Computer Simulation of Catheter Cryoablation for Pulmonary Vein Isolation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Software
2.2. Simulation Parameters
2.3. Dataset
2.4. Simulation Model
2.5. Estimation of Lesion
2.6. Temperature Parameters
3. Results
3.1. Temperature Distribution
3.2. CBA Simulation
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Whig, P.; Velu, A.; Nadikattu, R.R.; Alkali, Y.J. Computational science role in medical and healthcare-related approach. In Handbook of Computational Sciences: A Multi and Interdisciplinary Approach; Elgnar, A.A., Vigneshwar, M., Singh, K.K., Polkowski, Z., Eds.; Wiley: Hoboken, NJ, USA, 2023; pp. 245–272. [Google Scholar]
- Krishnamoorthy, S.; Dua, A.; Gupta, S. Role of emerging technologies in future IoT-driven Healthcare 4.0 technologies: A survey, current challenges and future directions. J. Ambient. Intell. Humaniz. Comput. 2023, 14, 361–407. [Google Scholar] [CrossRef]
- Baladron, C.; de Diego, J.J.G.; Amat-Santos, I.J. Big data and new information technology: What cardiologists need to know. Rev. Esp. Cardiol. 2021, 74, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Mena, L.J.; Félix, V.G.; Ostos, R.; González, A.J.; Martínez-Peláez, R.; Melgarejo, J.D.; Maestre, G.E. Mobile personal health care system for noninvasive, pervasive, and continuous blood pressure monitoring: Development and usability study. JMIR mHealth uHealth 2020, 8, e18012. [Google Scholar] [CrossRef] [PubMed]
- del Álamo, J.C.; Marsden, A.L.; Lasheras, J.C. Avances en mecánica computacional para el diagnóstico y tratamiento de la enfermedad cardiovascular. Rev. Esp. Cardiol. 2009, 62, 781–805. [Google Scholar] [CrossRef] [PubMed]
- Mena, L.J.; Félix, V.G.; Ochoa, A.; Ostos, R.; González, E.; Aspuru, J.; Velarde, P.; Maestre, G.E. Mobile personal health monitoring for automated classification of electrocardiogram signals in elderly. Comput. Math. Methods Med. 2018, 2018, 9128054. [Google Scholar] [CrossRef] [PubMed]
- Kwong, R.Y.; Yucel, E.K. Computed tomography scan and magnetic resonance imaging. Circulation 2003, 108, e104–e106. [Google Scholar] [CrossRef] [PubMed]
- Cleary, K.; Peters, T.M. Image-guided interventions: Technology review and clinical applications. Annu. Rev. Biomed. Eng. 2010, 12, 119–142. [Google Scholar] [CrossRef] [PubMed]
- Salifu, D.A.; Heymans, Y.; Christmals, C.D. A simulation-based clinical nursing education framework for a low-resource setting: A multimethod study. Healthcare 2022, 10, 1639. [Google Scholar] [CrossRef] [PubMed]
