Comparing the Effectiveness of Cognitive Rehabilitation and Binaural Beats on Craving and Comorbidities of Sexual Hyperactivity: A Pilot, Exploratory Quasi-Experimental Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study’s Procedures
2.3. Study Instruments and Questionnaires Section
2.3.1. Carnes et al.’s Sexual Addiction Screening Test
2.3.2. Depression, Anxiety, and Stress 21-Item Questionnaire
2.3.3. Stop-Signal Task
2.3.4. The Go/No-Go Task
2.4. Statistical Analyses
3. Results
3.1. Population Recruited
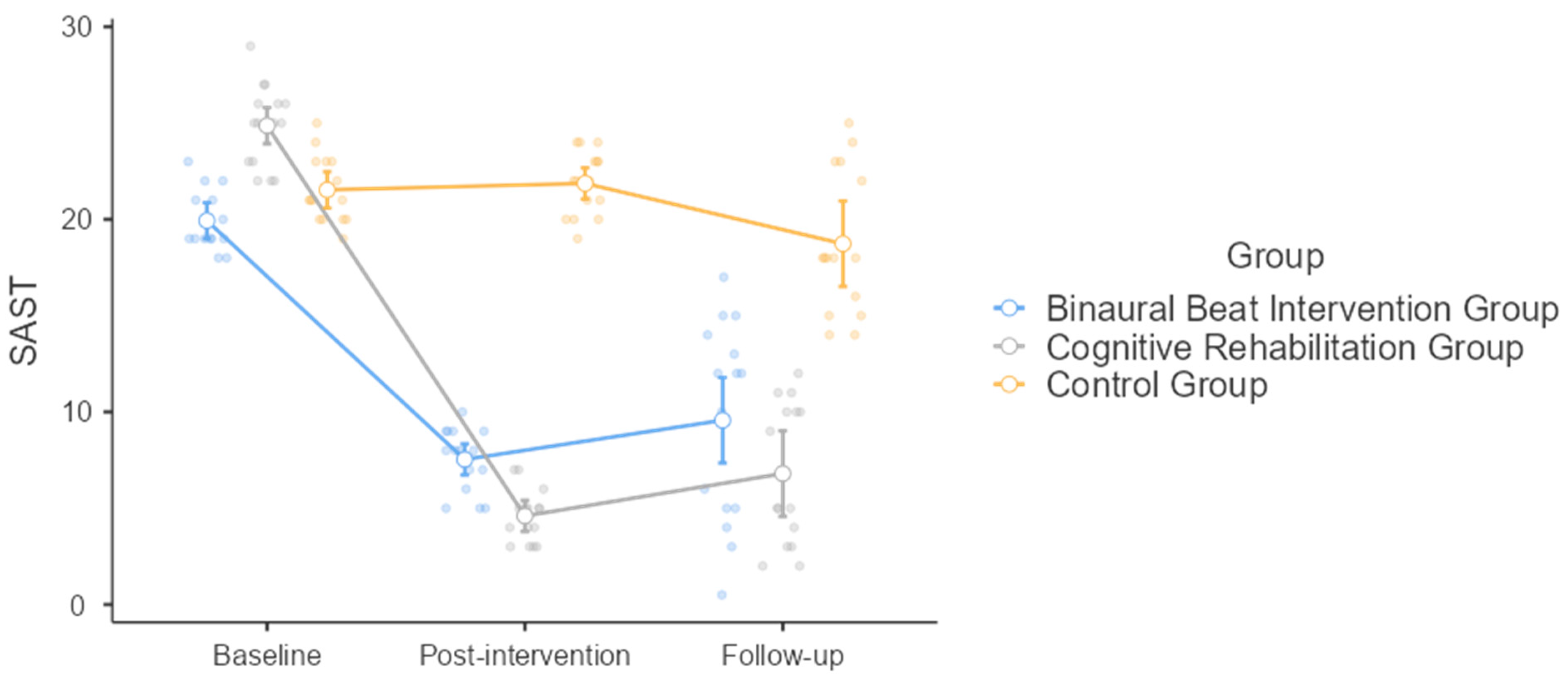
3.2. The Sex Addiction Screening Test
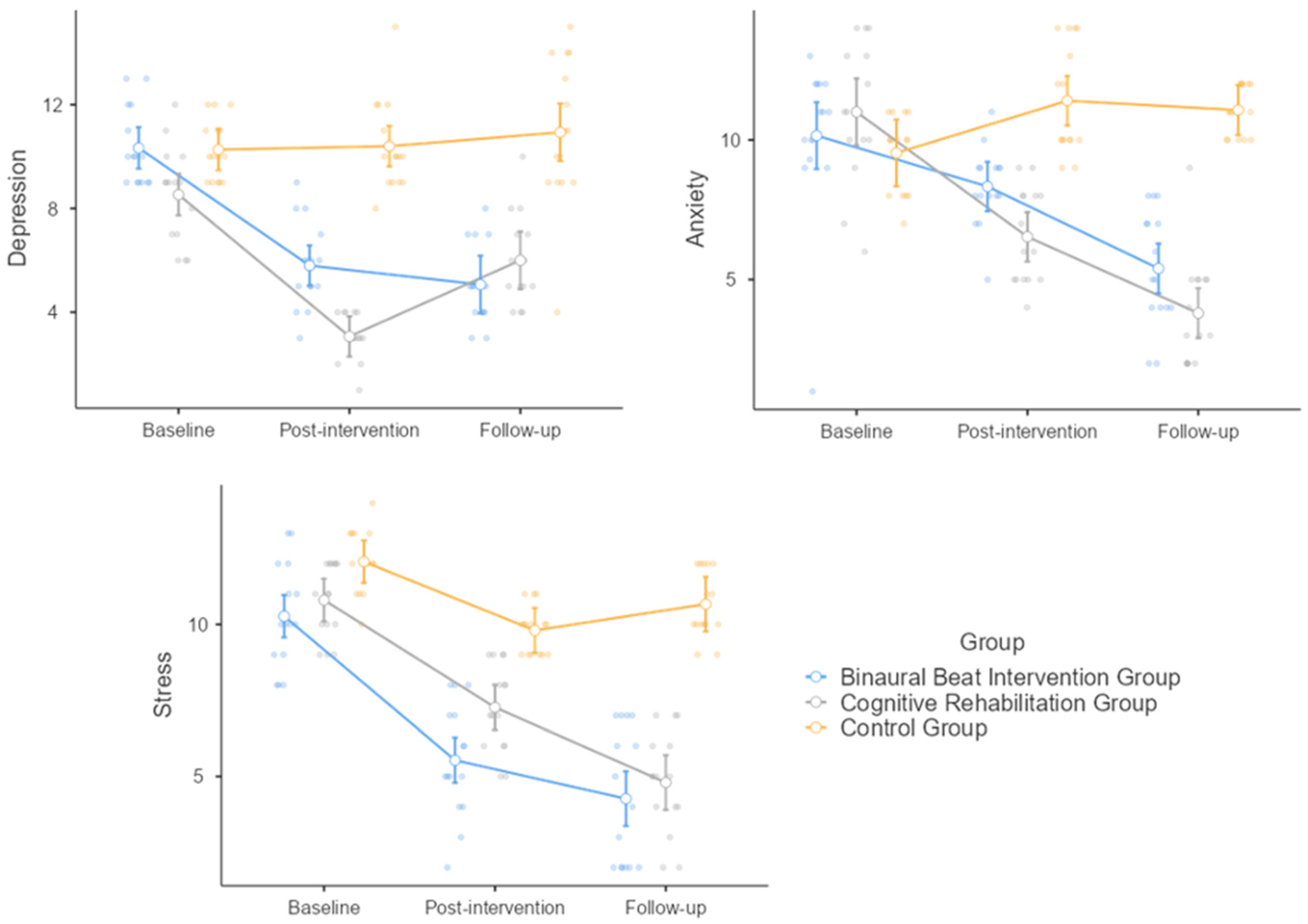
3.3. Depression
3.4. Anxiety
3.5. Stress
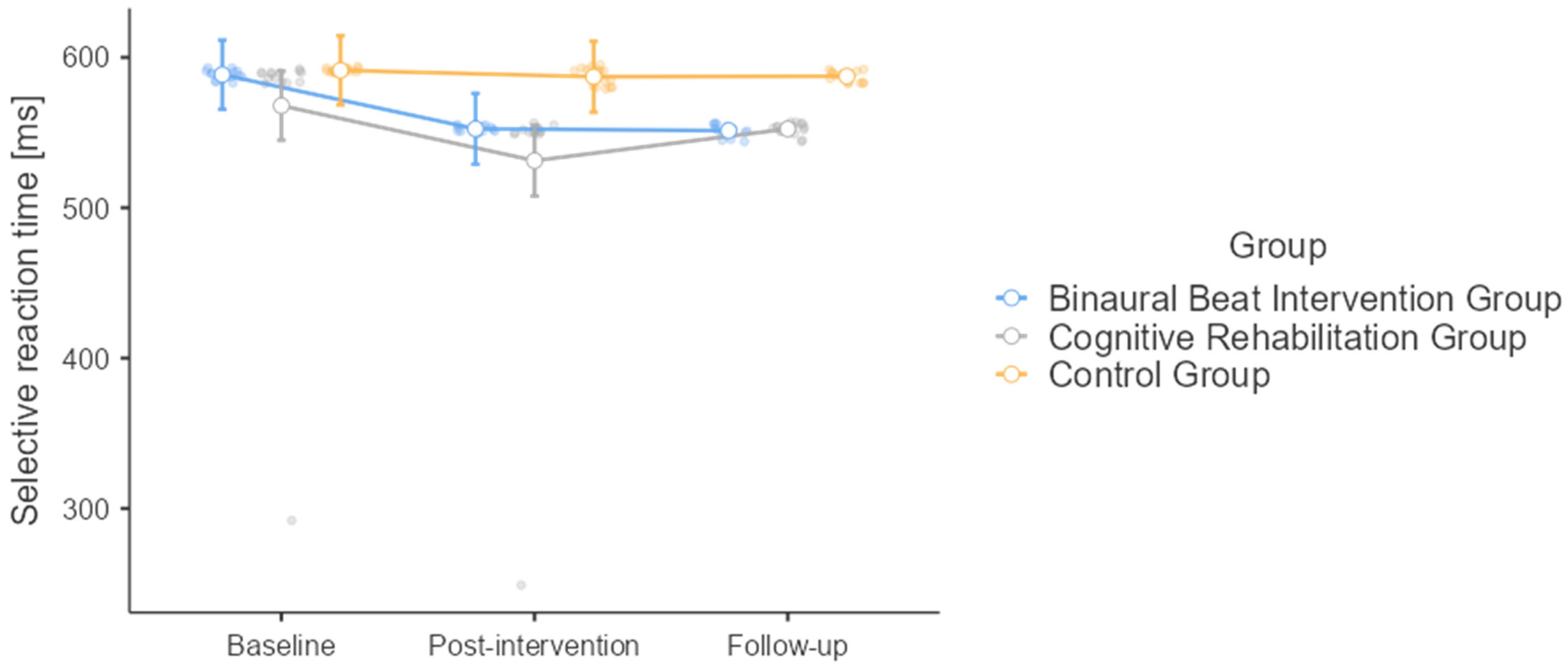
3.6. Selective Reaction Time
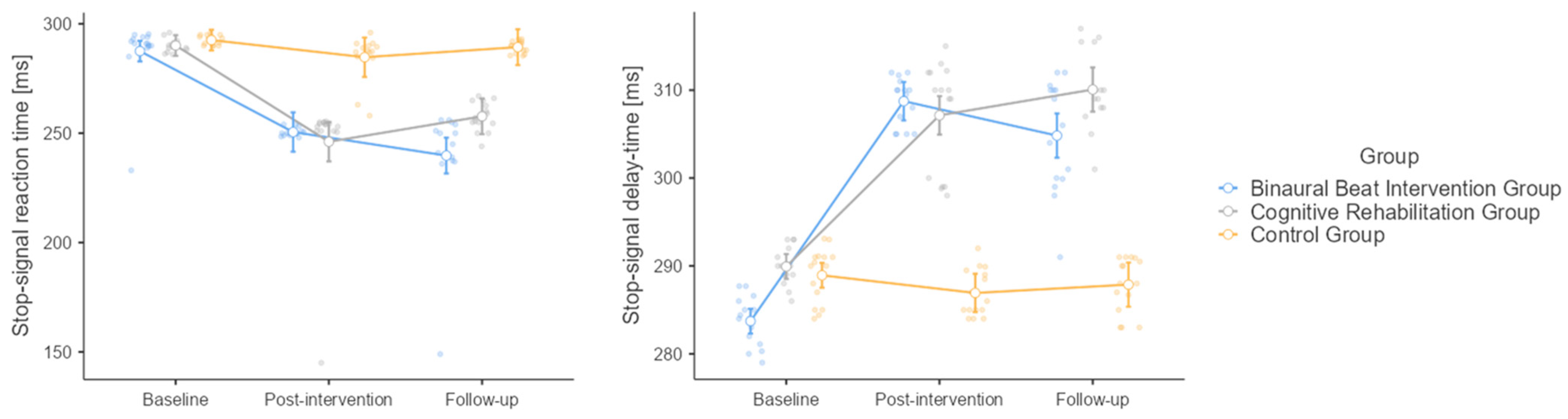
3.7. Stop-Signal Task
3.8. Stop-Signal Delay Time
4. Discussion
5. Study’s Strengths and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kafka, M.P. Hypersexual disorder: A proposed diagnosis for DSM-V. Arch. Sex. Behav. 2010, 39, 377–400. [Google Scholar] [CrossRef] [PubMed]
- Kingston, D.A.; Firestone, P. Problematic Hypersexuality: A review of conceptualization and diagnosis. Sex. Addict. Compulsivity 2008, 4, 284–310. [Google Scholar] [CrossRef]
- Grubbs, J.B.; Hoagland, K.C.; Lee, B.N.; Grant, J.T.; Davison, P.; Reid, R.C.; Kraus, S.W. Sexual addiction 25 years on: A systematic and methodological review of empirical literature and an agenda for future research. Clin. Psychol. Rev. 2020, 82, 101925. [Google Scholar] [CrossRef] [PubMed]
- Halpern, A.L. The proposed diagnosis of hypersexual disorder for inclusion in DSM-5: Unnecessary and harmful. Arch. Sex. Behav. 2011, 40, 487–488. [Google Scholar] [CrossRef] [PubMed]
- Winters, J. Hypersexual disorder: A more cautious approach. Arch. Sex. Behav. 2010, 39, 594–596. [Google Scholar] [CrossRef] [PubMed]
