Evaluation of the Oral Microbiome before and after Treatments for Halitosis with Photodynamic Therapy and Probiotics—Pilot Study
Abstract
1. Introduction
2. Materials and Methods
- Group 1—Tongue Scraping
- Group 2—Antimicrobial Photodynamic Therapy (aPDT)
- Group 3—Probiotics
- Group 4—Antimicrobial Photodynamic Therapy (aPDT) and Probiotics
3. Results
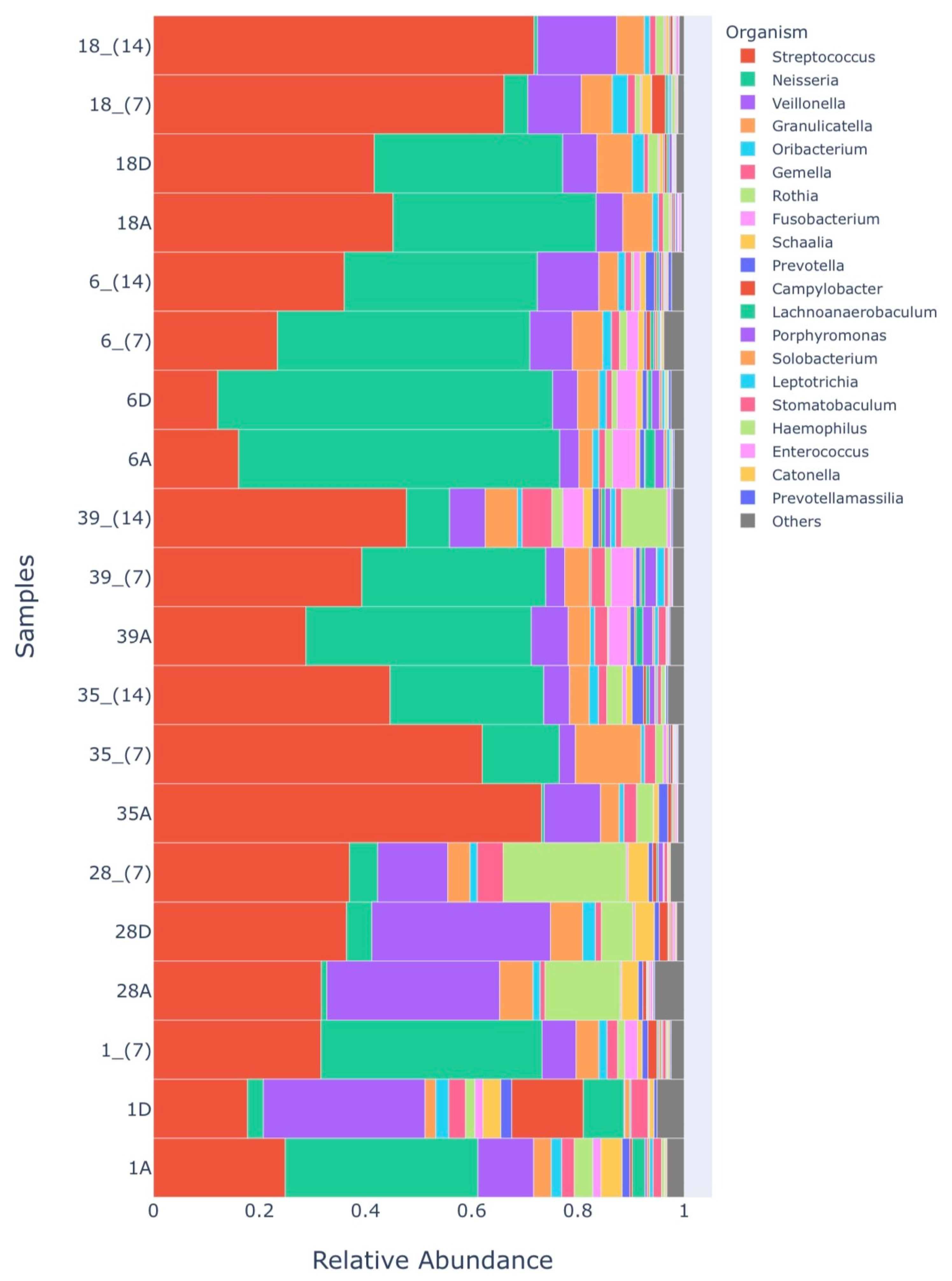
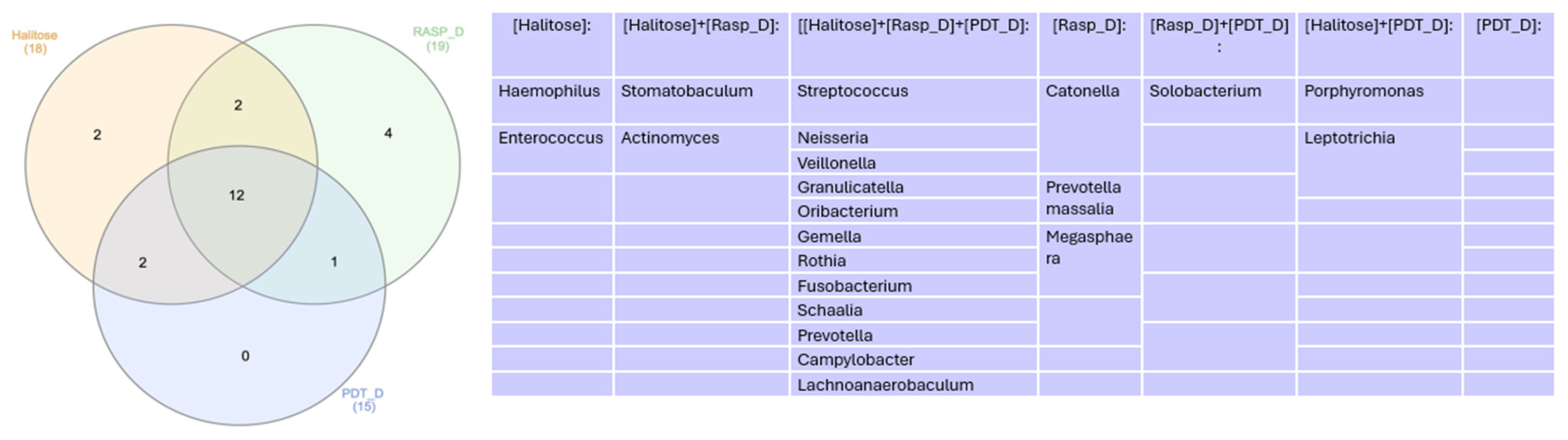
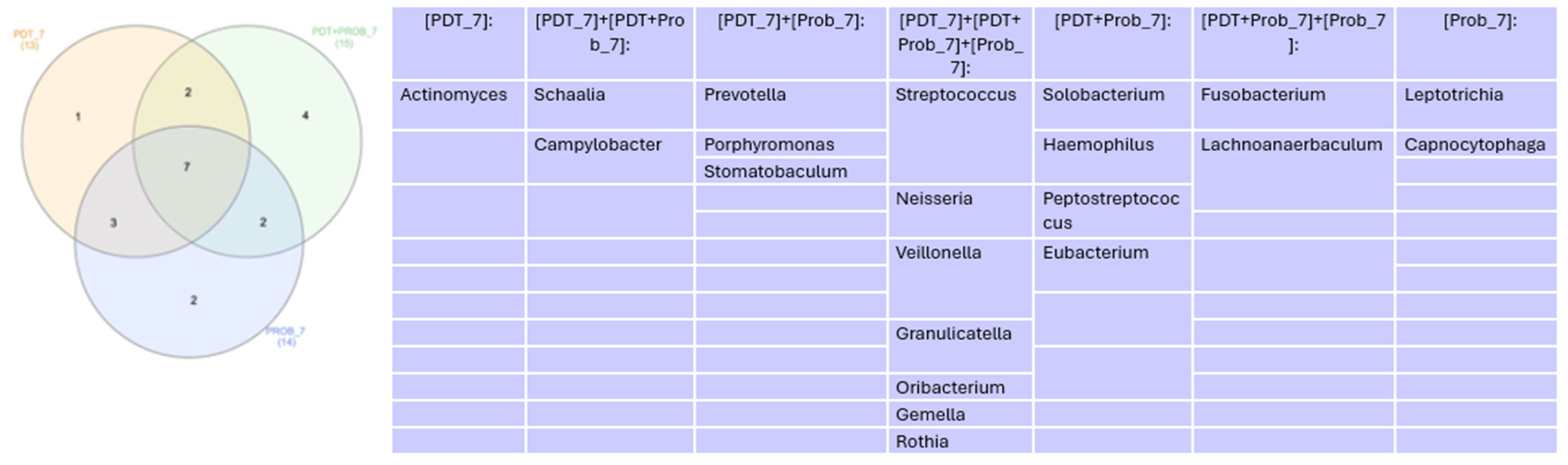
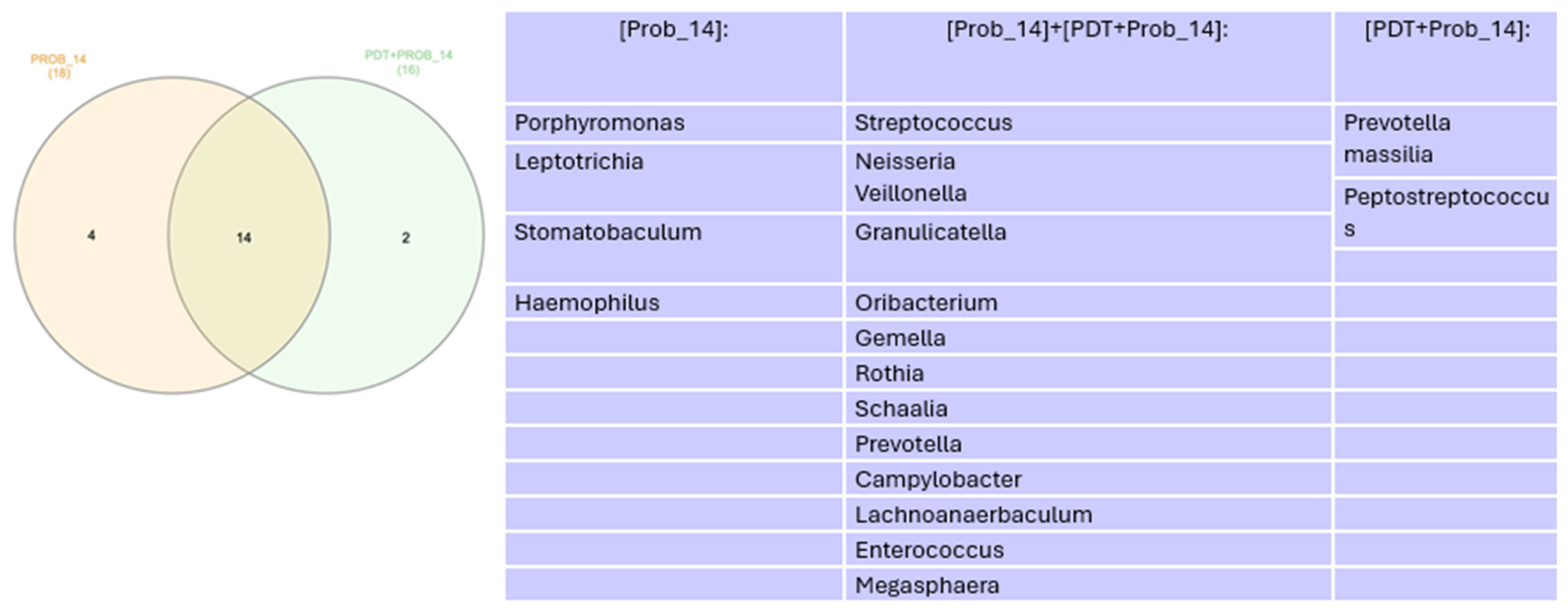
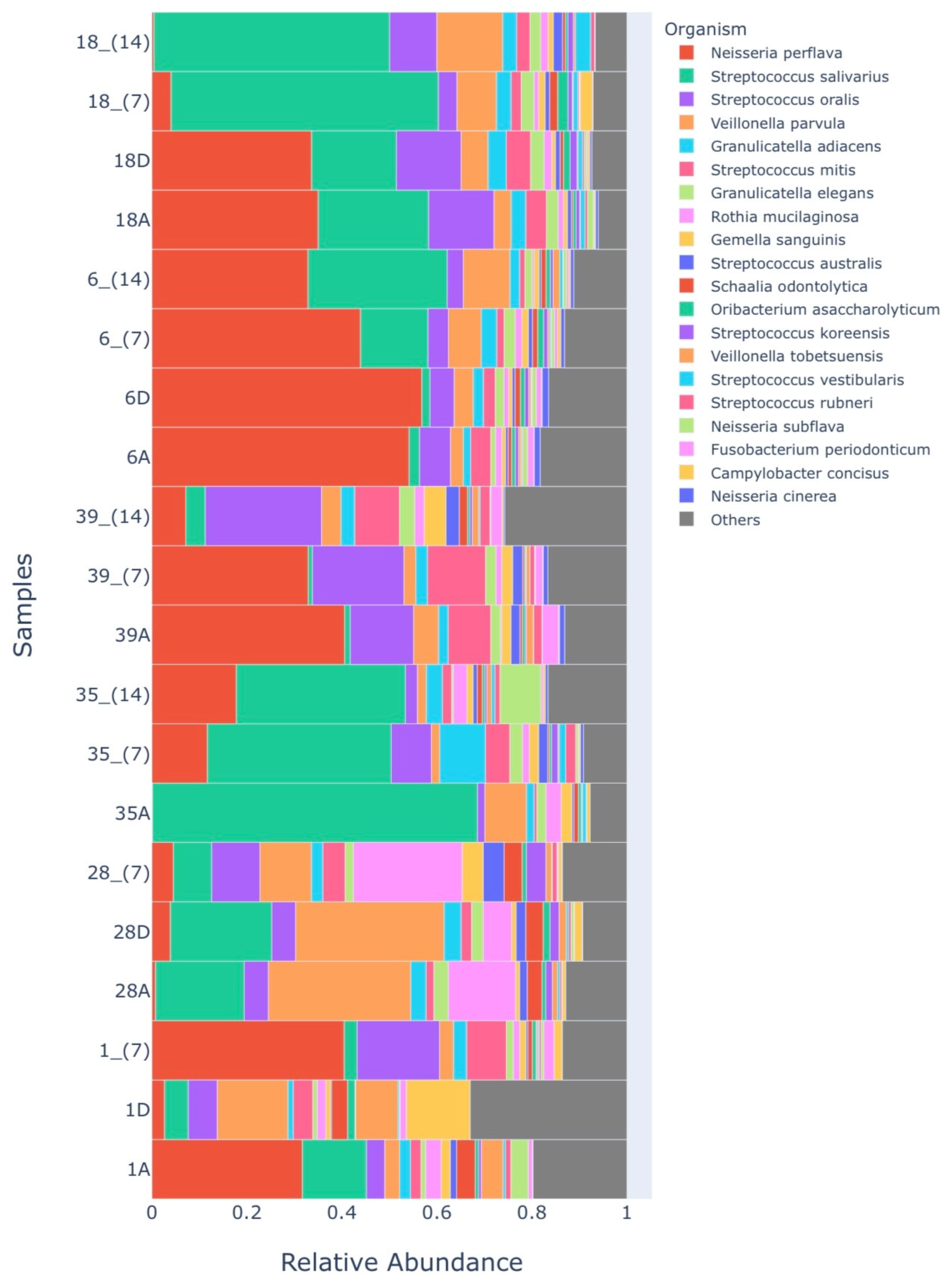
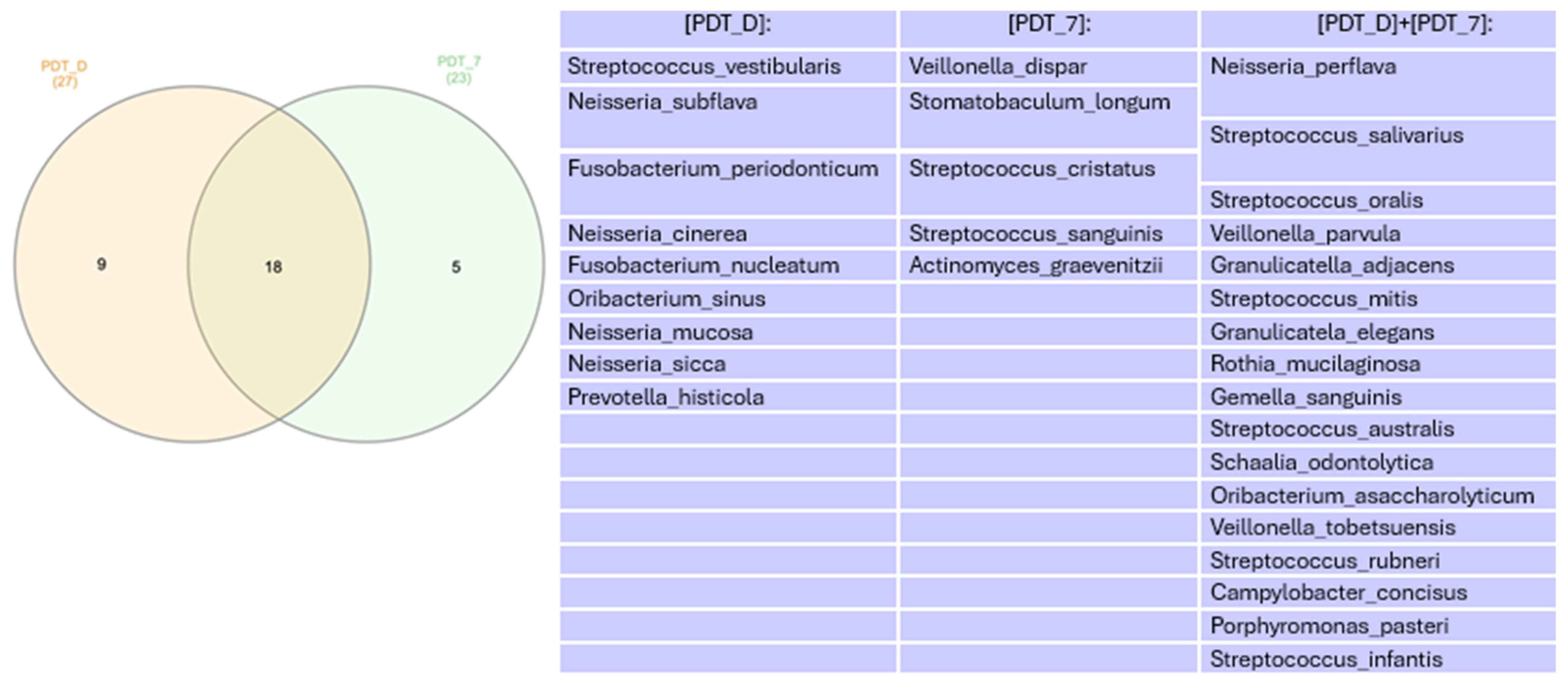
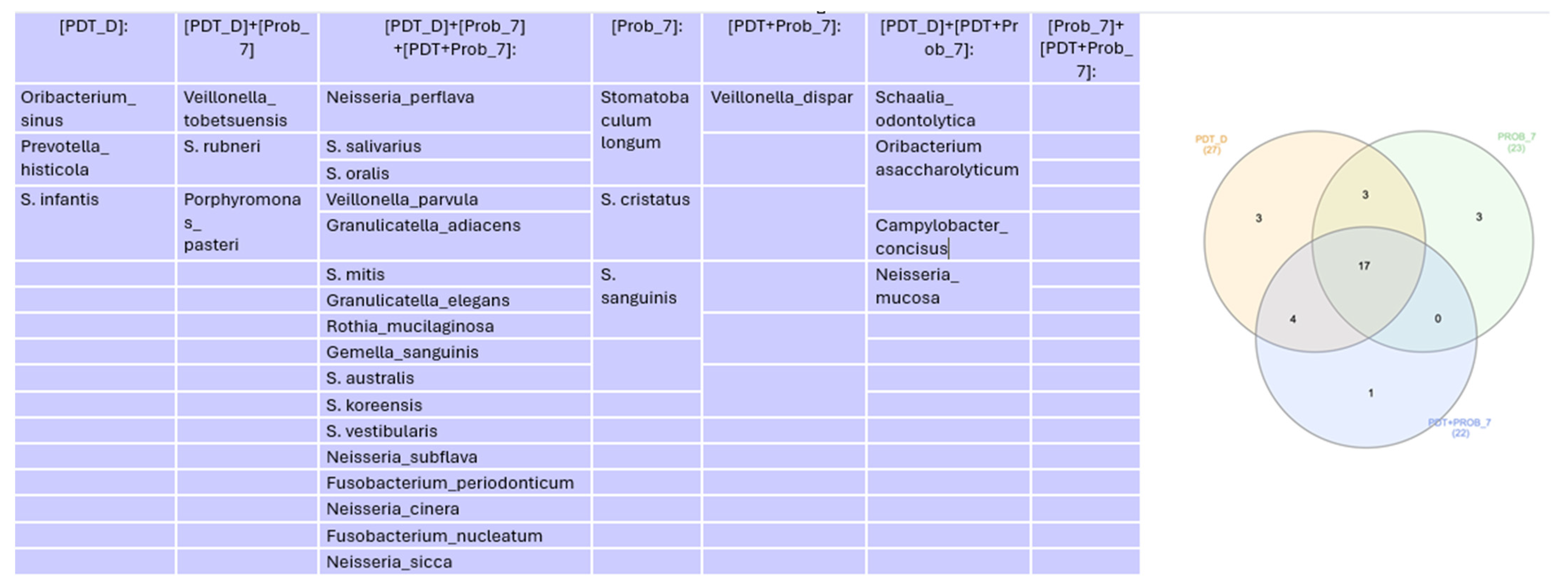
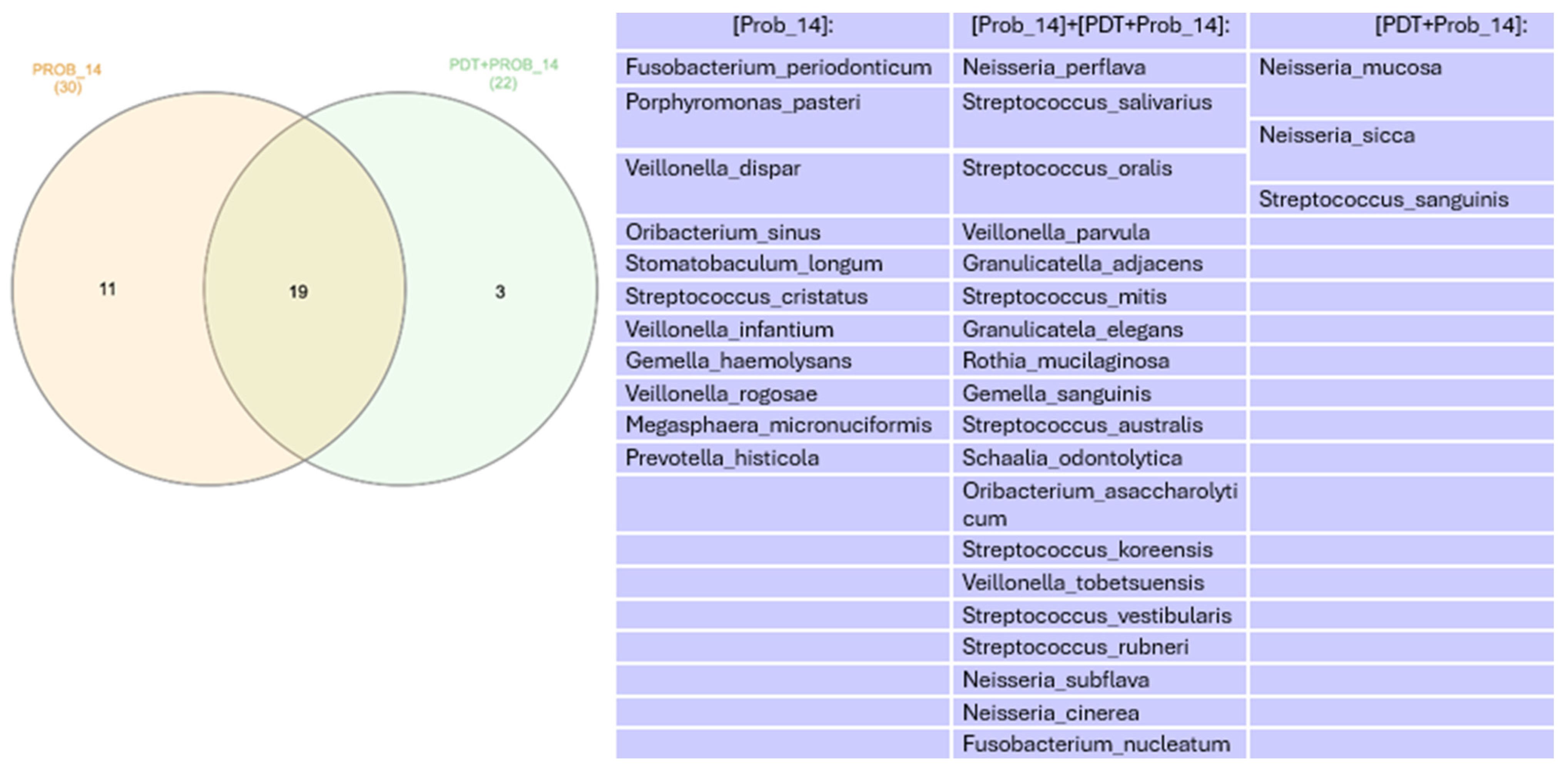
Microbiome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Zhu, C.; Cao, G.; Zhan, J.; Feng, X.; Chen, X. Dynamic Alterations of Oral Microbiota Related to Halitosis in Preschool Children. Front. Cell. Infect. Microbiol. 2021, 11, 599467. [Google Scholar] [CrossRef] [PubMed]
- Hampelska, K.; Jaworska, M.M.; Babalska, Z.Ł.; Karpiński, T.M. The Role of Oral Microbiota in Intra-Oral Halitosis. J. Clin. Med. 2020, 9, 2484. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Izidoro, C.; Botelho, J.; Machado, V.; Reis, A.M.; Proença, L.; Alves, R.C.; Mendes, J.J. Revisiting Standard and Novel Therapeutic Approaches in Halitosis: A Review. Int. J. Environ. Res. Public Health 2022, 19, 11303. [Google Scholar] [CrossRef] [PubMed]