- Chaer, R.A.; DeRubertis, B.G.; Lin, S.C.; Bush, H.L.; Karwowski, J.K.; Birk, D.; Morrissey, N.J.; Faries, P.L.; McKinsey, J.F.; Kent, K.C. Simulation improves resident performance in catheter-based intervention: Results of a randomized, controlled study. Ann. Surg. 2006, 244, 343–352. [Google Scholar] [CrossRef]
- Westerdahl, D.E. The necessity of high-fidelity simulation in cardiology training programs. J. Am. Coll. Cardiol. 2016, 67, 1375–1378. [Google Scholar] [CrossRef]
- Trayanova, N.A.; Chang, K.C. How computer simulations of the human heart can improve anti-arrhythmia therapy. J. Physiol. 2016, 594, 2483–2502. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Shih, C.L. Modeling an emergency medical services system using computer simulation. Int. J. Med. Inform. 2003, 72, 57–72. [Google Scholar] [CrossRef] [PubMed]
- Bruppacher, H.R.; Alam, S.K.; LeBlanc, V.R.; Naik, V.N.; Savoldelli, G.L.; Mazer, C.D.; Kurrek, M.M.; Joo, H.S. Simulation-based training improves physicians’ performance in patient care in high-stakes clinical setting of cardiac surgery. Anesthesiology 2010, 112, 985–992. [Google Scholar] [CrossRef] [PubMed]
- El Faquir, N.; Ren, B.; Van Mieghem, N.M.; Bosmans, J.; De Jaegere, P.P. Patient-specific computer modelling–its role in the planning of transcatheter aortic valve implantation. Neth. Heart J. 2017, 25, 100–105. [Google Scholar] [CrossRef] [PubMed]
- de Jaegere, P.; Rocatello, G.; Prendergast, B.D.; de Backer, O.; Van Mieghem, N.M.; Rajani, R. Patient-specific computer simulation for transcatheter cardiac interventions: What a clinician needs to know. Heart 2019, 105, s21–s27. [Google Scholar] [CrossRef] [PubMed]
- Højager, A.; Schoos, M.M.; Tingsgaard, P.K.; Bock, T.G.; Homøe, P. Prevalence of silent atrial fibrillation and cardiovascular disease in patients with obstructive sleep apnea. Sleep Med. 2022, 100, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Magnani, J.W.; Rienstra, M.; Lin, H.; Sinner, M.F.; Lubitz, S.A.; McManus, D.D.; Dupuis, J.; Ellinor, P.T.; Benjamin, E.J. Atrial fibrillation: Current knowledge and future directions in epidemiology and genomics. Circulation 2011, 124, 1982–1993. [Google Scholar] [CrossRef] [PubMed]