- Bőthe, B.; Koós, M.; Demetrovics, Z. Contradicting classification, nomenclature, and diagnostic criteria of Compulsive Sexual Behavior Disorder (CSBD) and future directions. J. Behav. Addict. 2022, 11, 204–209. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Karila, L.; Wéry, A.; Weinstein, A.; Cottencin, O.; Petit, A.; Reynaud, M.; Billieux, J. Sexual addiction or hypersexual disorder: Different terms for the same problem? A review of the literature. Curr. Pharm. Des. 2014, 20, 4012–4020. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, I. The Structure of Compulsive Sexual Behavior: A Network Analysis Study. Arch. Sex. Behav. 2023, 52, 1271–1284. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Långström, N.; Hanson, R.K. High rates of sexual behavior in the general population: Correlates and predictors. Arch. Sex. Behav. 2006, 35, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Sahithya, B.R.; Kashyap, R.S. Sexual Addiction Disorder—A Review With Recent Updates. J. Psychosexual Health 2022, 4, 95–101. [Google Scholar] [CrossRef]
- Ajegena, B.K.; Victor, O.B.; Usman, B.A. Sex and Sexual Addiction in the United States of America: An Overview of Its Epidemiology, Management and Prevention Strategies. J. Addict. Res. Ther. 2018, 9, 366. [Google Scholar] [CrossRef]
- Gold, S.N.; Heffner, C.L. Sexual addiction: Many conceptions, minimal data. Clin. Psychol. Rev. 1998, 18, 367–381. [Google Scholar] [CrossRef]
- Nenad, P.; Jonas, H. Conceptualization of Hypersexual Disorder with the Behavioral-Cognitive Inhibition Theory. Psychology 2014, 5, 151–159. [Google Scholar] [CrossRef]
- Du, R.; Knight, R.A. The Structure of Hypersexuality and Its Relation to Impulsivity. Arch. Sex. Behav. 2024. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Shao, X.; Shen, C.; Wang, W. Development of a structure-validated hypersexuality scale in Chinese university students. BMC Psychiatry 2021, 21, 352. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kingston, D.A.; Walters, G.D.; Olver, M.E.; Levaque, E.; Sawatsky, M.; Lalumière, M.L. Understanding the Latent Structure of Hypersexuality: A Taxometric Investigation. Arch. Sex. Behav. 2018, 47, 2207–2221. [Google Scholar] [CrossRef] [PubMed]