- Bollen, C.M.L.; Beikler, T. Halitosis: The multidisciplinary approach. Int. J. Oral Sci. 2012, 4, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.; Wu, Z.X.; Chen, X.Y.; Wang, J.Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Chung, S.W.; Auh, Q.S.; Hong, S.J.; Lee, Y.A.; Jung, J.; Lee, G.J.; Park, H.J.; Shin, S.I.; Hong, J.Y. Progress in Oral Microbiome Related to Oral and Systemic Diseases: An Update. Diagnostics 2021, 1, 1283. [Google Scholar] [CrossRef] [PubMed]
- Marsh, P.D.; Zaura, E. Dental biofilm: Ecological interactions in health and disease. J. Clin. Periodontol. 2017, 44, S12–S22. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Zhang, Y.; He, M.; Zhu, C.; Fenget, X.P. Relationship of tongue coating microbiome on volatile sulfur compounds in healthy and halitosis adults. J. Breath Res. 2019, 14, 016005. [Google Scholar] [CrossRef] [PubMed]
- Zanetti, F.; Zivkovic Semren, T.; Battey, J.N.D.; Guy, P.A.; Ivanov, N.V.; van der Plas, A.; Hoeng, J. A Literature Review and Framework Proposal for Halitosis Assessment in Cigarette Smokers and Alternative Nicotine-Delivery Products Users. Front. Oral Health 2021, 2, 777442. [Google Scholar] [CrossRef]
- Conceicao, M.; Marocchio, L.; Giudice, F. Diagnostic Technique for Assessing Halitosis Origin Using Oral and Nasal Organoleptic Tests, Including Safety Measures Post COVID-19. J. Dent. Oral Sci. 2020, 2, 1–19. [Google Scholar] [CrossRef]
- Motta, P.D.B.; Motta, L.J.; Campos, T.M.; Gonçalves, M.L.L.; Santos, E.M.; Martimbianco, A.L.C.; de Andrade, D.J.C.; Mesquita-Ferrari, R.A.; Fernandes, K.P.S.; Horliana, A.C.R.T.; et al. Effect of Photodynamic Therapy on Halitosis: A Systematic Review of Randomized Controlled Trials. Sensors 2022, 22, 469. [Google Scholar] [CrossRef] [PubMed]
