Renal and Genitourinary Ultrasound Evaluation in Emergency and Critical Care: An Overview
Abstract
:1. Introduction
2. Materials and Methods
3. Results
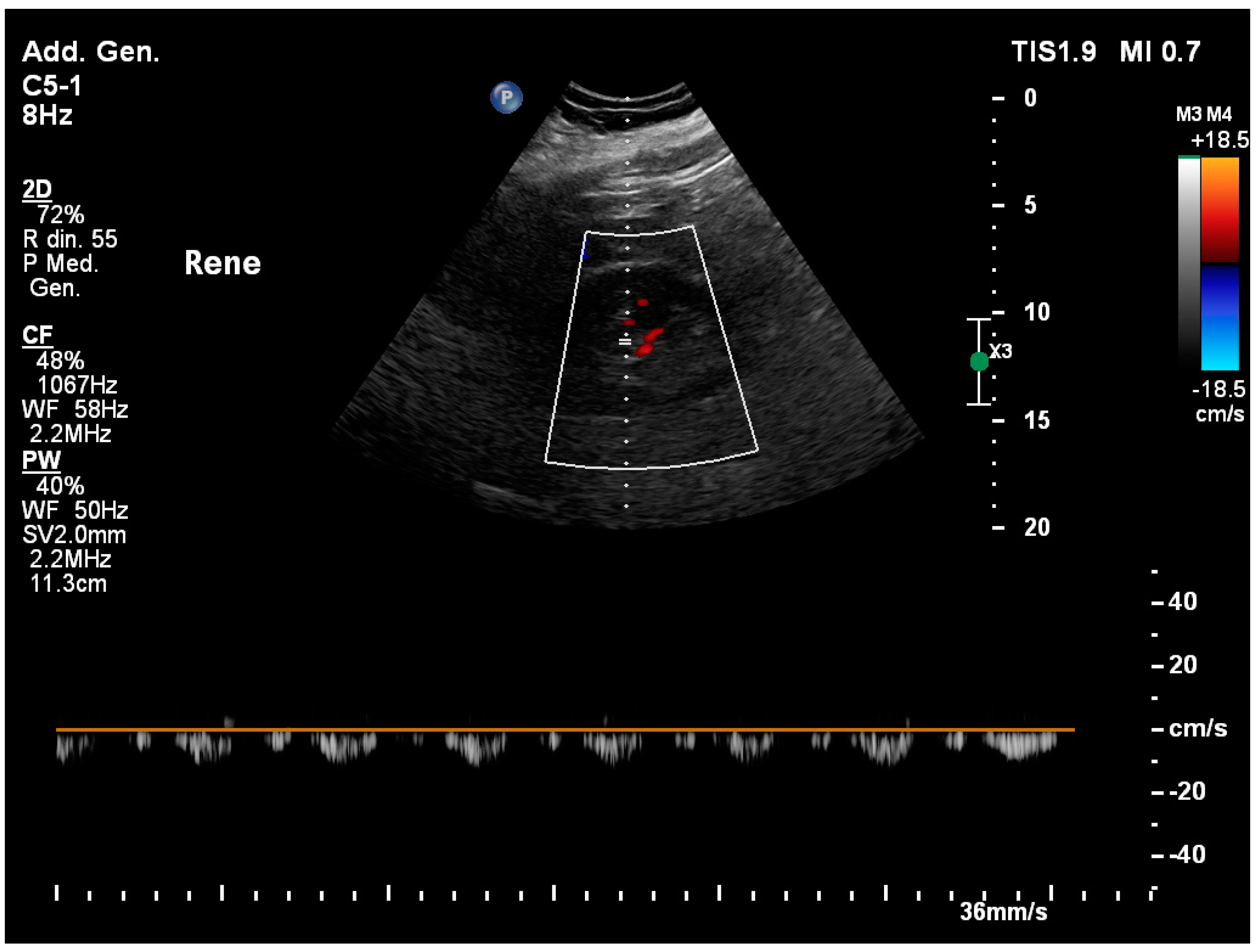
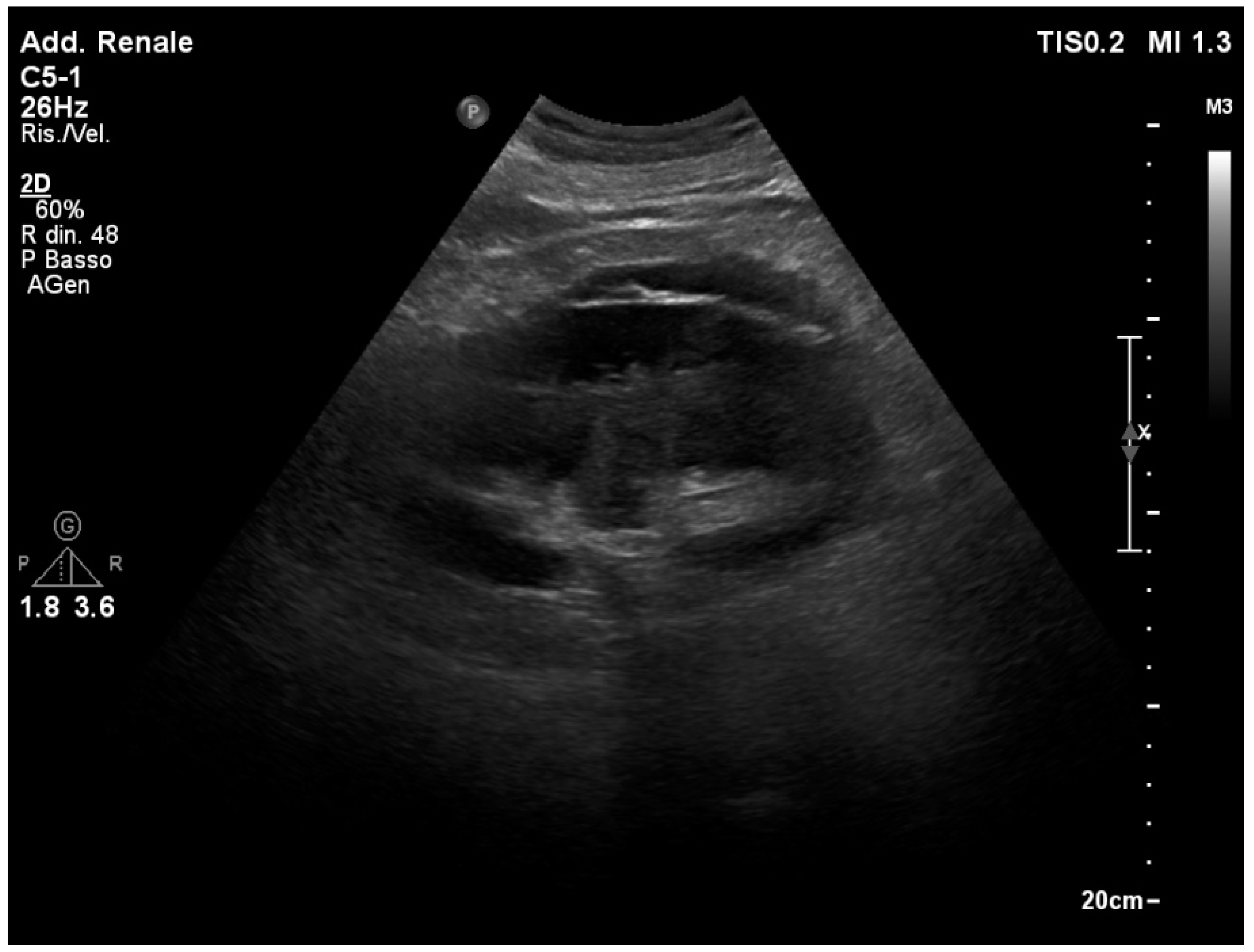
3.1. Normal Sonographic Anatomy
3.2. Acute Kidney Injury
3.3. Renal Colic
3.4. Ureteral Stone
3.5. The “Twinkling” Artifact
3.6. The “Swinging Kidney” Sign
3.7. Identification of the Ureteral Jet
3.8. Ureteropelvic Junction Obstruction
3.9. Acute Renal Infection
3.10. Masses and Cysts
3.11. Acute Renal Venous Thrombosis
3.12. Renal Artery Stenosis
3.13. Renal Resistivity Index
3.14. Hemodinamyc Management Guided by Renal Ultrasound: The VExUS Protocol
3.15. Renal Trauma
3.16. Differential Diagnosis and Ruling out of Life-Threatening Diagnosis
3.17. Bladder Ultrasound in Emergency
3.18. Ultrasound-Guided Procedures for Genitourinary and Kidney System
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Robba, C.; Wong, A.; Poole, D.; Al Tayar, A.; Arntfield, R.T.; Chew, M.S.; Corradi, F.; Douflé, G.; Goffi, A.; Lamperti, M.; et al. Basic ultrasound head-to-toe skills for intensivists in the general and neuro intensive care unit population: Consensus and expert recommendations of the European Society of Intensive Care Medicine. Intensive Care Med. 2021, 47, 1347–1367. [Google Scholar] [CrossRef] [PubMed]
- Barr, L.; Hatch, N.; Roque, P.J.; Wu, T.S. Basic ultrasound-guided procedures. Crit. Care Clin. 2014, 30, 275–304. [Google Scholar] [CrossRef] [PubMed]
- Quaia, E.; Correas, J.M.; Mehta, M.; Murchison, J.T.; Gennari, A.G.; van Beek, E.J.R. Gray Scale Ultrasound, Color Doppler Ultrasound, and Contrast-Enhanced Ultrasound in Renal Parenchymal Diseases. Ultrasound Q. 2018, 34, 250–267. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H. History of ultrasound in nephrourology. Ultrasound Med. Biol. 2001, 27, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Green, B.N.; Johnson, C.D.; Adams, A. Writing narrative literature reviews for peer-reviewed journals: Secrets of the trade. J. Chiropr. Med. 2006, 5, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Baethge, C.; Goldbeck-Wood, S.; Mertens, S. SANRA—A scale for the quality assessment of narrative review articles. Res. Integr. Peer Rev. 2019, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Drelich-Zbroja, A.; Kuczyńska, M.; Światłowski, Ł.; Szymańska, A.; Elwertowski, M.; Marianowska, A. Recommendations for ultrasonographic assessment of renal arteries. J. Ultrason. 2018, 18, 338–343. [Google Scholar] [CrossRef]