- Montgomery-Graham, S. Conceptualization and Assessment of Hypersexual Disorder: A Systematic Review of the Literature. Sex. Med. Rev. 2017, 5, 146–162. [Google Scholar] [CrossRef] [PubMed]
- Öberg, K.G.; Hallberg, J.; Kaldo, V.; Dhejne, C.; Arver, S. Hypersexual Disorder According to the Hypersexual Disorder Screening Inventory in Help-Seeking Swedish Men and Women With Self-Identified Hypersexual Behavior. Sex. Med. 2017, 5, e229–e236. [Google Scholar] [CrossRef]
- Bőthe, B.; Kovács, M.; Tóth-Király, I.; Reid, R.C.; Griffiths, M.D.; Orosz, G.; Demetrovics, Z. The Psychometric Properties of the Hypersexual Behavior Inventory Using a Large-Scale Nonclinical Sample. J. Sex. Res. 2019, 56, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Olver, M.E.; Kingston, D.A.; Laverty, E.K.; Seto, M.C. Psychometric Properties of Common Measures of Hypersexuality in an Online Canadian Sample. J. Sex. Med. 2022, 19, 331–346. [Google Scholar] [CrossRef] [PubMed]
- Koós, M.; Bőthe, B.; Orosz, G.; Potenza, M.N.; Reid, R.C.; Demetrovics, Z. The negative consequences of hypersexuality: Revisiting the factor structure of the Hypersexual Behavior Consequences Scale and its correlates in a large, non-clinical sample. Addict. Behav. Rep. 2020, 13, 100321. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Efrati, Y.; Shukron, O.; Epstein, R. Compulsive sexual behavior and sexual offending: Differences in cognitive schemas, sensation seeking, and impulsivity. J. Behav. Addict. 2019, 8, 432–441. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Duffy, A.; Dawson, D.L.; das Nair, R. Pornography Addiction in Adults: A Systematic Review of Definitions and Reported Impact. J. Sex. Med. 2016, 13, 760–777. [Google Scholar] [CrossRef] [PubMed]
- Hald, G.M.; Malamuth, N.M.; Yuen, C. Pornography and attitudes supporting violence against women: Revisiting the relationship in nonexperimental studies. Aggress. Behav. 2010, 36, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Turban, J.L.; Potenza, M.N.; Hoff, R.A.; Martino, S.; Kraus, S.W. Psychiatric disorders, suicidal ideation, and sexually transmitted infections among post-deployment veterans who utilize digital social media for sexual partner seeking. Addict. Behav. 2017, 66, 96–100. [Google Scholar] [CrossRef] [PubMed]