- Porter, S.R.; Scully, C. Oral malodour (halitosis). BMJ 2006, 333, 632–635. [Google Scholar] [CrossRef] [PubMed]
- Kara, C.; Tezel, A.; Orbak, R. Effect of oral hygiene instruction and scaling on oral malodour in a population of Turkish children with gingival inflammation. Int. J. Paediatr. Dent. 2006, 16, 399–404. [Google Scholar] [CrossRef]
- Lopes, R.G.; Costa da Mota, A.C.; Deana, A.M.; Prates, R.A.; França, C.M.; Fernandes, K.P.S.; Ferrari, R.A.M.; Bussadori, S.K. Immediate results of photodynamic therapy for the treatment of halitosis in adolescents: A randomized, controlled, clinical trial. Lasers Med. Sci. 2016, 31, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Costa da Mota, A.C.; França, C.M.; Prates, R.; Deana, A.M.; Santos, L.C.; Garcia, R.L.; Gonçalves, M.L.L.; Ferrari, R.A.M.F.; Fernandes, K.P.S.; Bussadori, S.K. Effect of photodynamic therapy for the treatment of halitosis in adolescents—A controlled, microbiological, clinical trial. J. Biophotonics 2016, 9, 1337–1343. [Google Scholar] [CrossRef]
- Gonçalves, M.L.L.; Costa da Mota, A.C.; Deana, A.M.; Cavalcante, L.A.S.; Horliana, A.C.R.T.; Pavani, C.; Motta, L.J.; Fernandes, K.P.S.; Mesquita-Ferrari, R.A.; da Silva, D.F.T.; et al. Antimicrobial photodynamic therapy with Bixa orellana extract and blue LED in the reduction of halitosis—A randomized, controlled clinical trial. Photodiagn. Photodyn. Ther. 2020, 30, 101751. [Google Scholar] [CrossRef]
