.; Huang, A.; Lee, T.C.; Jennings, A.; Choudhri, O.; Backman, C. Study of a multisite prospective adverse event surveillance system. BMJ Qual. Saf. 2020, 29, 277–285. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Akiba, C.F.; Powell, B.J.; Pence, B.W.; Muessig, K.; Golin, C.E.; Go, V. “We start where we are”: A qualitative study of barriers and pragmatic solutions to the assessment and reporting of implementation strategy fidelity. Implement Sci. Commun. 2022, 3, 117. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Hospital/Location | Ward Description | Included Population(s) | Before Implementation Data Collection | Implementation Period (6 Month) | After Implementation Data Collection | Maintenance Period (6 Months) and after Maintenance Data Collection |
|---|---|---|---|---|---|---|
| A Major city | 20 bed rehab ward | Rehab | Completed | Completed | Completed | Completed while operating under COVID-19 conditions |
| 20 bed rehab ward | Rehab | Completed | Completed | Completed | Completed while operating under COVID-19 conditions | |
| 28 bed rehab ward: 20 rehab, 8 neurological. 2 overflow beds | Acute stroke, acute medicine, rehab | Completed | Completed | Completed | Completed while operating under COVID-19 conditions | |
| B Major city | 12 bed ward: 8 general medicine, 4 Acute SU | Acute stroke, acute medicine | Completed | Completed | Completed: 1 of 3 months under COVID-19 conditions | Completed while operating under COVID-19 conditions |
| C Major city | 30 bed ward: 26 general medicine, 4 comprehensive SU | Acute stroke, acute medicine | Completed | Completed | Completed: 2 of 3 months under COVID-19 conditions | Completed while operating under COVID-19 conditions |
| D Regional | 32 bed ward: medical and rehab | Acute stroke, rehab | Completed | Completed | Completed: 2 of 3 months under COVID-19 conditions | Completed while operating under COVID-19 conditions |
| E Major city | 32 bed rehab ward | Rehab | Completed | Completed | Study disbanded due to onset of COVID-19 with ward lockdown/closure and furloughing of staff. | |
| 28 bed general medical ward | Acute stroke, acute medicine | Completed | 5 months completed | Study disbanded due to onset of COVID-19 with ward lockdown/closure and furloughing of staff | ||
| F Regional | 22 bed rehab ward | Rehab | Completed | Completed | Completed: 1 of 3 months under COVID-19 conditions | Completed while operating under COVID-19 conditions |
| G Regional | 28 bed ward: 24 general medical, 4 Acute SU | Acute stroke | Completed | Completed | Completed: 2 of 3 months under COVID-19 conditions | Completed while operating under COVID-19 conditions |
| H Regional | 16 bed rehab hospital | Rehab | Completed | Completed | Completed: 2 of 3 months under COVID-19 conditions | Completed while operating under COVID-19 conditions |
| I Regional | 28 bed ward: 4 Acute SU, 8 MAU, 16 respiratory/cardiac | Acute stroke | Completed | Completed | Completed: 3 of 3 months under COVID-19 conditions | Completed while operating under COVID-19 conditions |
| J Regional | 24 bed ward: 20 general rehab, 4 comprehensive SU | Acute stroke | Completed | Completed | Completed: 1 of 3 months under COVID-19 conditions | Completed while operating under COVID-19 conditions |
| K Regional | 18 bed hospital: 8 rehab, 10 general medical | Rehab | Completed | Study disbanded due to onset of COVID-19 | ||
| L Regional | 16 bed rehab ward | Rehab | Completed | Study disbanded due to onset of COVID-19 | ||
| Key Barriers | COM-B Model | Strategy | Strategy Adopted by Ward [n/13 (%)] |
|---|---|---|---|