- American Institute of Ultrasound in Medicine. AIUM practice guideline for the performance of native renal artery duplex sonography. J. Ultrasound Med. 2013, 32, 1331–1340. [Google Scholar] [CrossRef]
- Rha, S.E.; Byun, J.Y.; Jung, S.E.; Oh, S.N.; Choi, Y.J.; Lee, A.; Lee, J.M. The renal sinus: Pathologic spectrum and multimodality imaging approach. Radiographics 2004, 24 (Suppl. S1), S117–S131. [Google Scholar] [CrossRef]
- Gulati, M.; Cheng, J.; Loo, J.T.; Skalski, M.; Malhi, H.; Duddalwar, V. Pictorial review: Renal ultrasound. Clin. Imaging 2018, 51, 133–154. [Google Scholar] [CrossRef]
- Ronco, C.; Bellomo, R.; Kellum, J.A. Acute kidney injury. Lancet 2019, 394, 1949–1964. [Google Scholar] [CrossRef]
- Nepal, S.; Dachsel, M.; Smallwood, N. Point-of-care ultrasound rapidly and reliably diagnoses renal tract obstruction in patients admitted with acute kidney injury. Clin. Med. 2020, 20, 541–544. [Google Scholar] [CrossRef] [PubMed]
- Beland, M.D.; Walle, N.L.; Machan, J.T.; Cronan, J.J. Renal cortical thickness measured at ultrasound: Is it better than renal length as an indicator of renal function in chronic kidney disease? AJR Am. J. Roentgenol. 2010, 195, W146–W149. [Google Scholar] [CrossRef]
- Korkmaz, M.; Aras, B.; Güneyli, S.; Yılmaz, M. Clinical significance of renal cortical thickness in patients with chronic kidney disease. Ultrasonography 2018, 37, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Pruijm, M.; Milani, B.; Pivin, E.; Podhajska, A.; Vogt, B.; Stuber, M.; Burnier, M. Reduced cortical oxygenation predicts a progressive decline of renal function in patients with chronic kidney disease. Kidney Int. 2018, 93, 932–940. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, X. Clinical utility of ultrasonographic evaluation in acute kidney injury. Transl. Androl. Urol. 2020, 9, 1345–1355. [Google Scholar] [CrossRef]
- Wei, Q.; Zhu, Y.; Zhen, W.; Zhang, X.; Shi, Z.; Zhang, L.; Zhou, J. Performance of resistive index and semi-quantitative power doppler ultrasound score in predicting acute kidney injury: A meta-analysis of prospective studies. PLoS ONE 2022, 17, e0270623. [Google Scholar] [CrossRef] [PubMed]
- Teichman, J.M. Clinical practice. Acute renal colic from ureteral calculus. N. Engl. J. Med. 2004, 350, 684–693. [Google Scholar] [CrossRef] [PubMed]
- Dahm, P.; Koziarz, A.; Gerardo, C.J.; Nishijima, D.K.; Jung, J.H.; Benipal, S.; Raja, A.S. A systematic review and meta-analysis of clinical signs, symptoms, and imaging findings in patients with suspected renal colic. J. Am. Coll. Emerg. Physicians Open 2022, 3, e12831. [Google Scholar] [CrossRef]
