Comparing Cancer Risks and Mortality between Phytopharmaceuticals and Estrogen-Progestogen Medications for Menopausal Women: A Population-Based Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
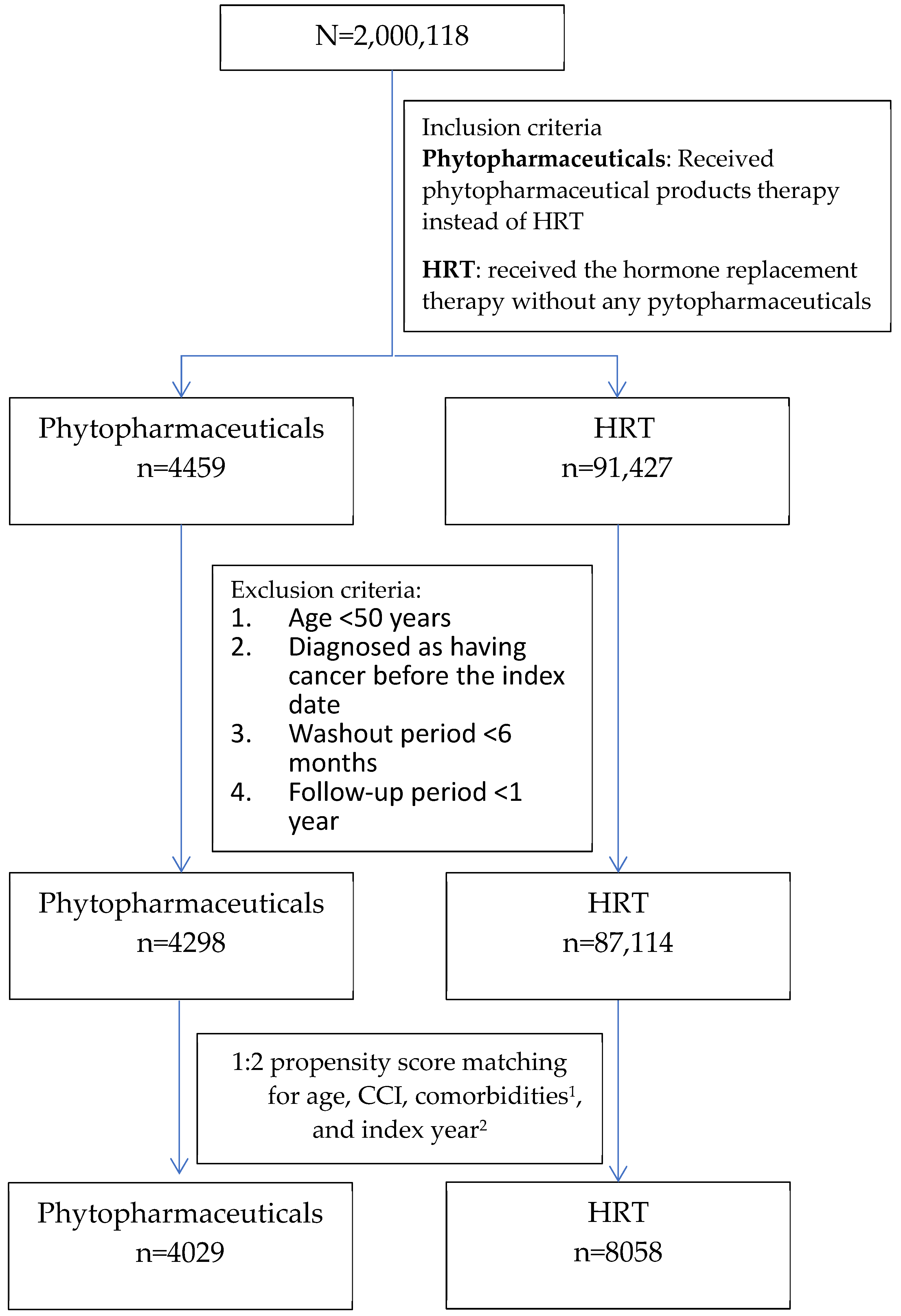
2.2. Study Design and Study Participants
2.3. Potential Confounding Factors
2.4. Covariate Measurements
2.5. Primary Outcome Measures
2.6. Exposure Definition and Follow-Up
2.7. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Comparison of Each Cancer Prevalence and Mortality Rate between Phytopharmaceuticals and HRT
3.3. Phytopharmaceuticals Duration Effect on Cancer Development and Mortality
3.4. Effect of Top Five Phytopharmaceuticals Prescriptions on Cancer Development and Mortality
4. Discussion
4.1. New User Design for Balanced Characteristics in Groups
4.2. Principal Outcomes
4.3. Endometrial and Cervical Cancer
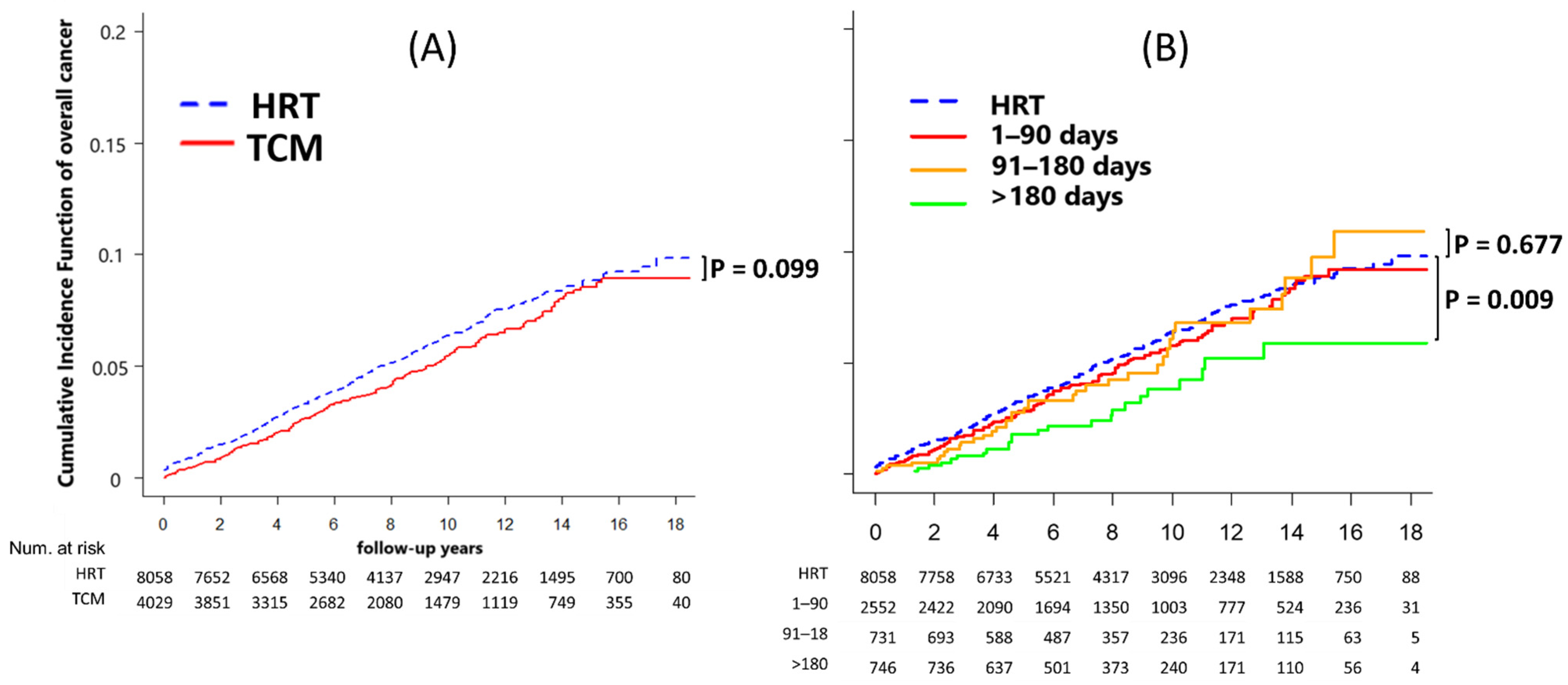
4.4. Overall Cancer
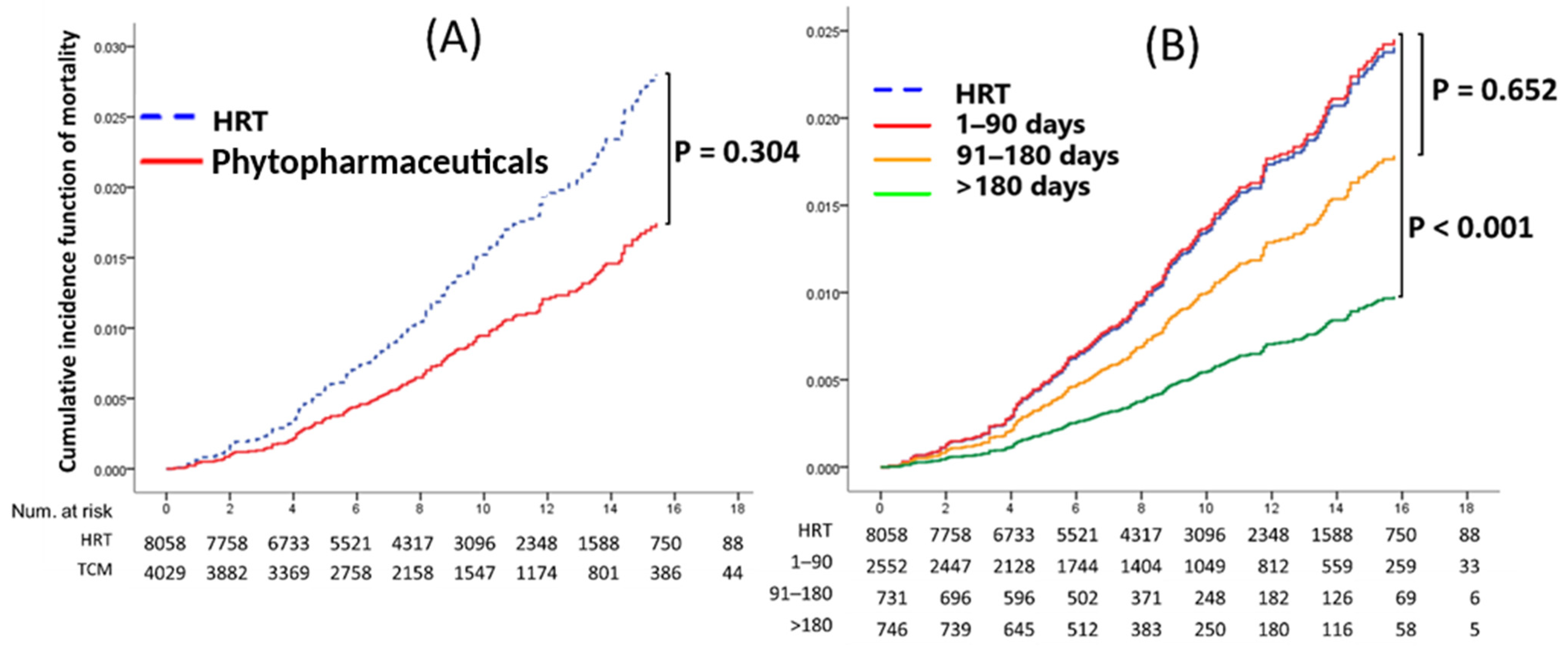
4.5. All-Cause Mortality
4.6. Comparison between Subgroups of the Top Five Polyherbal Formulations
4.7. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wassertheil-Smoller, S.; Hendrix, S.; Limacher, M.; Heiss, G.; Kooperberg, C.; Baird, A.; Kotchen, T.; Curb, J.D.; Black, H.; Rossouw, J.E. Effect of estrogen plus progestin on stroke in postmenopausal women: The Women’s Health Initiative: A randomized trial. Jama 2003, 289, 2673–2684. [Google Scholar] [CrossRef]