- Costa da Mota, A.C.; Gonçalves, M.L.L.; Horliana, A.C.R.T.; Deana, A.M.; Cavalcante, L.A.S.; Gomes, A.O.; Mayer, M.P.A.; Suguimoto, E.S.A.; Fernandes, K.P.S.; Mesquita-Ferrari, R.A.; et al. Effect of antimicrobial photodynamic therapy with red led and methylene blue on the reduction of halitosis: Controlled microbiological clinical trial. Lasers Med. Sci. 2022, 37, 877–886. [Google Scholar] [CrossRef]
- Iwamoto, T.; Suzuki, N.; Tanabe, K.; Takeshita, T.; Hirofuji, T. Effects of probiotic Lactobacillus salivarius WB21 on halitosis and oral health: An open-label pilot trial. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2010, 110, 201–208. [Google Scholar] [CrossRef] [PubMed]
- López-Valverde, N.; López-Valverde, A.; Macedo de Sousa, B.; Rodríguez, C.; Suárez, A.; Aragoneses, J.M. Role of Probiotics in Halitosis of Oral Origin: A Systematic Review and Meta-Analysis of Randomized Clinical Studies. Front. Nutr. 2022, 8, 787908. [Google Scholar] [CrossRef]
- Shimizu, T.; Ueda, T.; Sakurai, K. New method for evaluation of tongue-coating status. J. Oral Rehabil. 2007, 34, 442–447. [Google Scholar] [CrossRef]
- Park, D.Y.; Park, J.Y.; Lee, D.; Hwang, I.; Kim, H.S. Leaky gum: The revisited origin of systemic diseases. Cells 2022, 11, 1079. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Cheng, L.; You, Y.; Tang, C.; Ren, B.; Li, Y.; Xu, X.; Zhou, X. Oral microbiota in human systematic diseases. Int. J. Oral Sci. 2022, 14, 14. [Google Scholar] [CrossRef] [PubMed]