- Naser, N.; Kulic, M.; Dilic, M.; Dzubur, A.; Durak, A.; Pepic, E.; Smajic, E.; Kusljugic, Z. The cumulative incidence of stroke, myocardial infarction, heart failure and sudden cardiac death in patients with atrial fibrillation. Med. Arch. 2017, 71, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Ruiz, L.A.; Rodríguez-Piña, H.; Martínez-Flores, J.E. Fibrilación auricular evaluación y tratamiento. Rev. Med. Inst. Mex. Seguro Soc. 2012, 50, 273–284. [Google Scholar]
- Rojas-Durán, A.M.; Sáenz-Morales, O.A.; Garay-Fernández, M.; Vergara-Vela, E. Evaluación del tratamiento de la fibrilación auricular valvular y no valvular y su relación con eventos adversos en pacientes hospitalizados en el servicio de urgencias de un hospital de tercer nivel. Rev. Colomb. Cardiol. 2020, 27, 529–537. [Google Scholar] [CrossRef]
- Lara-Vaca, S.; Cordero-Cabra, A.; Martinez-Flores, E.; Iturralde-Torres, P. The Mexican Registry of Atrial Fibrillation (ReMeFa). Gac. Med. Mex. 2014, 150, 48–59. [Google Scholar] [PubMed]
- Rodríguez-Reyes, H.; Laguna-Muñoz, C.I.; Gallegos de Luna, C.F.; de los Ríos-Ibarra, M.O.; Salas-Pacheco, J.L.; Leyva-Pons, J.L.; Muñoz-Gutiérrez, L.M.; Vargas-Hernandez, A.; Rodríguez-Muñoz, K.M.; Barragán-Luna, J.; et al. Fibrilación auricular en población mexicana: Diferencias en presentación, comorbilidades y factores de riesgo entre hombres y mujeres. Arch. Cardiol. Mex. 2022, 92, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Prystowsky, E.N.; Padanilam, B.J.; Fogel, R.I. Treatment of atrial fibrillation. J. Am. Med. Assoc. 2015, 314, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Reiffel, J.A. Oral anticoagulation and antiarrhythmic drug therapy for atrial fibrillation. J. Innov. Card. Rhythm. Manag. 2018, 9, 3446. [Google Scholar] [CrossRef] [PubMed]
- Hachem, A.H.; Marine, J.E.; Tahboub, H.A.; Kamdar, S.; Kanjwal, S.; Soni, R.; Kanjwal, K. Radiofrequency ablation versus cryoablation in the treatment of paroxysmal atrial fibrillation: A meta-analysis. Cardiol. Res. Pract. 2018, 2018, 6276241. [Google Scholar] [CrossRef]
- Rottner, L.; Bellmann, B.; Lin, T.; Reissmann, B.; Tönnis, T.; Schleberger, R.; Nies, M.; Jungen, C.; Dinshaw, L.; Klatt, N.; et al. Catheter ablation of atrial fibrillation: State of the art and future perspectives. Cardiol. Ther. 2020, 9, 45–58. [Google Scholar] [CrossRef]