- Rossouw, J.E.; Anderson, G.L.; Prentice, R.L.; LaCroix, A.Z.; Kooperberg, C.; Stefanick, M.L.; Jackson, R.D.; Beresford, S.A. Howard BV, Johnson KC: Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the Women’s Health Initiative randomized controlled trial. Jama 2002, 288, 321–333. [Google Scholar]
- Rossouw, J.E.; Prentice, R.L.; Manson, J.E.; Wu, L.; Barad, D.; Barnabei, V.M.; Ko, M.; LaCroix, A.Z.; Margolis, K.L.; Stefanick, M.L. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. Jama 2007, 297, 1465–1477. [Google Scholar] [CrossRef]
- Chen, F.-P.; Chen, T.-J.; Kung, Y.-Y.; Chen, Y.-C.; Chou, L.-F.; Chen, F.-J.; Hwang, S.-J. Use frequency of traditional Chinese medicine in Taiwan. BMC Health Serv. Res. 2007, 7, 26. [Google Scholar] [CrossRef]
- Freeman, E.W.; Sammel, M.D.; Lin, H.; Liu, Z.; Gracia, C.R. Duration of menopausal hot flushes and associated risk factors. Obstet. Gynecol. 2011, 117, 1095–1104. [Google Scholar] [CrossRef]
- Santoro, N.; Roeca, C.; Peters, B.A.; Neal-Perry, G. The menopause transition: Signs, symptoms, and management options. J. Clin. Endocrinol. Metab. 2021, 106, 1–15. [Google Scholar] [CrossRef]
- Nelson, H.D.; Walker, M.; Zakher, B.; Mitchell, J. Menopausal hormone therapy for the primary prevention of chronic conditions: A systematic review to update the US Preventive Services Task Force recommendations. Ann. Intern. Med. 2012, 157, 104–113. [Google Scholar] [CrossRef]