- HOMD: Human Oral Microbiome Database. Available online: http://www.homd.org/ (accessed on 19 June 2020).
- Krishnan, K.; Chen, T.; Paster, B.J. A practical guide to the oral microbiome and its relation to health and disease. Oral Dis. 2017, 23, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Socransky, S.S.; Gibbons, R.J.; Dale, A.C.; Bortnick, L.; Rosenthal, E.; MacDonald, J.B. The microbiota of the gingival crevice area of man. I. Total microscopic and viable counts and counts of specific organisms. Arch. Oral Biol. 1963, 8, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Olsen, I.; Preza, D.; Aas, J.A.; Paster, B.J. Cultivated and not-yetcultivated bacteria in oral biofilms. Microb. Ecol. Health Dis. 2009, 21, 65–71. [Google Scholar] [CrossRef]
- Takeshita, T.; Suzuki, N.; Nakano, Y.; Yasui, M.; Yoneda, M.; Shimazaki, Y.; Hirofuji, T.; Yamashita, Y. Discrimination of the oral microbiota associated with high hydrogen sulfide and methyl mercaptan production. Sci. Rep. 2012, 2, 215. [Google Scholar] [CrossRef] [PubMed]
- Seerangaiyan, K.; van Winkelhoff, A.J.; Harmsen, H.J.M.; Rossen, J.W.A.; Winkel, E.G. The tongue microbiome in healthy subjects and patients with intra-oral halitosis. J. Breath Res. 2017, 11, 036010. [Google Scholar] [CrossRef]
- Bernardi, S.; Karygianni, L.; Filippi, A.; Anderson, A.C.; Zürcher, A.; Hellwig, E.; Vach, K.; Macchiarelli, G.; Al-Ahmad, A. Combining culture and culture-independent methods reveals new microbial composition of halitosis patients’ tongue biofilm. Microbiologyopen 2020, 9, e958. [Google Scholar] [CrossRef]