- George, M.; Maheshwari, S.; Chandran, S.; Rao, S.S.; Shivanand, M.J.; Sathyanarayana Rao, T.S. Psychosocial intervention for sexual addiction. Indian J. Psychiatry 2018, 60 (Suppl. 4), S510–S513. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Efrati, Y.; Gola, M. Compulsive sexual behavior: A twelve-step therapeutic approach. J. Behav. Addict. 2018, 7, 445–453. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thom, R.P.; Grudzinskas, A.J., Jr.; Saleh, F.M. Sexual Behavior Among Persons With Cognitive Impairments. Curr. Psychiatry Rep. 2017, 19, 25. [Google Scholar] [CrossRef] [PubMed]
- Momtaz, Y.A.; Hamid, T.A.; Ibrahim, R. The impact of mild cognitive impairment on sexual activity. Am. J. Alzheimers. Dis. Other Demen. 2013, 28, 759–762. [Google Scholar] [CrossRef] [PubMed]
- Carien, H.; Hannie, C.; Cees, J. Cognitive functioning and its influence on sexual behavior in normal aging and dementia. Int. J. Geriatr. Psychiatry 2014, 29, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Reid, R.C.; Woolley, S.R. Using Emotionally Focused Therapy for couples to resolve attachment ruptures created by hypersexual behavior. Sex. Addict. Compulsivity 2006, 13, 219–239. [Google Scholar] [CrossRef]
- Engel, J.; Veit, M.; Sinke, C.; Heitland, I.; Kneer, J.; Hillemacher, T.; Hartmann, U.; Kruger, T. Same but Different: A Clinical Characterization of Men with Hypersexual Disorder in the Sex@Brain Study. J. Clin. Med. 2019, 8, 157. [Google Scholar] [CrossRef] [PubMed]
- Ingendoh, R.M.; Posny, E.S.; Heine, A. Binaural beats to entrain the brain? A systematic review of the effects of binaural beat stimulation on brain oscillatory activity, and the implications for psychological research and intervention. PLoS ONE 2023, 18, e0286023. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wahbeh, H.; Calabrese, C.; Zwickey, H. Binaural beat technology in humans: A pilot study to assess psychologic and physiologic effects. J. Altern. Complement. Med. 2007, 13, 25–32. [Google Scholar] [CrossRef]