- Wong, C.; Teitge, B.; Ross, M.; Young, P.; Robertson, H.L.; Lang, E. The Accuracy and Prognostic Value of Point-of-care Ultrasound for Nephrolithiasis in the Emergency Department: A Systematic Review and Meta-analysis. Acad. Emerg. Med. 2018, 25, 684–698. [Google Scholar] [CrossRef]
- Moore, C.L.; Carpenter, C.R.; Heilbrun, M.L.; Klauer, K.; Krambeck, A.C.; Moreno, C.; Remer, E.M.; Scales, C.; Shaw, M.M.; Sternberg, K.M. Imaging in Suspected Renal Colic: Systematic Review of the Literature and Multispecialty Consensus. J. Urol. 2019, 202, 475–483. [Google Scholar] [CrossRef]
- Keskin, E.T.; Bozkurt, M.; Özdemir, H.; Uğur, R.; Savun, M.; Özdemir, M.; Topal, C.; Canat, H.L. The severity of renal colic pain: Can it be predicted? Can. Urol. Assoc. J. 2023, 17, e257–e262. [Google Scholar] [CrossRef]
- Innes, G.D.; Scheuermeyer, F.X.; McRae, A.D.; Teichman, J.M.H.; Lane, D.J. Hydronephrosis severity clarifies prognosis and guides management for emergency department patients with acute ureteral colic. Cjem 2021, 23, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Jendeberg, J.; Geijer, H.; Alshamari, M.; Cierzniak, B.; Lidén, M. Size matters: The width and location of a ureteral stone accurately predict the chance of spontaneous passage. Eur. Radiol. 2017, 27, 4775–4785. [Google Scholar] [CrossRef] [PubMed]
- Sfoungaristos, S.; Kavouras, A.; Perimenis, P. Predictors for spontaneous stone passage in patients with renal colic secondary to ureteral calculi. Int. Urol. Nephrol. 2012, 44, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Abou Heidar, N.; Labban, M.; Najdi, J.; Al Shami, A.; Nasrallah, O.; Nasr, R. Spontaneous ureteral stone passage: A novel and comprehensive nomogram. Minerva Urol. Nephrol. 2022, 74, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Abushamma, F.; Ktaifan, M.; Abdallah, A.; Alkarajeh, M.; Maree, M.; Awadghanem, A.; Jaradat, A.; Aghbar, A.; Zyoud, S.H.; Keeley, F.X., Jr. Clinical and Radiological Predictors of Early Intervention in Acute Ureteral Colic. Int. J. Gen. Med. 2021, 14, 4051–4059. [Google Scholar] [CrossRef]
- Ahmed, F.; Askarpour, M.R.; Eslahi, A.; Nikbakht, H.A.; Jafari, S.H.; Hassanpour, A.; Makarem, A.; Salama, H.; Ayoub, A. The role of ultrasonography in detecting urinary tract calculi compared to CT scan. Res. Rep. Urol. 2018, 10, 199–203. [Google Scholar] [CrossRef]
- Shabana, W.; Bude, R.O.; Rubin, J.M. Comparison between color Doppler twinkling artifact and acoustic shadowing for renal calculus detection: An in vitro study. Ultrasound Med. Biol. 2009, 35, 339–350. [Google Scholar] [CrossRef]