- Posadzki, P.; Watson, L.K.; Ernst, E. Adverse effects of herbal medicines: An overview of systematic reviews. Clin. Med. 2013, 13, 7. [Google Scholar] [CrossRef]
- Kargozar, R.; Azizi, H.; Salari, R. A review of effective herbal medicines in controlling menopausal symptoms. Electron. Physician 2017, 9, 5826. [Google Scholar] [CrossRef]
- Scheid, V.; Ward, T.; Tuffrey, V. Comparing TCM textbook descriptions of menopausal syndrome with the lived experience of London women at midlife and the implications for Chinese medicine research. Maturitas 2010, 66, 408–416. [Google Scholar] [CrossRef]
- Hsieh, C.-Y.; Su, C.-C.; Shao, S.-C.; Sung, S.-F.; Lin, S.-J.; Kao Yang, Y.-H.; Lai, E.C.-C. Taiwan’s national health insurance research database: Past and future. Clin. Epidemiol. 2019, 11, 349–358. [Google Scholar] [CrossRef]
- Schneeweiss, S. A basic study design for expedited safety signal evaluation based on electronic healthcare data. Pharmacoepidemiol. Drug Saf. 2010, 19, 858–868. [Google Scholar] [CrossRef]
- Greifer, N. Estimating Effects after Matching. MatchIt: Nonparametric Preprocessing for Parametric Causal Inference. 2022. Available online: https://cran.r-project.org/web/packages/MatchIt/vignettes/estimating-effects.html (accessed on 1 August 2022).