- Perez, H.D.O.; Dumas, G.; Lehmann, A. Binaural Beats through the Auditory Pathway: From Brainstem to Connectivity Patterns. Eneuro 2020, 7, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Wahbeh, H.; Calabrese, C.; Zwickey, H.; Zajdel, D. Binaural beat technology in humans: A pilot study to assess neuropsychologic, physiologic, and electroencephalographic effects. J. Altern. Complement. Med. 2007, 13, 199–206. [Google Scholar] [CrossRef]
- Ala, T.S.; Ahmadi-Pajouh, M.A.; Nasrabadi, A.M. Cumulative effects of theta binaural beats on brain power and functional connectivity. Biomed. Signal Proc. Control 2018, 42, 242–252. [Google Scholar] [CrossRef]
- Memon, M.A.; Ting, H.; Cheah, J.H.; Thurasamy, R.; Chuah, F.; Cham, T.H. Sample size for survey research: Review and recommendations. J. Appl. Struct. Equ. Model. 2020, 4, 1–20. [Google Scholar] [CrossRef]
- Carnes, P.; Green, B.; Carnes, S. The same yet different refocusing the sexual Addiction Screening Test (SAST) to reflect orientation and gender. Sex. Addict. Compulsivity 2010, 17, 3–7. [Google Scholar] [CrossRef]
- Zahedian, S.; Mohammadi, M.; Samani, S. The role of attachment styles, Parental bonding and self concept in sexual addiction. J. Clin. Psychol. 2011, 3, 65–73. (In Persian) [Google Scholar]
- Lovibond, S.H.; Lovibond, P.F. Manual for the Depression Anxiety and Stress Scales (DASS21), 2nd ed.; Psychology Foundation of Australia: Sydney, Australia, 1995; pp. 1–3. [Google Scholar]
- Verbruggen, F.; Logan, G.D.; Steven, M.A. STOP-IT: Windows executable software for the stop-signal paradigm. Behav. Res. Methods 2008, 40, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Falkenstein, M.; Hoormann, J.; Hohnsbein, J. ERP components in Go/Nogo tasks and their relation to inhibition. Acta Psychol. 1999, 101, 267–291. [Google Scholar] [CrossRef]