- Laher, A.E.; McDowall, J.; Gerber, L.; Aigbodion, S.J.; Enyuma, C.O.A.; Buchanan, S.; Adam, A. The ultrasound ‘twinkling artefact’ in the diagnosis of urolithiasis: Hocus or valuable point-of-care-ultrasound? A systematic review and meta-analysis. Eur. J. Emerg. Med. 2020, 27, 13–20. [Google Scholar] [CrossRef]
- Nabheerong, P.; Kengkla, K.; Saokaew, S.; Naravejsakul, K. Diagnostic accuracy of Doppler twinkling artifact for identifying urolithiasis: A systematic review and meta-analysis. J. Ultrasound 2023, 26, 321–331. [Google Scholar] [CrossRef]
- Castelletto, S.; Amore, G.; Giudice, C.A.; Orso, D.; Copetti, R. A Preliminary Investigation on the “Swinging Kidney”: A Sonographic Sign Useful for Diagnosing Renal Colic. J. Diagn. Med. Sonogr. 2022, 38, 331–337. [Google Scholar] [CrossRef]
- Öğreden, E.; Oǧuz, U.; Karadayı, M.; Demirelli, E.; Tosun, A.; Günaydın, M. Factors associated with urinoma accompanied by ureteral calculi. Arch. Ital. Urol. Androl. 2019, 91, 11–15. [Google Scholar] [CrossRef]
- Bambrick, C.; Khader, D.; Mead, T. Ureteral Obstruction and Ureteral Jet Identification—A Case Report. J. Educ. Teach. Emerg. Med. 2021, 6, v12–v14. [Google Scholar] [CrossRef] [PubMed]
- Ucar, A.K.; Kurugoglu, S. Urinary Ultrasound and Other Imaging for Ureteropelvic Junction Type Hydronephrosis (UPJHN). Front. Pediatr. 2020, 8, 546. [Google Scholar] [CrossRef] [PubMed]
- Ellerkamp, V.; Kurth, R.R.; Schmid, E.; Zundel, S.; Warmann, S.W.; Fuchs, J. Differences between intrinsic and extrinsic ureteropelvic junction obstruction related to crossing vessels: Histology and functional analyses. World J. Urol. 2016, 34, 577–583. [Google Scholar] [CrossRef]
- Wendler, J.J.; Baumunk, D.; Liehr, U.B.; Schostak, M. Kidney dislocation in a monstrous inguinal intestinal hernia with ureteropelvic junction obstruction and acute on chronic renal failure. Urol. Int. 2013, 91, 370–372. [Google Scholar] [CrossRef] [PubMed]
- Wenwei, C.; Xiangzhi, Y.; Huazhi, X.; Yirong, Y.; Chengdi, L. Left nutcracker syndrome and right ureteropelvic junction obstruction. Asian J. Surg. 2014, 37, 167–169. [Google Scholar] [CrossRef]
- Smith, A.D.; Nikolaidis, P.; Khatri, G.; Chong, S.T.; De Leon, A.D.; Ganeshan, D.; Gore, J.L.; Gupta, R.T.; Kwun, R.; Lyshchik, A.; et al. ACR Appropriateness Criteria® Acute Pyelonephritis: 2022 Update. J. Am. Coll. Radiol. 2022, 19, S224–S239. [Google Scholar] [CrossRef]
- Hosokawa, T.; Tanami, Y.; Sato, Y.; Oguma, E. Comparison of imaging findings between acute focal bacterial nephritis (acute lobar nephronia) and acute pyelonephritis: A preliminary evaluation of the sufficiency of ultrasound for the diagnosis of acute focal bacterial nephritis. Emerg. Radiol. 2020, 27, 405–412. [Google Scholar] [CrossRef]