- Khargi, K.; Hutten, B.A.; Lemke, B.; Deneke, T. Surgical treatment of atrial fibrillation; a systematic review. Eur. J. Cardiothorac. 2005, 27, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Tomaiko, E.; Su, W.W. Comparing radiofrequency and cryoballoon technology for the ablation of atrial fibrillation. Curr. Opin. Cardiol. 2019, 34, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.B.; Rossvoll, O.; Tande, P.; Schuster, P.; Solheim, E.; Chen, J. Cryoballoon vs. radiofrequency catheter ablation: Insights from NOrwegian randomized study of PERSistent Atrial Fibrillation (NO-PERSAF study). Europace 2022, 24, 226–233. [Google Scholar] [CrossRef]
- Kotadia, I.D.; Williams, S.E.; O’Neill, M. High-power, short-duration radiofrequency ablation for the treatment of AF. Arrhythm. Electrophysiol. Rev. 2019, 8, 265–272. [Google Scholar] [CrossRef]
- Habibi, M.; Berger, R.D.; Calkins, H. Radiofrequency ablation: Technological trends, challenges, and opportunities. Europace 2021, 23, 511–519. [Google Scholar] [CrossRef]
- Tao, T.; Zheng, J.; Xu, H. An abnormal left ventricular-atrial perforation after radiofrequency catheter ablation: A case report. J. Cardiothorac. Surg. 2019, 14, 185. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Li, L.; Liu, N.; Zhang, M.; Wu, K.; Ruan, Y.; Bai, R.; Du, X.; Dong, J.; Ma, C. Early versus delayed removal of the pericardial drain in patients with cardiac tamponade complicating radiofrequency ablation of atrial fibrillation. J. Cardiovasc. Electrophysiol. 2020, 31, 597–603. [Google Scholar] [CrossRef]
- Schoene, K.; Sepehri-Shamloo, A.; Sommer, P.; Jahnke, C.; Paetsch, I.; Hindricks, G.; Arya, A. Natural course of acquired pulmonary vein stenosis after radiofrequency ablation for atrial fibrillation-Is routine follow-up imaging indicated or not? J. Cardiovasc. Electrophysiol. 2019, 30, 1786–1791. [Google Scholar] [CrossRef]
- Liu, X.H.; Gao, X.F.; Jin, C.L.; Chen, C.F.; Chen, B.; Xu, Y.Z. Cryoballoon versus radiofrequency ablation for persistent atrial fibrillation: A systematic review and meta-analysis. Kardiol. Pol. 2020, 78, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Kuck, K.H.; Brugada, J.; Fürnkranz, A.; Metzner, A.; Ouyang, F.; Chun, J.; Elvan, A. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N. Engl. J. Med. 2016, 374, 2235–2245. [Google Scholar] [CrossRef]
- Andrade, J.G.; Khairy, P.; Dubuc, M. Catheter cryoablation: Biology and clinical uses. Circ. Arrhythm. Electrophysiol. 2013, 6, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Andrade, J.G.; Wazni, O.M.; Kuniss, M.; Hawkins, N.M.; Deyell, M.W.; Chierchia, G.B.; Nissen, S.; Verma, A.; Wells, G.A.; Turgeon, R.D. Cryoballoon ablation as initial treatment for atrial fibrillation: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2021, 78, 914–930. [Google Scholar] [CrossRef] [PubMed]