- Yitzhaki, S.; Reshef, L.; Gophna, U.; Rosenberg, M.; Sterer, N. Microbiome associated with denture malodour. J. Breath Res. 2018, 12, 027103. [Google Scholar] [CrossRef]
- Ren, W.; Zhang, Q.; Liu, X.; Zheng, S.; Ma, L.; Chen, F.; Xu, T.; Xu, B. Supragingival Plaque Microbial Community Analysis of Children with Halitosis. J. Microbiol. Biotechnol. 2016, 26, 2141–2147. [Google Scholar] [CrossRef]
- Ademovski, S.E.; Persson, G.R.; Winkel, E.; Tangerman, A.; Lingström, P.; Renvert, S. The short-term treatment effects on the microbiota at the dorsum of the tongue in intra-oral halitosis patients—A randomized clinical trial. Clin. Oral Investig. 2013, 17, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Riggio, M.P.; Lennon, A.; Rolph, H.J.; Hodge, P.J.; Donaldson, A.; Maxwell, A.J.; Bagg, J. Molecular identification of bacteria on the tongue dorsum of subjects with and without halitosis. Oral Dis. 2008, 14, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Persson, S.; Edlund, M.B.; Claesson, R.; Carlsson, J. The formation of hydrogen sulfide and methyl mercaptan by oral bacteria. Oral Microbiol. Immunol. 1990, 5, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Haraszthy, V.I.; Zambon, J.J.; Sreenivasan, P.K.; Zambon, M.M.; Gerber, D.; Rego, R.; Parker, C. Identification of oral bacterial species associated with halitosis. J. Am. Dent. Assoc. 2007, 138, 1113–1120. [Google Scholar] [CrossRef]