| Ward leads and champions have limited knowledge and experience in conducting implementation projects | Capability—Physical and Psychological | Identify and prepare—2 implementation workshops conducted | 13 (100%) |
| Motivation—Reflective and Automatic | Monthly virtual community of practice meetings, out of session phone calls, and emails with project leads | 13 (100%) | |
| - UI/LUTS usually a comorbidity, not the main reason for admission so may be overlooked - Clinicians not aware of UI/LUTS evidence–practice gap - Need to change local processes to adopt formalised UI/LUTS care | Motivation—Reflective Capability—Psychological | Audit and feedback of before-implementation results to raise awareness/highlight evidence–practice gap—via ward meetings, emails | 13 (100%) |
| Motivation—Reflective | Conduct BIM tool to identify local barriers and facilitators | 13 (100%) | |
| Opportunity—Physical | Develop local action plan | 12 (92%) | |
| Motivation—Reflective Opportunity—Social | Audit and feedback—spot check audits to determine what part of the process performed well and by who and what can be improved. Feedback via safety huddles, ward meetings, emails | 13 (100%) | |
| Opportunity—Physical | SCAMP decision support tool embedded into routine practice | 13 (100%) | |
| Capability—Physical | Intensive education and upskilling phase to achieve a critical mass prior to launch | 13 (100%) | |
| Motivation—Automatic | Launch/promotional activities | 12 (92%) | |
| - Ward clinicians are not experts in continence, with no/little access to community-based continence nurses - Clinicians perceive they lack knowledge, skills, and confidence in continence care, particularly diagnosis and management plans | Capability—Psychological | Education (meetings, web-based modules) to increase knowledge UI/LUTS types, using SCAMP tool | 13 (100%) |
| Capability—Physical | Upskilling 1:1 with ward champion/lead | 13 (100%) | |
| Capability—PhysicalMotivation—Reflective | Local champions identified and trained as resource people | 12 (92%) | |
| Opportunity—Physical | Local champions available throughout implementation | 4 (31%) | |
| Motivation—Automatic | Recognition from manager/local project lead of individual staff who did well | 11 (85%) | |
| Clinicians need to remember to use SCAMP tool | Capability—Psychological | Written reminders—including posters displayed in ward/emails/SCAMP resource folder | 13 (100%) |
| Opportunity—Physical | Verbal reminders—including safety huddles/1:1s/ward meetings | 13 (100%) | |
| Maintaining improvements | Capability—Psychological | SCAMP education embedded into onboarding of new nursing staff | 13 (100%) |
| Motivation—Reflective | Spot check audit and feedback—via safety huddles, ward meetings, emails | 10/11 (91%) * |
| Demographic Characteristic | Before Implementation (n = 283) | After Implementation (n = 214) | Maintenance (n = 232) | p-Value | |
|---|---|---|---|---|---|
| Age at admission (years) | Median (Q1, Q3) | 83 (72, 88) | 81 (69, 87) | 78 (70, 85) | 0.004 ** |
| Age group | 18–64 | 36 (13%) | 37 (17%) | 40 (17%) | 0.020 ** |
| 65–74 | 47 (17%) | 42 (20%) | 46 (20%) | ||
| 75–84 | 83 (29%) | 65 (30%) | 86 (37%) | ||
| 85+ | 117 (41%) | 70 (33%) | 60 (26%) | ||
| Sex | Female | 161 (57%) | 119 (56%) | 134 (58%) | 0.631 |
| Male | 122 (43%) | 93 (44%) | 97 (42%) | ||
| Other | 0 | 1 (0.5%) | 0 | ||
| Indigenous status * | Indigenous | 3 (1.1%) | 9 (4.2%) | 9 (3.9%) | 0.065 |
| Location of hospital | Large city | 199 (70%) | 164 (77%) | 163 (70%) | 0.220 |
| Regional | 84 (30%) | 50 (23%) | 69 (30%) | ||