- Abadie, A.; Spiess, J. Robust post-matching inference. J. Am. Stat. Assoc. 2022, 117, 983–995. [Google Scholar] [CrossRef]
- Higbee, J.D.; Lefler, J.S.; Burnett, R.T.; Ezzati, M.; Marshall, J.D.; Kim, S.-Y.; Bechle, M.; Robinson, A.L. Pope III CA: Estimating long-term pollution exposure effects through inverse probability weighting methods with Cox proportional hazards models. Environ. Epidemiol. 2020, 4, e085. [Google Scholar] [CrossRef]
- Stocker, T.; Qin, D.; Plattner, G.; Tignor, M.; Allen, S.; Boschung, J.; Nauels, A.; Xia, Y.; Bex, V.; Midgley, P. Climate Change 2013: The Physical Science Basis; Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Pintilie, M. An introduction to competing risks analysis. Rev. Española De Cardiol. Engl. Ed. 2011, 64, 599–605. [Google Scholar] [CrossRef]
- Zhu, X.; Liew, Y.; Liu, Z.L. Chinese herbal medicine for menopausal symptoms. Cochrane Database Syst. Rev. 2016, 3, 1–90. [Google Scholar] [CrossRef]
- Li, M.; Hung, A.; Lenon, G.B.; Yang, A.W.H. Chinese herbal formulae for the treatment of menopausal hot flushes: A systematic review and meta-analysis. PLoS ONE 2019, 14, e0222383. [Google Scholar] [CrossRef]
- Casper, R.F. Clinical manifestations and diagnosis of menopause. In UpToDate. Edn.; UpToDate Inc.: Waltham, MA, USA, 2009. [Google Scholar]
- Chen, H.-Y.; Lin, Y.-H.; Wu, J.-C.; Chen, Y.-C.; Yang, S.-H.; Chen, J.-L.; Chen, T.-J. Prescription patterns of Chinese herbal products for menopausal syndrome: Analysis of a nationwide prescription database. J. Ethnopharmacol. 2011, 137, 1261–1266. [Google Scholar] [CrossRef]
- Chen, H. Cases with menopausal syndrome treated by Kun Tai capsule. China Pharm. 2014, 23, 108–109. [Google Scholar]
- Sun, A.-J.; Wang, Y.-P.; Gu, B.; Zheng, T.-P.; Lin, S.-Q.; Bai, W.-P.; Wei, Y.; Zhang, S.-F.; Zhang, Y. A multi-center, randomized, controlled and open clinical trial of heyan kuntai capsule (和颜坤泰胶囊) and hormone therapy in perimenopausal women. Chin. J. Integr. Med. 2018, 24, 487–493. [Google Scholar] [CrossRef]
- Azizi, H.; Liu, Y.F.; Wang, C.H.; Du, L.; Bahrami-Taghanaki, H.; Ollah Esmaily, H.; Azizi, H.; Xue, X.O. Menopause-related symptoms: Traditional Chinese medicine vs hormone therapy. Altern. Ther. Health Med. 2011, 17, 48. [Google Scholar]
- Shen, L.-L.; Muo, C.-H.; Su, S.-Y.; Morisky, D.E. Use of chinese medicine reduces the development of cervical cancer from pap smear-diagnosed cervical dysplasia: A case-control study. Evid. Based Complement. Altern. Med. 2017, 2017, 4082630. [Google Scholar] [CrossRef]
- Manson, J.E.; Aragaki, A.K.; Rossouw, J.E.; Anderson, G.L.; Prentice, R.L.; LaCroix, A.Z.; Chlebowski, R.T.; Howard, B.V. Thomson CA, Margolis KL: Menopausal hormone therapy and long-term all-cause and cause-specific mortality: The Women’s Health Initiative randomized trials. Jama 2017, 318, 927–938. [Google Scholar] [CrossRef]
- Grady, D.; Herrington, D.; Bittner, V.; Blumenthal, R.; Davidson, M.; Hlatky, M.; Hsia, J.; Hulley, S.; Herd, A.; Khan, S. Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II). Jama 2002, 288, 49–57. [Google Scholar] [CrossRef]