- Jin, E.S.; Wang, P.J. Cryoballoon ablation for atrial fibrillation: A comprehensive review and practice guide. Korean Circ. J. 2018, 48, 114–123. [Google Scholar] [CrossRef]
- Andrade, J.G. Cryoablation for atrial fibrillation. Heart Rhythm O2 2020, 1, 44–58. [Google Scholar] [CrossRef]
- Tokuda, M.; Yamashita, S.; Sato, H.; Oseto, H.; Ikewaki, H.; Yokoyama, M.; Isogai, R.; Tokutake, K.; Yokoyama, K.; Kato, M.; et al. Long-term course of phrenic nerve injury after cryoballoon ablation of atrial fibrillation. Sci. Rep. 2021, 11, 6226. [Google Scholar] [CrossRef] [PubMed]
- Molenaar, M.M.; Timmermans, C.C.; Hesselink, T.; Scholten, M.F.; ter Bekke, R.M.; Luermans, J.G.; Brusse-Keizer, M.; Kraaier, K.; ten Haken, B.; Grandjean, J.G.; et al. Shorter cryoballoon applications times do effect efficacy but result in less phrenic nerve injury: Results of the randomized 123 study. Pacing Clin. Electrophysiol. 2019, 42, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Anwar, O.; Gunawardene, M.A.; Dickow, J.; Scherschel, K.; Jungen, C.; Münkler, P.; Eickholt, C.; Willems, S.; Gessler, N.; Meyer, C. Contemporary analysis of phrenic nerve injuries following cryoballoon-based pulmonary vein isolation: A single-centre experience with the systematic use of compound motor action potential monitoring. PLoS ONE 2020, 15, e0235132. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, J.; Sepahpour, A.; Chan, K.H.; Singarayar, S.; McGuire, M.A. Immediate balloon deflation for prevention of persistent phrenic nerve palsy during pulmonary vein isolation by balloon cryoablation. Heart Rhythm. 2013, 10, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Rieder, C.; Schwenke, M.; Pätz, T.; Georgii, J.; Ballhausen, H.; Schwen, L.O.; Haase, S.; Preusser, T. Evaluation of a numerical simulation for cryoablation–comparison with bench data, clinical kidney and lung cases. Int. J. Hyperth. 2020, 37, 1268–1278. [Google Scholar] [CrossRef] [PubMed]
- González-Suárez, A.; Pérez, J.J.; Irastorza, R.M.; D’Avila, A.; Berjano, E. Computer modeling of radiofrequency cardiac ablation: 30 years of bioengineering research. Comput. Methods Programs Biomed. 2022, 214, 106546. [Google Scholar] [CrossRef] [PubMed]
- Pérez, J.J.; D’Avila, A.; Aryana, A.; Trujillo, M.; Berjano, E. Can fat deposition after myocardial infarction alter the performance of RF catheter ablation of scar-related ventricular tachycardia?: Results from a computer modeling study. J. Cardiovasc. Electrophysiol. 2016, 27, 947–952. [Google Scholar] [CrossRef]