- Enikeev, D.V.; Glybochko, P.; Alyaev, Y.; Enikeev, M.; Rapoport, L. Imaging technologies in the diagnosis and treatment of acute pyelonephritis. Urologia 2017, 84, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Nicolau, C.; Antunes, N.; Paño, B.; Sebastia, C. Imaging Characterization of Renal Masses. Medicina 2021, 57, 51. [Google Scholar] [CrossRef] [PubMed]
- Asghar, M.; Ahmed, K.; Shah, S.S.; Siddique, M.K.; Dasgupta, P.; Khan, M.S. Renal vein thrombosis. Eur. J. Vasc. Endovasc. Surg. 2007, 34, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Zeller, T.; Macharzina, R.; Rastan, A.; Beschorner, U.; Noory, E. Renal artery stenosis: Up-date on diagnosis and treatment. Vasa 2014, 43, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Hinton, T.C.; Adams, Z.H.; Baker, R.P.; Hope, K.A.; Paton, J.F.R.; Hart, E.C.; Nightingale, A.K. Investigation and Treatment of High Blood Pressure in Young People: Too Much Medicine or Appropriate Risk Reduction? Hypertension 2020, 75, 16–22. [Google Scholar] [CrossRef]
- Chen, P.; Maklad, N.; Redwine, M. Color and power Doppler imaging of the kidneys. World J. Urol. 1998, 16, 41–45. [Google Scholar] [CrossRef]
- Park, B.K. Gray-Scale, Color Doppler, Spectral Doppler, and Contrast-Enhanced Renal Artery Ultrasound: Imaging Techniques and Features. J. Clin. Med. 2022, 11, 3961. [Google Scholar] [CrossRef]
- Di Nicolò, P.; Granata, A. Renal Resistive Index: Not only kidney. Clin. Exp. Nephrol. 2017, 21, 359–366. [Google Scholar] [CrossRef]
- Ninet, S.; Schnell, D.; Dewitte, A.; Zeni, F.; Meziani, F.; Darmon, M. Doppler-based renal resistive index for prediction of renal dysfunction reversibility: A systematic review and meta-analysis. J. Crit. Care 2015, 30, 629–635. [Google Scholar] [CrossRef]
- Rozemeijer, S.; Haitsma Mulier, J.L.G.; Röttgering, J.G.; Elbers, P.W.G.; Spoelstra-de Man, A.M.E.; Tuinman, P.R.; de Waard, M.C.; Oudemans-van Straaten, H.M. Renal Resistive Index: Response to Shock and its Determinants in Critically Ill Patients. Shock 2019, 52, 43–51. [Google Scholar] [CrossRef]
- Deruddre, S.; Cheisson, G.; Mazoit, J.X.; Vicaut, E.; Benhamou, D.; Duranteau, J. Renal arterial resistance in septic shock: Effects of increasing mean arterial pressure with norepinephrine on the renal resistive index assessed with Doppler ultrasonography. Intensive Care Med. 2007, 33, 1557–1562. [Google Scholar] [CrossRef]