- Su, H.I.; Chen, Y.-C.; Hwang, W.-T.; Liu, Z.; Su, T.-P.; Chen, T.-J.; Barnhart, K.T.; Yang, Y.-X. Risks and benefits of menopausal hormone therapy in postmenopausal Chinese women. Menopause 2012, 19, 931. [Google Scholar] [CrossRef]
- Xu, F.; Peng, D.; Tao, C.; Yin, D.; Kou, J.; Zhu, D.; Yu, B. Anti-depression effects of Danggui-Shaoyao-San, a fixed combination of Traditional Chinese Medicine, on depression model in mice and rats. Phytomedicine 2011, 18, 1130–1136. [Google Scholar] [CrossRef]
- Manson, J.E.; Chlebowski, R.T.; Stefanick, M.L.; Aragaki, A.K.; Rossouw, J.E.; Prentice, R.L.; Anderson, G.; Howard, B.V.; Thomson, C.A.; LaCroix, A.Z. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. Jama 2013, 310, 1353–1368. [Google Scholar] [CrossRef]
- Shumaker, S.A.; Legault, C.; Rapp, S.R.; Thal, L.; Wallace, R.B.; Ockene, J.K.; Hendrix, S.L.; Jones, B.N., III; Assaf, A.R.; Jackson, R.D. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: The Women’s Health Initiative Memory Study: A randomized controlled trial. Jama 2003, 289, 2651–2662. [Google Scholar] [CrossRef]
- Anderson, G.L.; Limacher, M.; Assaf, A.R.; Bassford, T.; Beresford, S.A.; Black, H.; Bonds, D.; Brunner, R.; Brzyski, R.; Caan, B. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: The Women’s Health Initiative randomized controlled trial. Jama 2004, 291, 1701–1712. [Google Scholar]
- Koyama, T.; Ohara, M.; Ichimura, M.; Saito, M. Effects of Japanese Kampo Medicine on Hypothalamic-Pituitary-Ovarian Function in Women with Ovarian Insufficiency. Am. J. Chin. Med. 1988, 16, 47–55. [Google Scholar] [CrossRef]
- Hagino, N. An overview of Kampo medicine: Toki-Shakuyaku-San (TJ-23). Phytother. Res. 1993, 7, 391–394. [Google Scholar] [CrossRef]
- Shen, A.-Y.; Wang, T.-S.; Huang, M.-H.; Liao, C.-H.; Chen, S.-J.; Lin, C.-C. Antioxidant and antiplatelet effects of dang-gui-shao-yao-san on human blood cells. Am. J. Chin. Med. 2005, 33, 747–758. [Google Scholar] [CrossRef]
- Akase, T.; Akase, T.; Onodera, S.; Jobo, T.; Matsushita, R.; Kaneko, M.; Tashiro, S.-I. A comparative study of the usefulness of toki-shakuyaku-san and an oral iron preparation in the treatment of hypochromic anemia in cases of uterine myoma. Yakugaku Zasshi 2003, 123, 817–824. [Google Scholar] [CrossRef]
- Ushiroyama, T.; Ikeda, A.; Sakuma, K.; Ueki, M. Changes in serum tumor necrosis factor (TNF-α) with Kami-shoyo-san administration in depressed climacteric patients. Am. J. Chin. Med. 2004, 32, 621–629. [Google Scholar] [CrossRef]
- Liu, C.-M.; Chen, J.; Yang, S.; Mao, L.-G.; Jiang, T.-T.; Tu, H.-H.; Chen, Z.-L.; Hu, Y.-T.; Gan, L.; Li, Z.-J. The Chinese herbal formula Zhibai Dihuang Granule treat Yin-deficiency-heat syndrome rats by regulating the immune responses. J. Ethnopharmacol. 2018, 225, 271–278. [Google Scholar] [CrossRef]
- Zhao, L.; Gao, H.; Zhao, Y.; Lin, D. Metabonomic analysis of the therapeutic effect of Zhibai Dihuang Pill in treatment of streptozotocin-induced diabetic nephropathy. J. Ethnopharmacol. 2012, 142, 647–656. [Google Scholar] [CrossRef]