- Yoo, J.I.; Shin, I.S.; Jeon, J.G.; Yang, Y.M.; Kim, J.G.; Lee, D.W. The Effect of Probiotics on Halitosis: A Systematic Review and Meta-analysis. Probiotics Antimicrob. Proteins 2019, 11, 150–157. [Google Scholar] [CrossRef]






| Participant and Treatment That Was Performed | Coated Tong Index (CTI) | Sulfide in ppb—Initial | Sulfide in ppb— Immediately after Treatments | Sulfide in ppb—after 7 Days | Sulfide in ppb—after 14 Days | Sulfide in ppb—after 30 Days |
|---|---|---|---|---|---|---|
| 1—Tongue scraping | 16.66% | 1436 | 0 | 592 | - | 1224 |
| 28—aPDT | 50% | 2175 | 7 | 1751 | - | 599 |
| 35—Probiotics | 66.66% | 1354 | - | 279 | 780 | 1648 |
| 39—Probiotics | 66.66% | 437 | - | 65 | 95 | - |
| 6 aPDT + Probiotics | 16.66% | 621 | 32 | 173 | 523 | 342 |
| 18 aPDT + Probiotics | 16.66% | 482 | 0 | 7 | 497 | 282 |
| Groups Microbiome 1 | Sample Identification |
|---|---|
| HALITOSE | 1A |
| RASP_D | 1D |
| RASP_7 | 1_ (7) |
| HALITOSE | 28A |
| PDT_D | 28D |
| PDT_7 | 28_ (7) |
| HALITOSE | 35A |
| PROB_7 | 35_ (7) |
| PROB_14 | 35_ (14) |
| HALITOSE | 39A |
| PROB_7 | 39_ (7) |
| PROB_14 | 39_ (14) |
| HALITOSE | 6A |
| PDT_D | 6D |
| PDT + PROB_7 | 6_ (7) |
| PDT + PROB_14 | 6_ (14) |
| HALITOSE | 18A |
| PDT_D | 18D |
| PDT + PROB_7 | 18_ (7) |
| PDT + Prob_14 | 18_ (14) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Barros Motta, P.; Gonçalves, M.L.L.; Gallo, J.M.A.S.; Sobral, A.P.T.; Motta, L.J.; Santos, E.M.; de Andrade, D.J.C.; Duran, C.C.G.; Fernandes, K.P.S.; Mesquita-Ferrari, R.A.; et al. Evaluation of the Oral Microbiome before and after Treatments for Halitosis with Photodynamic Therapy and Probiotics—Pilot Study. Healthcare 2024, 12, 1123. https://doi.org/10.3390/healthcare12111123