- González-Suárez, A.; Berjano, E.; Guerra, J.M.; Gerardo-Giorda, L. Computational modeling of open-irrigated electrodes for radiofrequency cardiac ablation including blood motion-saline flow interaction. PLoS ONE 2016, 11, e0150356. [Google Scholar] [CrossRef] [PubMed]
- Petras, A.; Leoni, M.; Guerra, J.M.; Gerardo-Giorda, L. A computational model of open-irrigated radiofrequency catheter ablation accounting for mechanical properties of the cardiac tissue. Int. J. Numer. Method. Biomed. Eng. 2019, 35, e3232. [Google Scholar] [CrossRef]
- Yan, S.; Gu, K.; Wu, X.; Wang, W. Computer simulation study on the effect of electrode–tissue contact force on thermal lesion size in cardiac radiofrequency ablation. Int. J. Hyperth. 2020, 37, 37–48. [Google Scholar] [CrossRef]
- Müssig, R.; Müssig, R.; Hörth, J. Cryoballoon model and simulation of catheter ablation for pulmonary vein isolation in atrial fibrillation. Curr. Dir. Biomed. Eng. 2018, 4, 473–475. [Google Scholar] [CrossRef]
- Getman, M.K.; Wissner, E.; Ranjan, R.; Lalonde, J.P. Relationship between time-to-isolation and freeze duration: Computational modeling of dosing for Arctic Front Advance and Arctic Front Advance Pro cryoballoons. J. Cardiovasc. Electrophysiol. 2019, 30, 2274–2282. [Google Scholar] [CrossRef] [PubMed]
- COMSOL Multiphysics. Introduction to Heat Transfer Module. Available online: https://doc.comsol.com/5.4/doc/com.comsol.help.heat/IntroductionToHeatTransferModule.pdf (accessed on 6 July 2024).
- Patel, T.; Li, C.; Raissi, F.; Kassab, G.S.; Gao, T.; Lee, L.C. Coupled thermal-hemodynamics computational modeling of cryoballoon ablation for pulmonary vein isolation. Comput. Biol. Med. 2023, 157, 106766. [Google Scholar] [CrossRef] [PubMed]
- Lenhardt, R.; Sessler, D.I. Estimation of mean body temperature from mean skin and core temperature. Anesthesiology 2006, 105, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Elwassif, M.M.; Kong, Q.; Vazquez, M.; Bikson, M. Bio-heat transfer model of deep brain stimulation-induced temperature changes. J. Neural Eng. 2006, 3, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Potenza, M.; Pisano, C.; Coppa, P.; Bovesecchi, G.; Corasaniti, S.; Barbero, F. Numerical simulations of temperature inside the heart tissues to evaluate the performances of cryoablative probe. Int. J. Heat. Mass. Transf. 2023, 146, 106877. [Google Scholar] [CrossRef]