- Yokozawa, T.; Kim, H.Y.; Yamabe, N. Amelioration of diabetic nephropathy by dried Rehmanniae Radix (Di Huang) extract. Am. J. Chin. Med. 2004, 32, 829–839. [Google Scholar] [CrossRef]
- Liu, H.-R.; Tang, X.-Y.; Dai, D.-Z.; Dai, Y. Ethanol extracts of Rehmannia complex (Di Huang) containing no Corni fructus improve early diabetic nephropathy by combining suppression on the ET-ROS axis with modulate hypoglycemic effect in rats. J. Ethnopharmacol. 2008, 118, 466–472. [Google Scholar] [CrossRef]
- Kang, N.; Luan, Y.; Jiang, Y.; Cheng, W.; Liu, Y.; Su, Z.; Liu, Y.; Tan, P. Neuroprotective effects of oligosaccharides in rehmanniae radix on transgenic caenorhabditis elegans models for alzheimer’s disease. Front. Pharmacol. 2022, 13, 1861. [Google Scholar] [CrossRef]
- Shergis, J.L.; Ni, X.; Sarris, J.; Zhang, A.L.; Guo, X.; Xue, C.C.; Lu, C. Hugel H: Ziziphus spinosa seeds for insomnia: A review of chemistry and psychopharmacology. Phytomedicine 2017, 34, 38–43. [Google Scholar] [CrossRef]
- Yang, R.; Yuan, B.-C.; Ma, Y.-S.; Zhou, S.; Liu, Y. The anti-inflammatory activity of licorice, a widely used Chinese herb. Pharm. Biol. 2017, 55, 5–18. [Google Scholar] [CrossRef]
- Xie, B.; Gong, T.; Gao, R.; Liu, J.; Zuo, J.; Wang, X.; Zhang, Z. Development of rat urinary HPLC-UV profiling for metabonomic study on Liuwei Dihuang Pills. J. Pharm. Biomed. Anal. 2009, 49, 492–497. [Google Scholar] [CrossRef]



| Phytopharmaceuticals | % | HRT | % | p | ||
|---|---|---|---|---|---|---|
| Age | 50–59 | 2452 | 60.9% | 4904 | 60.9% | 1.000 |
| 60–69 | 1381 | 34.3% | 2762 | 34.3% | ||
| >70 | 196 | 4.9% | 392 | 4.9% | ||
| CCI | 0 | 1830 | 45.4% | 3660 | 45.4% | 1.000 |
| 1–2 | 1445 | 35.9% | 2890 | 35.9% | ||
| 3–4 | 428 | 10.6% | 856 | 10.6% | ||
| >5 | 326 | 8.1% | 652 | 8.1% | ||
| DM | No | 3528 | 87.6% | 7056 | 87.6% | 1.000 |
| Yes | 501 | 12.4% | 1002 | 12.4% | ||
| HCD | No | 2881 | 71.5% | 5762 | 71.5% | 1.000 |
| Yes | 1148 | 28.5% | 2296 | 28.5% | ||
| Hyperlipidemia | No | 2828 | 70.2% | 5656 | 70.2% | 1.000 |
| Yes | 1201 | 29.8% | 2402 | 29.8% | ||
| Statins | No | 3115 | 77.3% | 6184 | 76.7% | 0.482 |
| Yes | 914 | 22.7% | 1874 | 23.3% | ||
| Aspirin | No | 3222 | 80.0% | 5987 | 74.3% | 0.000 |
| Yes | 807 | 20.0% | 2071 | 25.7% | ||
| β-blocker | No | 2240 | 55.6% | 3605 | 44.7% | 0.000 |
| Yes | 1789 | 44.4% | 4453 | 55.3% | ||
| Metformin | No | 3603 | 89.4% | 7225 | 89.7% | 0.689 |
| Yes | 426 | 10.6% | 833 | 10.3% | ||
| Glucocorticoids | No | 866 | 21.5% | 1021 | 12.7% | 0.000 |
| Yes | 3163 | 78.5% | 7037 | 87.3% |
| End Point | Phytopharmaceuticals | Incidence Rate * | (95% CI) | HRT | Incidence Rate * | (95% CI) |
|---|---|---|---|---|---|---|
| Ovarian cancer | 24 | 0.68 | 0.46–1.02 | 44 | 0.63 | 0.47–0.85 |
| Breast cancer | 78 | 2.23 | 1.78–2.77 | 138 | 1.98 | 1.67–2.34 |
| Endometrial cancer | 6 | 0.17 | 0.08–0.38 | 67 | 0.96 | 0.76–1.22 |