- Rola, P.; Miralles-Aguiar, F.; Argaiz, E.; Beaubien-Souligny, W.; Haycock, K.; Karimov, T.; Dinh, V.A.; Spiegel, R. Clinical applications of the venous excess ultrasound (VExUS) score: Conceptual review and case series. Ultrasound J. 2021, 13, 32. [Google Scholar] [CrossRef] [PubMed]
- Beaubien-Souligny, W.; Rola, P.; Haycock, K.; Bouchard, J.; Lamarche, Y.; Spiegel, R.; Denault, A.Y. Quantifying systemic congestion with Point-of-Care ultrasound: Development of the venous excess ultrasound grading system. Ultrasound J. 2020, 12, 16. [Google Scholar] [CrossRef]
- Edwards, A.; Hammer, M.; Artunduaga, M.; Peters, C.; Jacobs, M.; Schlomer, B. Renal ultrasound to evaluate for blunt renal trauma in children: A retrospective comparison to contrast enhanced CT imaging. J. Pediatr. Urol. 2020, 16, e551–e557. [Google Scholar] [CrossRef] [PubMed]
- Galosi, A.B.; Grilli Cicilioni, C.; Sbrollini, G.; Angelini, A.; Maselli, G.; Carbonari, L. Inflammatory abdominal aortic aneurysm presenting as bilateral hydroureteronephrosis: A case report and review of literature. Arch. Ital. Urol. Androl. 2014, 86, 385–386. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, R.C.; Smith, D.; Feig, R.; Mulflur, M.; Costantino, T.G. The sonographic protocol for the emergent evaluation of aortic dissections (SPEED protocol): A multicenter, prospective, observational study. Acad. Emerg. Med. 2024, 31, 112–118. [Google Scholar] [CrossRef]
- Lerch, M.M.; Riehl, J.; Buechsel, R.; Kierdorf, H.; Winkeltau, G.; Matern, S. Bedside ultrasound in decision making for emergency surgery: Its role in medical intensive care patients. Am. J. Emerg. Med. 1992, 10, 35–38. [Google Scholar] [CrossRef]
- Artusi, N.; Bussani, R.; Cominotto, F. Pheochromocytoma-Induced Tako-Tsubo Syndrome: An Uncommon Presentation. J. Emerg. Med. 2022, 63, e1–e6. [Google Scholar] [CrossRef]
- Disick, G.I.; Palese, M.A. Extra-adrenal pheochromocytoma: Diagnosis and management. Curr. Urol. Rep. 2007, 8, 83–88. [Google Scholar] [CrossRef]
- Milošević, D.; Trkulja, V.; Turudić, D.; Batinić, D.; Spajić, B.; Tešović, G. Ultrasound bladder wall thickness measurement in diagnosis of recurrent urinary tract infections and cystitis cystica in prepubertal girls. J. Pediatr. Urol. 2013, 9, 1170–1177. [Google Scholar] [CrossRef]
- Tzou, D.T.; Usawachintachit, M.; Taguchi, K.; Chi, T. Ultrasound Use in Urinary Stones: Adapting Old Technology for a Modern-Day Disease. J. Endourol. 2017, 31, S89–S94. [Google Scholar] [CrossRef] [PubMed]