- Pennes, H.H. Analysis of tissue and arterial blood temperatures in the resting human forearm. J. Appl. Physiol. 1948, 1, 93–122. [Google Scholar] [CrossRef] [PubMed]
- Shih, T.C.; Yuan, P.; Lin, W.L.; Kou, H.S. Analytical analysis of the Pennes bioheat transfer equation with sinusoidal heat flux condition on skin surface. Med. Eng. Phys. 2007, 29, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Pérez, J.J.; González-Suárez, A.; Berjano, E. Numerical analysis of thermal impact of intramyocardial capillary blood flow during radiofrequency cardiac ablation. Int. J. Hyperth. 2017, 34, 243–249. [Google Scholar] [CrossRef]
- Kozlov, M.; Horner, M.; Kainz, W.; Weiskopf, N.; Möller, H.E. Modeling radio-frequency energy-induced heating due to the presence of transcranial electric stimulation setup at 3T. Magn. Reson. Mater. Phys. Biol. Med. 2020, 33, 793–807. [Google Scholar] [CrossRef]
- Townsley, M.I. Structure and composition of pulmonary arteries, capillaries and veins. Compr. Physiol. 2012, 2, 675–709. [Google Scholar] [PubMed]
- Su, W.; Orme, G.J.; Hoyt, R.; Baker, J.; Compton, S.; Fellows, C.; Harding, J.; Svinarich, J.T.; Kowalski, M.; Piedad, B.; et al. Retrospective review of Arctic Front Advance Cryoballoon Ablation: A multicenter examination of second-generation cryoballoon (RADICOOL trial). J. Interv. Card. Electrophysiol. 2018, 51, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Marom, E.M.; Herndon, J.E.; McAdams, H.P. Pulmonary vein diameter, cross-sectional area, and shape: CT analysis. Radiology 2015, 235, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Garan, A.; Al-Ahmad, A.; Mihalik, T.; Cartier, C.; Capuano, L.; Holtan, D.; Song, C.; Homoud, M.K.; Link, M.S.; Mark Estes III, N.A.; et al. Cryoablation of the pulmonary veins using a novel balloon catheter. J. Interv. Card. Electrophysiol. 2006, 15, 79–81. [Google Scholar] [CrossRef]
- Barnett, A.S.; Bahnson, T.D.; Piccini, J.P. Recent advances in lesion formation for catheter ablation of atrial fibrillation. Circ. Arrhythm. Electrophysiol. 2016, 9, e003299. [Google Scholar] [CrossRef] [PubMed]
- Rothenborg, H.W. Cryoprotective properties of vasoconstriction. Cryobiology 1977, 14, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Avitall, B.; Kalinski, A. Cryotherapy of cardiac arrhythmia: From basic science to the bedside. Heart Rhythm. 2015, 2, 2195–2203. [Google Scholar] [CrossRef] [PubMed]