| Cervical cancer | 7 | 0.20 | 0.09–0.42 | 50 | 0.72 | 0.54–0.95 |
| Oral cancer | 3 | 0.09 | 0.03–0.27 | 5 | 0.07 | 0.03–0.17 |
| Thyroid cancer | 12 | 0.34 | 0.19–0.60 | 17 | 0.24 | 0.15–0.39 |
| Skin cancer | 6 | 0.17 | 0.08–0.38 | 14 | 0.20 | 0.12–0.34 |
| Colon cancer | 15 | 0.43 | 0.26–0.71 | 37 | 0.53 | 0.38–0.73 |
| Rectal cancer | 12 | 0.34 | 0.19–0.60 | 21 | 0.30 | 0.20–0.46 |
| Liver cancer | 9 | 0.26 | 0.13–0.49 | 12 | 0.17 | 0.10–0.30 |
| Lung cancer | 21 | 0.60 | 0.39–0.92 | 36 | 0.52 | 0.37–0.72 |
| Stomach cancer | 4 | 0.11 | 0.04–0.30 | 8 | 0.11 | 0.06–0.23 |
| pancreatic cancer | 0 | 0.00 | 0.00–0.00 | 7 | 0.10 | 0.05–0.21 |
| Kidney cancer | 5 | 0.14 | 0.06–0.34 | 7 | 0.10 | 0.05–0.21 |
| Bladder cancer | 3 | 0.09 | 0.03–0.27 | 12 | 0.17 | 0.10–0.30 |
| Overall cancer | 197 | 5.62 | 4.89–6.46 | 452 | 6.48 | 5.91–7.10 |
| All-cause mortality | 75 | 2.14 | 1.71–2.68 | 173 | 2.41 | 2.07–2.79 |
| End Point | Phytopharmaceuticals | % | HRT | % | p | HR (95% CI) | p |
|---|---|---|---|---|---|---|---|
| Ovarian cancer | 24 | 0.6% | 44 | 0.5% | 0.394 | 1.39 (0.61–3.17) | 0.437 |
| Breast cancer | 78 | 1.9% | 138 | 1.7% | 0.385 | 1.18 (0.89–1.57) | 0.248 |
| Endometrial cancer | 6 | 0.1% | 67 | 0.8% | 0.000 | 0.17 (0.07–0.38) | 0.000 |
| Cervical cancer | 7 | 0.2% | 50 | 0.6% | 0.000 | 0.32 (0.14–0.71) | 0.005 |
| Oral cancer | 3 | 0.1% | 5 | 0.1% | 0.804 | 1.29 (0.30–5.55) | 0.728 |
| Thyroid cancer | 12 | 0.3% | 17 | 0.2% | 0.366 | 1.42 (0.65–3.10) | 0.380 |
| Skin cancer | 6 | 0.1% | 14 | 0.2% | 0.749 | 0.79 (0.30–2.10) | 0.637 |
| Colon cancer | 15 | 0.4% | 37 | 0.5% | 0.486 | 0.75 (0.40–1.41) | 0.379 |
| Rectal cancer | 12 | 0.3% | 21 | 0.3% | 0.714 | 0.92 (0.44–1.94) | 0.825 |
| Liver cancer | 9 | 0.2% | 12 | 0.1% | 0.364 | 1.50 (0.61–3.69) | 0.373 |
| Lung cancer | 21 | 0.5% | 36 | 0.4% | 0.576 | 1.29 (0.73–2.28) | 0.381 |
| Stomach cancer | 4 | 0.1% | 8 | 0.1% | 1.000 | 1.08 (0.31–3.82) | 0.901 |
| pancreatic cancer | 0 | 0.0% | 7 | 0.1% | 0.017 | n/a | n/a |
| Kidney cancer | 5 | 0.1% | 7 | 0.1% | 0.547 | 1.51 (0.46–4.93) | 0.492 |
| Bladder cancer | 3 | 0.1% | 12 | 0.1% | 0.252 | 0.31 (0.07–1.44) | 0.136 |
| Overall cancers | 197 | 4.9% | 452 | 5.6% | 0.095 | 0.87 (0.73–1.03) | 0.111 |
| All-cause mortality | 75 | 1.9% | 173 | 2.1% | 0.293 | 0.91 (0.69–1.20) | 0.519 |
| End Point | 1–90 Days | p | 91–180 Days | p | Over 180 Days | p |
|---|---|---|---|---|---|---|
| Ovarian cancer | 1.75 (0.72–4.24) | 0.214 | 1.62 (0.36–7.18) | 0.527 | n/a | - |
| Breast cancer | 1.32 (0.96–1.82) | 0.085 | 1.11 (0.63–1.97) | 0.715 | 0.84 (0.44–1.60) | 0.595 |
| Endometrial cancer | 0.17 (0.06–0.47) | 0.001 | 0.30 (0.07–1.21) | 0.091 | n/a | - |
| Cervical cancer | 0.28 (0.10–0.78) | 0.015 | 0.26 (0.04–1.92) | 0.188 | 0.48 (0.12–2.00) | 0.316 |
| Oral cancer | 0.68 (0.08–5.88) | 0.726 | 2.21 (0.25–19.4) | 0.475 | 2.78 (0.32–24.5) | 0.356 |
| Thyroid cancer | 1.81 (0.79–4.17) | 0.162 | 0.76 (0.10–5.74) | 0.786 | 0.71 (0.09–5.44) | 0.745 |