- Doble, A.; Carter, S.S. Ultrasonographic findings in prostatitis. Urol. Clin. N. Am. 1989, 16, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, P.A.; Choi, T.; Durham, B.A. Ultrasound-guided suprapubic cystostomy catheter placement in the emergency department. J. Emerg. Med. 2004, 26, 319–321. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, A.S.; Agwu, N.P.; Abdulwahab-Ahmed, A.; Abdullahi, K.; Mungadi, I.A. Safety and efficacy of ultrasound-guided percutaneous suprapubic cystostomy in resource-poor setting: A 7-year review. Urol. Ann. 2018, 10, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.H.; Wen, Y.C.; Chen, K.C.; Chen, C. Ultrasound-guided versus fluoroscopy-guided percutaneous nephrolithotomy: A systematic review and meta-analysis. World J. Urol. 2019, 37, 777–788. [Google Scholar] [CrossRef] [PubMed]
- Arabzadeh Bahri, R.; Maleki, S.; Shafiee, A.; Shobeiri, P. Ultrasound versus fluoroscopy as imaging guidance for percutaneous nephrolithotomy: A systematic review and meta-analysis. PLoS ONE 2023, 18, e0276708. [Google Scholar] [CrossRef] [PubMed]
- Romei, L.; Sabatini, A.; Biagioni, C. Nursing ultrasound examination in catheterization. Emerg. Care J. 2007, 3, 30. [Google Scholar] [CrossRef]
- Ricci, D.; Benetton, M. L’utilizzo dell’ecografia nell’assistenza infermieristica: Una revisione bibliografica. Scenario 2018, 31, 27–34. [Google Scholar] [CrossRef]
- Di Matteo, R.; Caccamo, I.; Arcidiacono, S.; Bertin, G.; Chiamosa, E.; Valenti, F.; Mugone, S.; De Piaggia, A.; Daniele, A.; Clara, M.; et al. Valutazione dell’impatto dell’ecografia vescicale sulle infezioni del tratto urinario associate al catetere e sui costi sanitari: Uno studio osservazionale pre-post. Assist. Inferm. E Ric. 2023, 42, 131–136. [Google Scholar] [CrossRef]
- Campos-Juanatey, F.; Osman, N.I.; Greenwell, T.; Martins, F.E.; Riechardt, S.; Waterloos, M.; Barratt, R.; Chan, G.; Esperto, F.; Ploumidis, A.; et al. European Association of Urology Guidelines on Urethral Stricture Disease (Part 2): Diagnosis, Perioperative Management, and Follow-up in Males. Eur. Urol. 2021, 80, 201–212. [Google Scholar] [CrossRef]
- Shen, Y.T.; Chen, L.; Yue, W.W.; Xu, H.X. Artificial intelligence in ultrasound. Eur. J. Radiol. 2021, 139, 109717. [Google Scholar] [CrossRef] [PubMed]
- Smith-Bindman, R.; Aubin, C.; Bailitz, J.; Bengiamin, R.N.; Camargo, C.A., Jr.; Corbo, J.; Dean, A.J.; Goldstein, R.B.; Griffey, R.T.; Jay, G.D.; et al. Ultrasonography versus computed tomography for suspected nephrolithiasis. N. Engl. J. Med. 2014, 371, 1100–1110. [Google Scholar] [CrossRef] [PubMed]