| Patient population | Acute stroke | 58 (20%) | 41 (19%) | 38 (16%) | 0.003 ** |
| Acute Medical | 92 (33%) | 104 (49%) | 95 (41%) | ||
| Rehabilitation | 133 (47%) | 69 (32%) | 99 (43%) | ||
| Intention-to-Treat Analysis * | ||||
|---|---|---|---|---|
| Outcome | Study Period Comparison | OR (95% CI) | p-Value | N in Model |
| Inpatient assessed for UI/LUTS | After implementation vs. Before implementation | 4.38 (2.73, 7.03) | <0.001 | 721 |
| Maintenance vs. Before implementation | 4.70 (2.94, 7.52) | <0.001 | ||
| Maintenance vs. After implementation | 1.07 (0.70, 1.65) | 0.745 | ||
| Inpatient received diagnosis of UI/LUTS type | After implementation vs. Before implementation | 6.49 (4.13, 10.20) | <0.001 | 729 |
| Maintenance vs. Before implementation | 6.01 (3.82, 9.48) | <0.001 | ||
| Maintenance vs. After implementation | 0.93 (0.60, 1.42) | 0.726 | ||
| Inpatient received UI/LUTS management plan | After implementation vs. Before implementation | 4.29 (2.32, 7.94) | <0.001 | 712 |
| Maintenance vs. Before implementation | 4.03 (2.16, 7.50) | <0.001 | ||
| Maintenance vs. After implementation | 0.94 (0.59, 1.49) | 0.788 | ||
| In-hospital complication often associated with UI/LUTS | After implementation vs. Before implementation | 1.42 (0.93, 2.16) | 0.106 | 729 |
| Maintenance vs. Before implementation | 1.48 (0.98, 2.22) | 0.061 | ||
| Maintenance vs. After implementation | 1.04 (0.69, 1.57) | 0.841 | ||
| Inpatient involved in the development of UI/LUTS management plan | After implementation vs. Before implementation | 0.95 (0.29, 3.08) | 0.925 | 127 |
| Maintenance vs. Before implementation | 0.48 (0.15, 1.57) | 0.224 | ||
| Maintenance vs. After implementation | 0.51 (0.21, 1.21) | 0.125 | ||
| Carer involved in the development of management plan | After implementation vs. Before implementation | 1.41 (0.32, 6.22) | 0.646 | 127 |
| Maintenance vs. Before implementation | 1.45 (0.34, 6.22) | 0.612 | ||
| Maintenance vs. After implementation | 1.03 (0.37, 2.84) | 0.956 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marsden, D.L.; Boyle, K.; Birnie, J.; Buzio, A.; Dizon, J.; Dunne, J.; Greensill, S.; Hill, K.; Lever, S.; Minett, F.; et al. Improving Practice for Urinary Continence Care on Adult Acute Medical and Rehabilitation Wards: A Multi-Site, Co-Created Implementation Study. Healthcare 2023, 11, 1241. https://doi.org/10.3390/healthcare11091241
Marsden DL, Boyle K, Birnie J, Buzio A, Dizon J, Dunne J, Greensill S, Hill K, Lever S, Minett F, et al. Improving Practice for Urinary Continence Care on Adult Acute Medical and Rehabilitation Wards: A Multi-Site, Co-Created Implementation Study. Healthcare. 2023; 11(9):1241. https://doi.org/10.3390/healthcare11091241
Chicago/Turabian StyleMarsden, Dianne Lesley, Kerry Boyle, Jaclyn Birnie, Amanda Buzio, Joshua Dizon, Judith Dunne, Sandra Greensill, Kelvin Hill, Sandra Lever, Fiona Minett, and et al. 2023. "Improving Practice for Urinary Continence Care on Adult Acute Medical and Rehabilitation Wards: A Multi-Site, Co-Created Implementation Study" Healthcare 11, no. 9: 1241. https://doi.org/10.3390/healthcare11091241
APA StyleMarsden, D. L., Boyle, K., Birnie, J., Buzio, A., Dizon, J., Dunne, J., Greensill, S., Hill, K., Lever, S., Minett, F., Ormond, S., Shipp, J., Steel, J., Styles, A., Wiggers, J., Cadilhac, D. A.-M., & Duff, J., on behalf of the I-SCAMP Project Team. (2023). Improving Practice for Urinary Continence Care on Adult Acute Medical and Rehabilitation Wards: A Multi-Site, Co-Created Implementation Study. Healthcare, 11(9), 1241. https://doi.org/10.3390/healthcare11091241