de Barros Motta P, Gonçalves MLL, Gallo JMAS, Sobral APT, Motta LJ, Santos EM, de Andrade DJC, Duran CCG, Fernandes KPS, Mesquita-Ferrari RA, et al. Evaluation of the Oral Microbiome before and after Treatments for Halitosis with Photodynamic Therapy and Probiotics—Pilot Study. Healthcare. 2024; 12(11):1123. https://doi.org/10.3390/healthcare12111123
Chicago/Turabian Stylede Barros Motta, Pamella, Marcela Leticia Leal Gonçalves, Juliana Maria Altavista Sagretti Gallo, Ana Paula Taboada Sobral, Lara Jansiski Motta, Elaine Marcílio Santos, David José Casimiro de Andrade, Cinthya Cosme Gutierrez Duran, Kristianne Porta Santos Fernandes, Raquel Agnelli Mesquita-Ferrari, and et al. 2024. "Evaluation of the Oral Microbiome before and after Treatments for Halitosis with Photodynamic Therapy and Probiotics—Pilot Study" Healthcare 12, no. 11: 1123. https://doi.org/10.3390/healthcare12111123
APA Stylede Barros Motta, P., Gonçalves, M. L. L., Gallo, J. M. A. S., Sobral, A. P. T., Motta, L. J., Santos, E. M., de Andrade, D. J. C., Duran, C. C. G., Fernandes, K. P. S., Mesquita-Ferrari, R. A., Horliana, A. C. R. T., & Bussadori, S. K. (2024). Evaluation of the Oral Microbiome before and after Treatments for Halitosis with Photodynamic Therapy and Probiotics—Pilot Study. Healthcare, 12(11), 1123. https://doi.org/10.3390/healthcare12111123