| Skin cancer | 0.77 (0.25–2.38) | 0.655 | 0.84 (0.11–6.47) | 0.869 | 0.77 (0.10–5.96) | 0.801 |
| Colon cancer | 0.97 (0.50–1.88) | 0.918 | 0.30 (0.04–2.20) | 0.237 | 0.32 (0.04–2.32) | 0.257 |
| Rectal cancer | 0.75 (0.30–1.87) | 0.532 | 1.23 (0.36–4.21) | 0.737 | 1.00 (0.23–4.37) | 0.999 |
| Liver cancer | 1.36 (0.46–4.02) | 0.576 | 3.37 (1.04–10.9) | 0.043 | n/a | - |
| Lung cancer | 1.42 (0.75–2.66) | 0.280 | 0.72 (0.17–3.00) | 0.649 | 1.23 (0.38–4.06) | 0.729 |
| Stomach cancer | 1.23 (0.31–4.89) | 0.773 | n/a | - | 1.45 (0.17–12.1) | 0.732 |
| Kidney cancer | 1.67 (0.48–5.84) | 0.422 | 1.46 (0.17–12.3) | 0.726 | n/a | - |
| Bladder cancer | 0.49 (0.11–2.23) | 0.357 | n/a | - | n/a | - |
| Overall cancers | 0.92 (0.75–1.11) | 0.377 | 0.92 (0.66–1.29) | 0.622 | 0.60 (0.40–0.90) | 0.015 |
| All-cause mortality | 1.02 (0.75–1.38) | 0.904 | 0.74 (0.42–1.31) | 0.302 | 0.40 (0.16–0.99) | 0.047 |
| Overall Cancers | p | All-Cause Mortality | p | |
|---|---|---|---|---|
| HRT (ref.) | ||||
| without BPF | 0.65 (0.29–1.46) | 0.294 | 0.68 (0.17–2.77) | 0.594 |
| BPF | 0.57 (0.36–0.92) | 0.021 | 0.33 (0.11–1.05) | 0.060 |
| without APR | 0.56 (0.33–0.96) | 0.034 | 0.41 (0.13–1.31) | 0.133 |
| with APR | 0.63 (0.34–1.19) | 0.153 | 0.42 (0.10–1.72) | 0.229 |
| without GZF | 0.66 (0.41–1.06) | 0.082 | 0.38 (0.12–1.18) | 0.095 |
| GZF | 0.45 (0.20–1.01) | 0.052 | 0.50 (0.12–2.04) | 0.337 |
| without ZC | 0.58 (0.35–0.95) | 0.031 | 0.24 (0.06–0.97) | 0.046 |
| ZC | 0.62 (0.31–1.24) | 0.176 | 0.83 (0.26–2.61) | 0.744 |
| without MGF | 0.60 (0.37–0.97) | 0.037 | 0.57 (0.23–1.41) | 0.227 |
| MGF | 0.57 (0.27–1.21) | 0.146 | n/a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, T.-B.; Hsieh, C.-C.; Wang, C.-H.; Chang, C.-H.; Hsueh, Y.-L.; Tseng, Y.-T.; Hsieh, M.-F. Comparing Cancer Risks and Mortality between Phytopharmaceuticals and Estrogen-Progestogen Medications for Menopausal Women: A Population-Based Cohort Study. Healthcare 2024, 12, 1220. https://doi.org/10.3390/healthcare12121220
Lin T-B, Hsieh C-C, Wang C-H, Chang C-H, Hsueh Y-L, Tseng Y-T, Hsieh M-F. Comparing Cancer Risks and Mortality between Phytopharmaceuticals and Estrogen-Progestogen Medications for Menopausal Women: A Population-Based Cohort Study. Healthcare. 2024; 12(12):1220. https://doi.org/10.3390/healthcare12121220
Chicago/Turabian StyleLin, Tsai-Bei, Chia-Chi Hsieh, Chun-Hsiang Wang, Chiung-Hung Chang, Yu-Ling Hsueh, Yuan-Tsung Tseng, and Men-Fong Hsieh. 2024. "Comparing Cancer Risks and Mortality between Phytopharmaceuticals and Estrogen-Progestogen Medications for Menopausal Women: A Population-Based Cohort Study" Healthcare 12, no. 12: 1220. https://doi.org/10.3390/healthcare12121220
APA StyleLin, T.-B., Hsieh, C.-C., Wang, C.-H., Chang, C.-H., Hsueh, Y.-L., Tseng, Y.-T., & Hsieh, M.-F. (2024). Comparing Cancer Risks and Mortality between Phytopharmaceuticals and Estrogen-Progestogen Medications for Menopausal Women: A Population-Based Cohort Study. Healthcare, 12(12), 1220. https://doi.org/10.3390/healthcare12121220