- Bagur, S.; Averseng, M.; Elgueda, D.; David, S.; Fritz, J.; Yin, P.; Shamma, S.; Boubenec, Y.; Ostojic, S. Go/No-Go task engagement enhances population representation of target stimuli in primary auditory cortex. Nat. Commun. 2018, 9, 2529. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Love, T.; Laier, C.; Brand, M.; Hatch, L.; Hajela, R. Neuroscience of Internet Pornography Addiction: A Review and Update. Behav. Sci. 2015, 5, 388–433. [Google Scholar] [CrossRef] [PubMed]
- Seyed Hashemi, S.; Shalchi, B.; Yaghoubi, H. Predicting Hypersexual Disorder Based on Difficulties in Emotion Regulation and Psychological Well-being in Male Students at Azarbaijan Shahid Madani University in 2016. JRUMS 2017, 16, 421–436. [Google Scholar]
- Seyed Hashemi, S.G. Sexual addiction: What clinicians and therapists need to know about it. In Proceedings of the Iran & World New Researches in Psychology and Educational Sciences Law and Social Sciences Conference, Shiraz, Iran, 18 May 2017. [Google Scholar]
- Lane, J.D.; Kasian, S.J.; Owens, J.E.; Marsh, G.R. Binaural Auditory Beats Affect Vigilance Performance and Mood. Physiol. Behav. 1998, 63, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Kennerly, R.C. An Empirical Investigation into the Effect of Beta Frequency Binaural Beat Audio Signals on Four Measures of Human Memory; Department of Psychology, West Georgia College: Carrolton, Georgia, 1994. [Google Scholar]
- Khoramabadi, Y.; Asadi, F.T. The Effect of Music Therapy on Reducing the Recurrence of Depression and Stress among Drug Addicts. Etiadpajohi 2016, 10, 151–162. [Google Scholar]
- Alipoor, A.; Oraki, M.; Yazdian Sabet, M. Efficiency of brainwave entrainment by binaural beats in reducing anxiety. J. Kermanshah. Univ. Med. Sci. 2014, 18, e74271. [Google Scholar] [CrossRef]
- Ann-Katrin, B.; Marlene, D.; Nikolai, A.; Leila, C.; Christian, E.; Juergen, F. Intracranial electroencephalography power and phase synchronization changes during monaural and binaural beat stimulation. Eur. J. Neurosci. 2014, 41, 254–263. [Google Scholar] [CrossRef]