- Kelahan, L.C.; Desser, T.S.; Troxell, M.L.; Kamaya, A. Ultrasound Assessment of Acute Kidney Injury. Ultrasound Q. 2019, 35, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, D.; Hu, Z.; Wang, X.; Chao, Y.; Wu, J.; Yin, W.; Zhang, H.; Zhang, L.; He, W.; et al. Renal hemodynamic evaluation protocol based on the pathophysiological mechanism of acute kidney injury: Critical Care UltraSound Guided-A((KI))BCDE. Ren. Fail. 2023, 45, 2284842. [Google Scholar] [CrossRef] [PubMed]
- Parulekar, P.; Harris, T.; Jarman, R. The Use of Lung Ultrasound in Acute Medicine. Acute Med. 2019, 18, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.B.; Søgaard, S.B.; Bech Andersen, S.; Skjoldbye, B.; Hansen, K.L.; Rafaelsen, S.; Nørgaard, N.; Carlsen, J.F. Highlights of the development in ultrasound during the last 70 years: A historical review. Acta Radiol. 2021, 62, 1499–1514. [Google Scholar] [CrossRef]
- Sippel, S.; Muruganandan, K.; Levine, A.; Shah, S. Review article: Use of ultrasound in the developing world. Int. J. Emerg. Med. 2011, 4, 72. [Google Scholar] [CrossRef]
- D’Andrea, A.; Del Giudice, C.; Fabiani, D.; Caputo, A.; Sabatella, F.; Cante, L.; Palermi, S.; Desiderio, A.; Tagliamonte, E.; Liccardo, B.; et al. The Incremental Role of Multiorgan Point-of-Care Ultrasounds in the Emergency Setting. Int. J. Environ. Res. Public Health 2023, 20, 2088. [Google Scholar] [CrossRef]
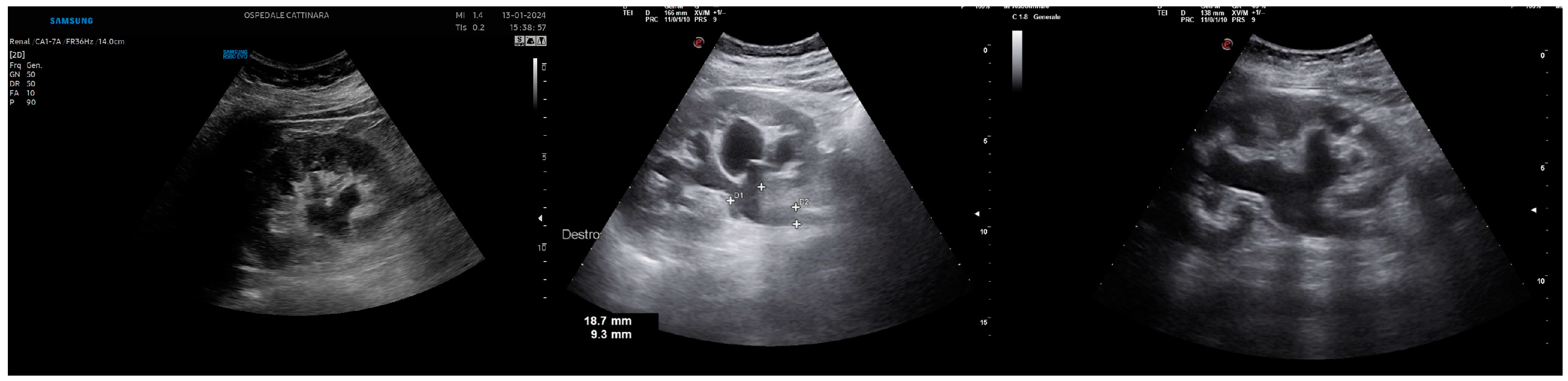

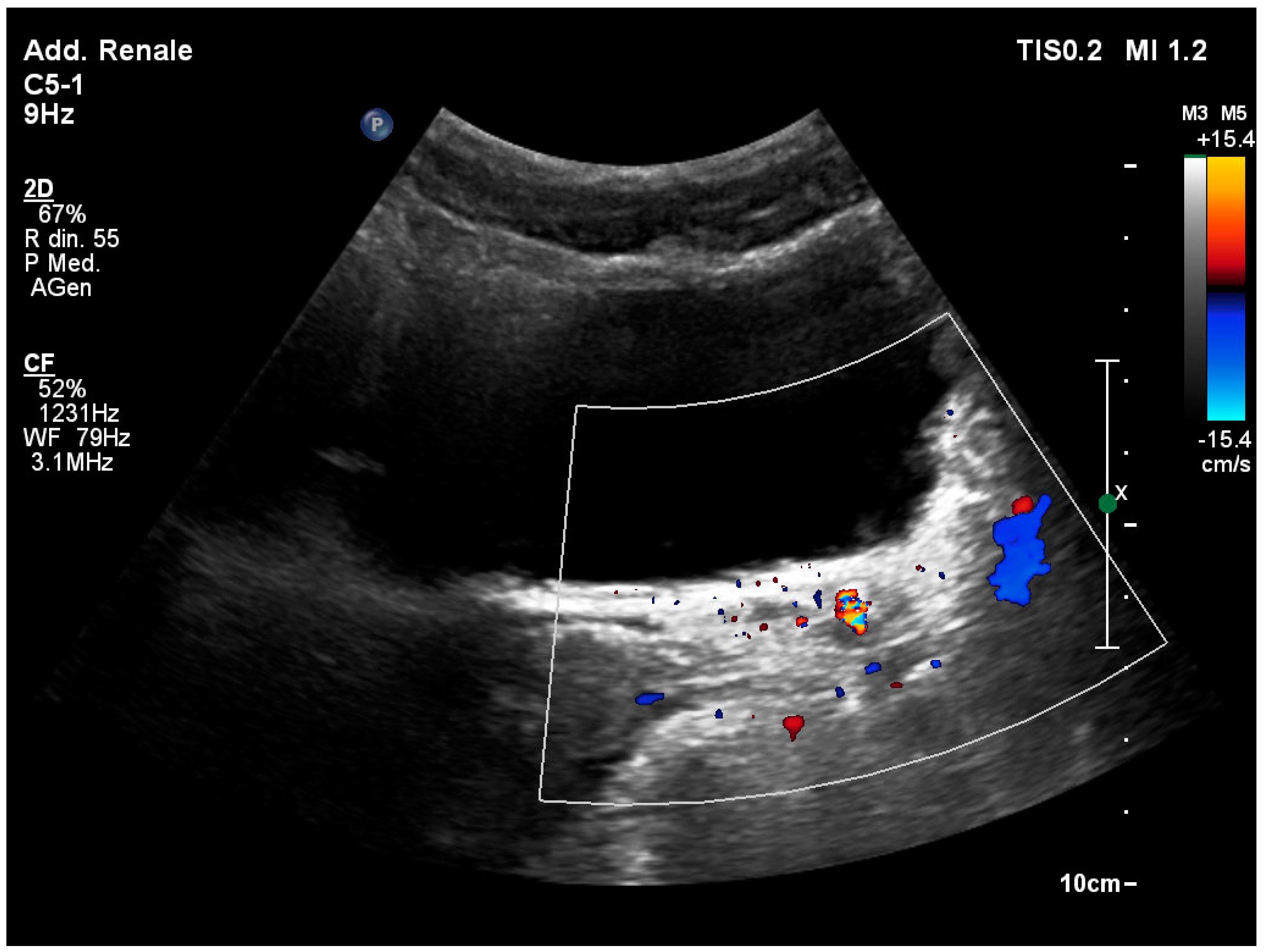

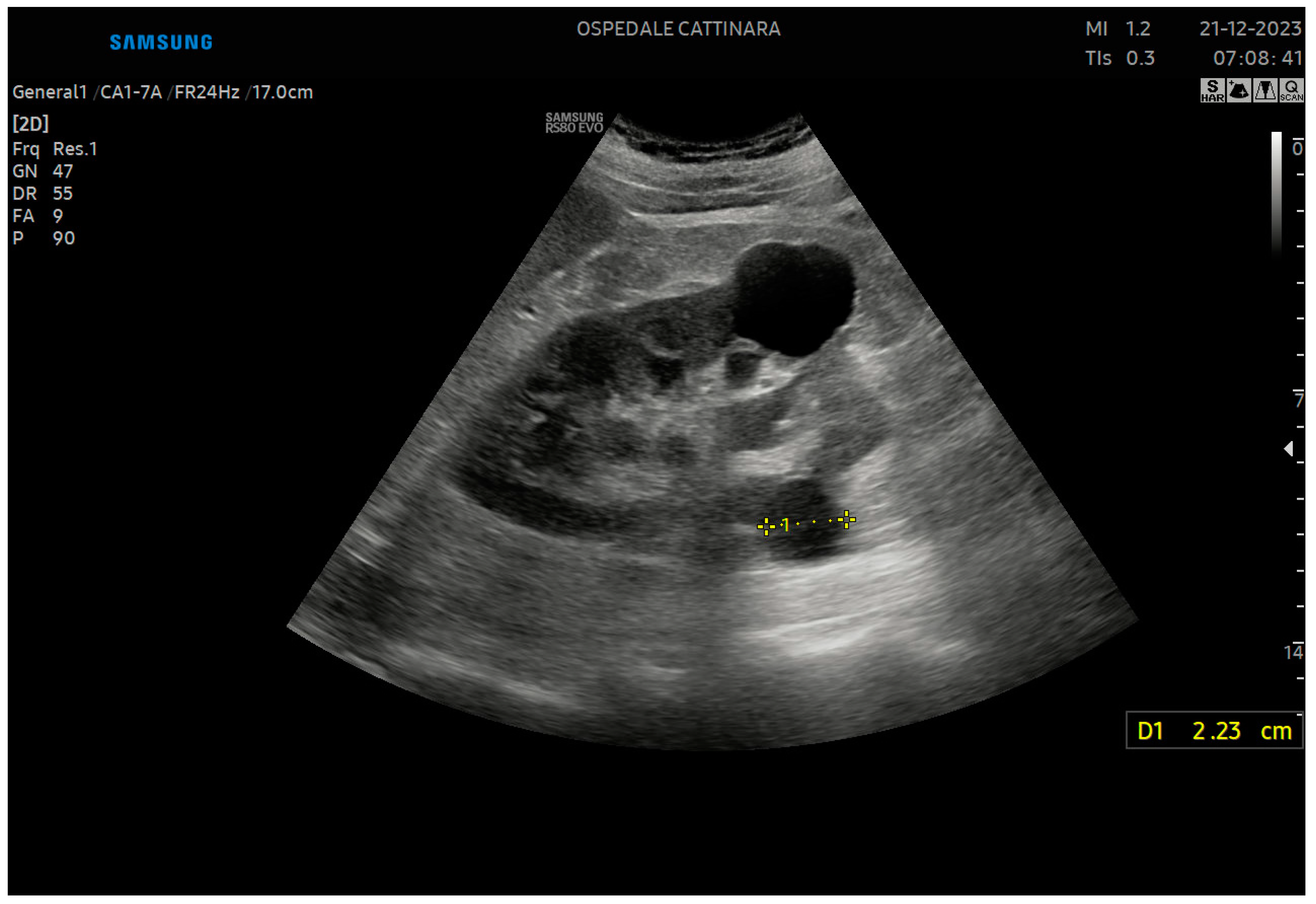
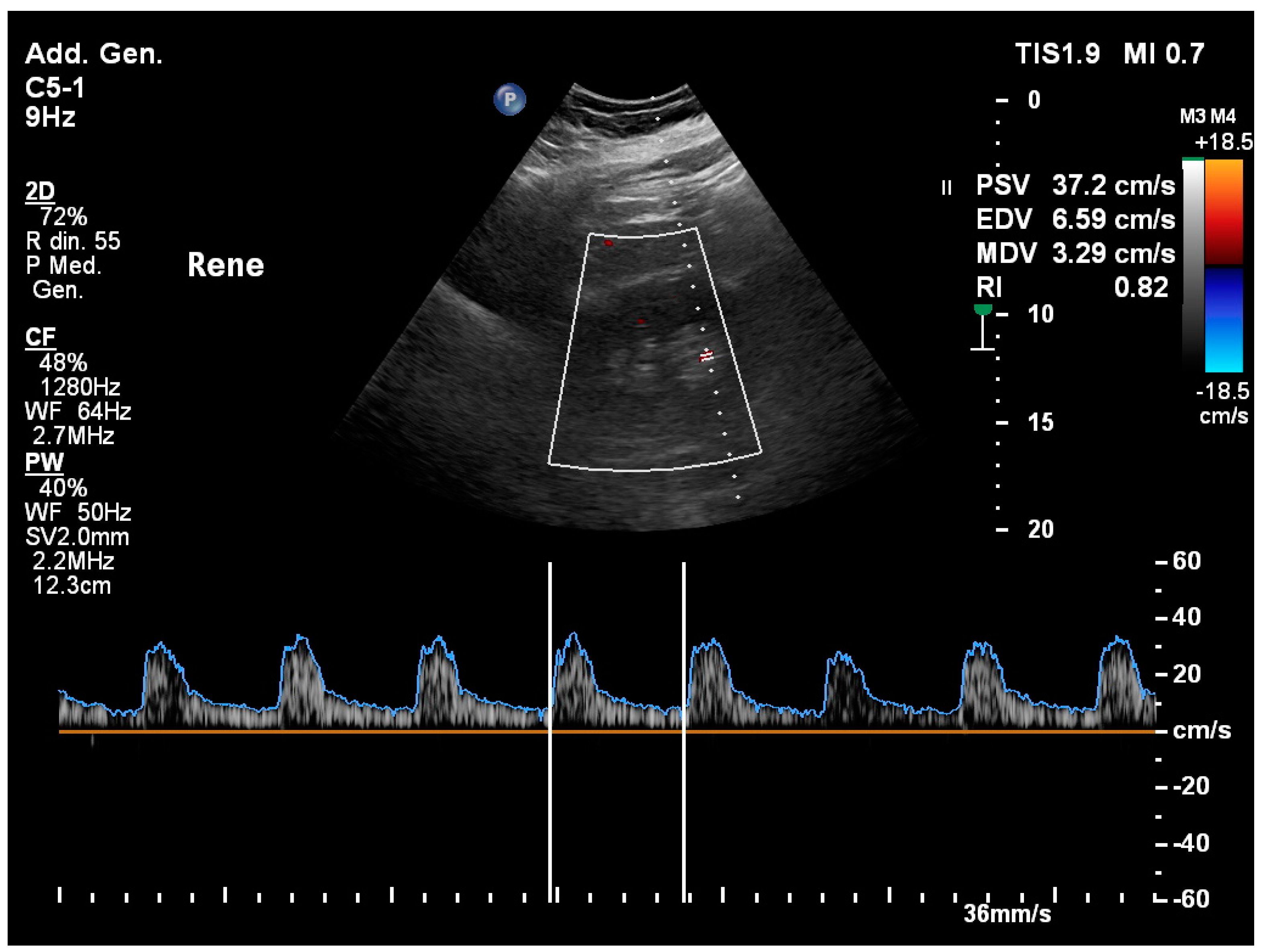

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orso, D.; Peric, D.; Di Gioia, C.C.; Comisso, I.; Bove, T.; Ban, A.; Fonda, F.; Federici, N. Renal and Genitourinary Ultrasound Evaluation in Emergency and Critical Care: An Overview. Healthcare 2024, 12, 1356. https://doi.org/10.3390/healthcare12131356
Orso D, Peric D, Di Gioia CC, Comisso I, Bove T, Ban A, Fonda F, Federici N. Renal and Genitourinary Ultrasound Evaluation in Emergency and Critical Care: An Overview. Healthcare. 2024; 12(13):1356. https://doi.org/10.3390/healthcare12131356
Chicago/Turabian StyleOrso, Daniele, Daniele Peric, Carmine Cristiano Di Gioia, Irene Comisso, Tiziana Bove, Alessio Ban, Federico Fonda, and Nicola Federici. 2024. "Renal and Genitourinary Ultrasound Evaluation in Emergency and Critical Care: An Overview" Healthcare 12, no. 13: 1356. https://doi.org/10.3390/healthcare12131356
APA StyleOrso, D., Peric, D., Di Gioia, C. C., Comisso, I., Bove, T., Ban, A., Fonda, F., & Federici, N. (2024). Renal and Genitourinary Ultrasound Evaluation in Emergency and Critical Care: An Overview. Healthcare, 12(13), 1356. https://doi.org/10.3390/healthcare12131356