- Gruber, T.; Müller, M.M.; Keil, A.; Elbert, T. Selective visual-spatial attention alters induced gamma band responses in the human EEG. Clin. Neurophysiol. 1999, 110, 2074–2085. [Google Scholar] [CrossRef]
- Sokolov, A.; Pavlova, M.; Lutzenberger, W.; Birbaumer, N. Reciprocal modulation of neuromagnetic induced gamma activity by attention in the human visual and auditory cortex. Neuroimage 2004, 22, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Reedijk, S.A.; Bolders, A.; Colzato, L.S.; Hommel, B. Eliminating the Attentional Blink through Binaural Beats: A Case for Tailored Cognitive Enhancement. Front. Psychiatry 2015, 6, 82. [Google Scholar] [CrossRef] [PubMed]
- Akram, A.; Kamran, Y.; Khodamarad, M. Investigating the effect of computerized cognitive rehabilitation on reducing cognitive avoidance of students with special learning disorder. Psychol. Except. People 2019, 33, 69–96. [Google Scholar]
- Jafari, R.; Bafandeh, H. The Effectiveness of Cognitive Rehabilitation on Anxiety Reduction and Brainwave Pattern in Patients with Anxiety Disorder. IJRN 2020, 7, 66–74. [Google Scholar] [CrossRef]
- Chaieb, L.; Wilpert, E.C.; Reber, T.P.; Fell, J. Auditory beat stimulation and its effects on cognition and mood States. Front. Psychiatry 2015, 6, 70. [Google Scholar] [CrossRef] [PubMed]
- Borges, L.R.; Arantes, A.P.B.B.; Naves, E.L.M. Influence of Binaural Beats Stimulation of Gamma Frequency over Memory Performance and EEG Spectral Density. Healthcare 2023, 11, 801. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, T.; Zhao, H.; Tang, Z. A study of brainwave entrainment based on EEG brain dynamics. Comput. Inf. Sci. 2009, 2, 80–86. [Google Scholar] [CrossRef]
- Le Scouarnec, R.P.; Poirier, R.M.; Owens, J.E.; Gauthier, J.; Taylor, A.G.; Foresman, P.A. Use of binaural beat tapes for treatment of anxiety: A pilot study of tape preference and outcomes. Altern. Ther. Health Med. 2001, 7, 58–63. [Google Scholar]
- Lee, M.; Song, C.B.; Shin, G.H.; Lee, S.W. Possible Effect of Binaural Beat Combined with Autonomous Sensory Meridian Response for Inducing Sleep. Front. Hum. Neurosci. 2019, 13, 425. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kienast, T.; Hariri, A.R.; Schlagenhauf, F.; Wrase, J.; Sterzer, P.; Buchholz, H.G.; Smolka, M.N.; Gründer, G.; Cumming, P.; Kumakura, Y.; et al. Dopamine in amygdala gates limbic processing of aversive stimuli in humans. Nat. Neurosci. 2008, 11, 1381–1382. [Google Scholar] [CrossRef]
- Berlucchi, G. Brain plasticity and cognitive neurorehabilitation. Neuropsychol. Rehabil. 2011, 21, 560–578. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Value |
|---|---|
| Age | 32.36 ± 6.00 years (range 20–40 years) |
| Socio-economic status | |
| Low | 13 (28.9%) |
| Medium | 23 (51.1%) |
| High | 9 (20.0%) |
| Job status | |
| Employed | 34 (75.6%) |
| Unemployed | 11 (24.4%) |
| Marital status | |
| Married | 24 (53.3%) |
| Not married | 21 (46.7%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mousavi, Z.; Samanipour, M.H.; Zarei, H.; Abharian, P.H.; Ceylan, H.İ.; Bragazzi, N.L. Comparing the Effectiveness of Cognitive Rehabilitation and Binaural Beats on Craving and Comorbidities of Sexual Hyperactivity: A Pilot, Exploratory Quasi-Experimental Study. Healthcare 2024, 12, 1116. https://doi.org/10.3390/healthcare12111116
Mousavi Z, Samanipour MH, Zarei H, Abharian PH, Ceylan Hİ, Bragazzi NL. Comparing the Effectiveness of Cognitive Rehabilitation and Binaural Beats on Craving and Comorbidities of Sexual Hyperactivity: A Pilot, Exploratory Quasi-Experimental Study. Healthcare. 2024; 12(11):1116. https://doi.org/10.3390/healthcare12111116
Chicago/Turabian StyleMousavi, Zeinab, Mohammad Hossein Samanipour, Hamed Zarei, Payman Hassani Abharian, Halil İbrahim Ceylan, and Nicola Luigi Bragazzi. 2024. "Comparing the Effectiveness of Cognitive Rehabilitation and Binaural Beats on Craving and Comorbidities of Sexual Hyperactivity: A Pilot, Exploratory Quasi-Experimental Study" Healthcare 12, no. 11: 1116. https://doi.org/10.3390/healthcare12111116