- Coulombe, N.; Paulin, J.; Su, W. Improved in vivo performance of second-generation cryoballoon for pulmonary vein isolation. J. Cardiovasc. Electrophysiol. 2013, 24, 919–925. [Google Scholar] [CrossRef]
- Nie, Z.; Zhao, D.; Ren, Z.; Hou, C.R.; Su, X.; Deng, C.; Yao, Y.; Tang, K.; Li, Y.; Fu, H.; et al. Liquid nitrogen cryoballoon ablation system for paroxysmal atrial fibrillation: A multicenter, prospective, single-arm clinical trial. J. Am. Coll. Cardiol. Asia 2023, 3, 805–816. [Google Scholar]
- Abugattas, J.P.; de Asmundis, C.; Iacopino, S.; Salghetti, F.; Takarada, K.; Coutiño, H.E.; Ströker, E.; De Regibus, V.; de Greef, Y.; Brugada, P.; et al. Phrenic nerve injury during right inferior pulmonary vein ablation with the second-generation cryoballoon: Clinical, procedural, and anatomical characteristics. Europace 2018, 20, e156–e163. [Google Scholar] [CrossRef]
- Heeger, C.H.; Popescu, S.Ș.; Sohns, C.; Pott, A.; Metzner, A.; Inaba, O.; Straube, F.; Kuniss, M.; Aryana, A.; Miyazaki, S.; et al. Impact of cryoballoon application abortion due to phrenic nerve injury on reconnection rates: A YETI subgroup analysis. Europace 2023, 25, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Sorguven, E.; Bozkurt, S.; Baldock, C. Computer simulations can replace in-vivo experiments for implantable medical devices. Phys. Eng. Sci. Med. 2021, 44, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Burks, A.C.; Keim-Malpass, J. Health literacy and informed consent for clinical trials: A systematic review and implications for nurses. Nursing Res. Rev. 2019, 9, 31–40. [Google Scholar] [CrossRef]
- Pietrzykowski, T.; Smilowska, K. The reality of informed consent: Empirical studies on patient comprehension—Systematic review. Trials 2021, 22, 57. [Google Scholar] [CrossRef] [PubMed]
- Indrayan, A.; Mishra, A. The importance of small samples in medical research. J. Postgrad. Med. 2021, 67, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, S.; Hasegawa, K.; Mukai, M.; Aoyama, D.; Nodera, M.; Shiomi, Y.; Tama, N.; Ikeda, H.; Ishida, K.; Uzui, H.; et al. The advantages and disadvantages of the novel fourth-generation cryoballoon as compared to the second-generation cryoballoon in the current short freeze strategy. J. Interv. Card. Electrophysiol. 2022, 63, 143–152. [Google Scholar] [CrossRef]
- Vennin, S. How modelling is transforming medicine. Phys. World 2020, 33, 41. [Google Scholar] [CrossRef]
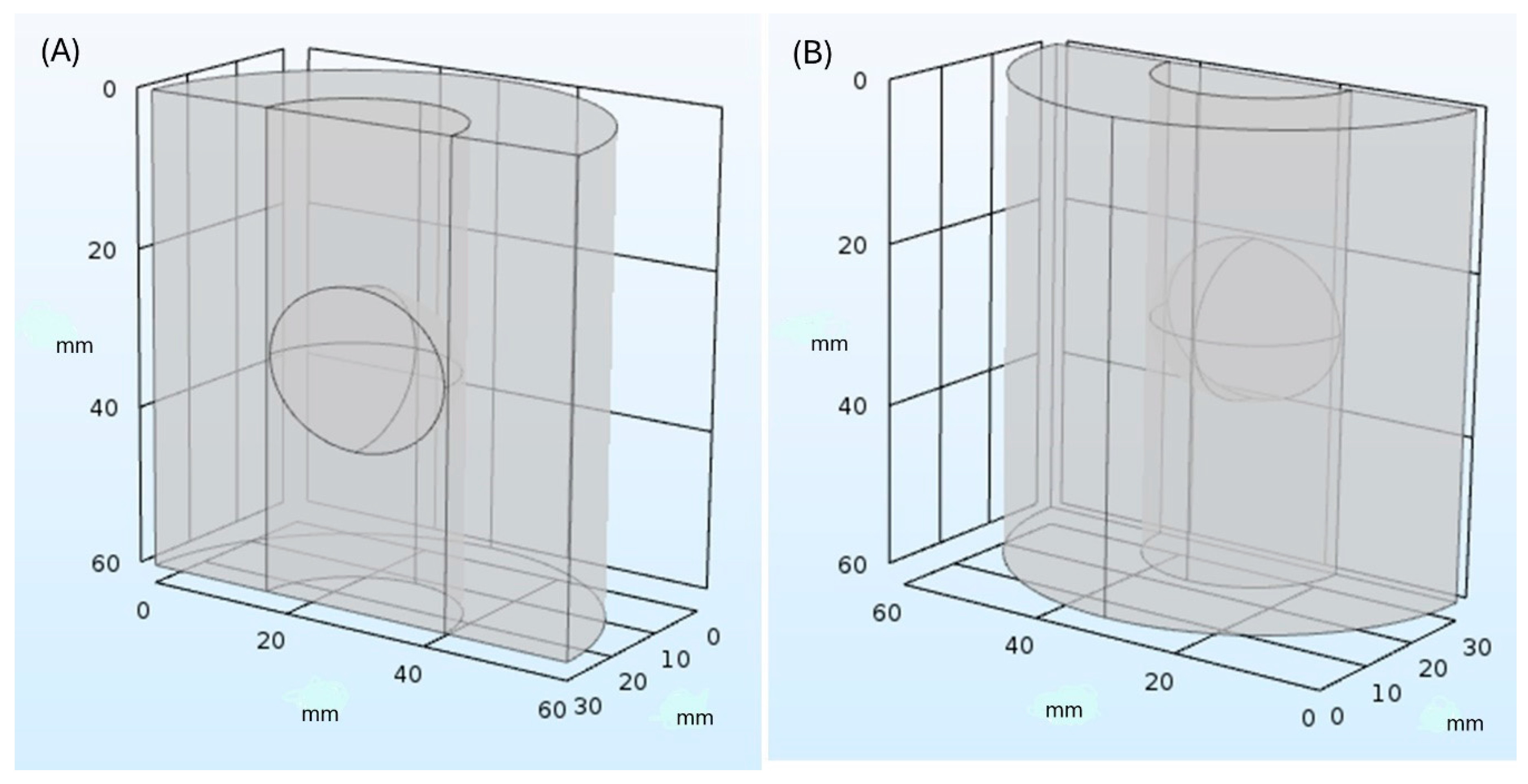
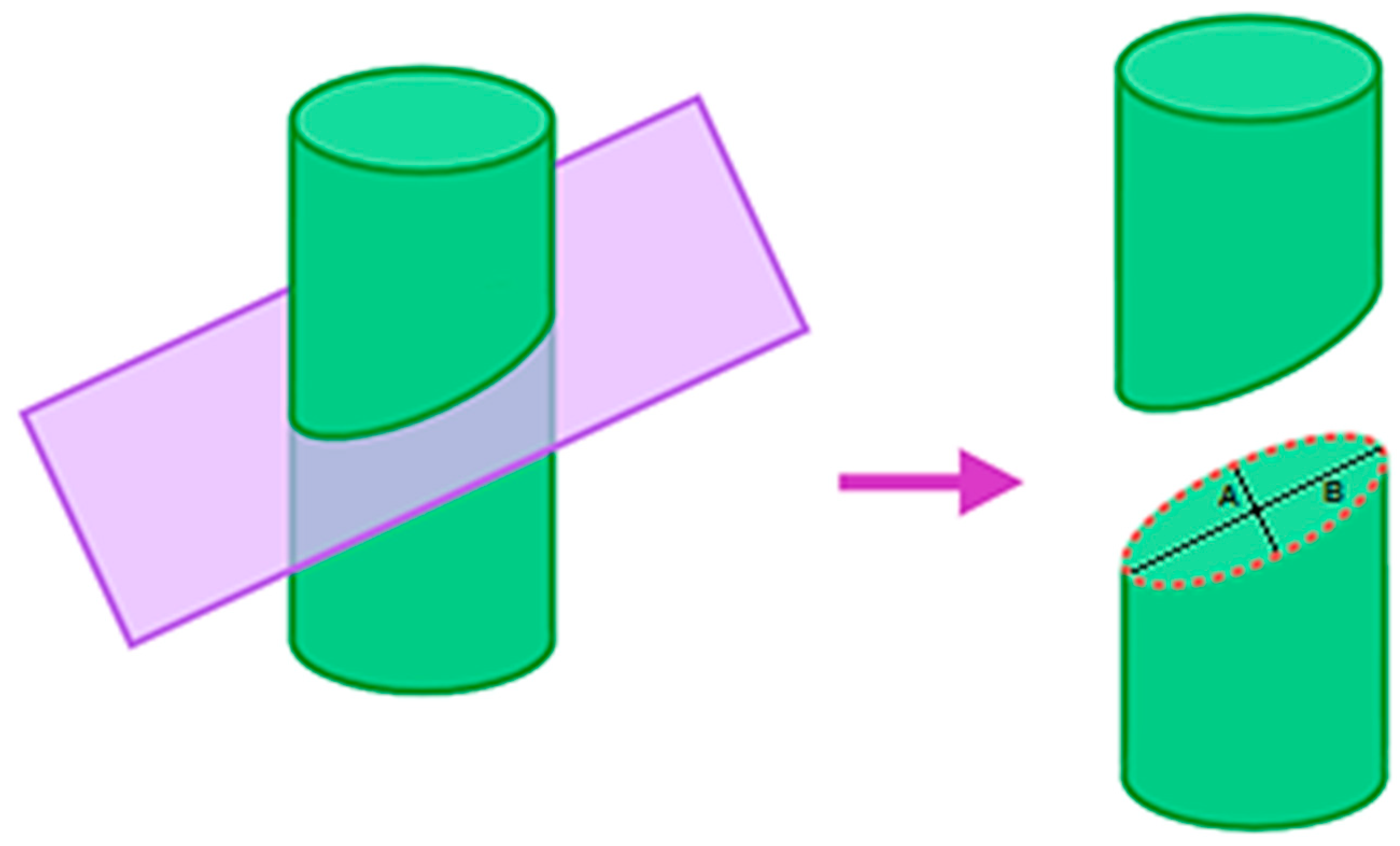
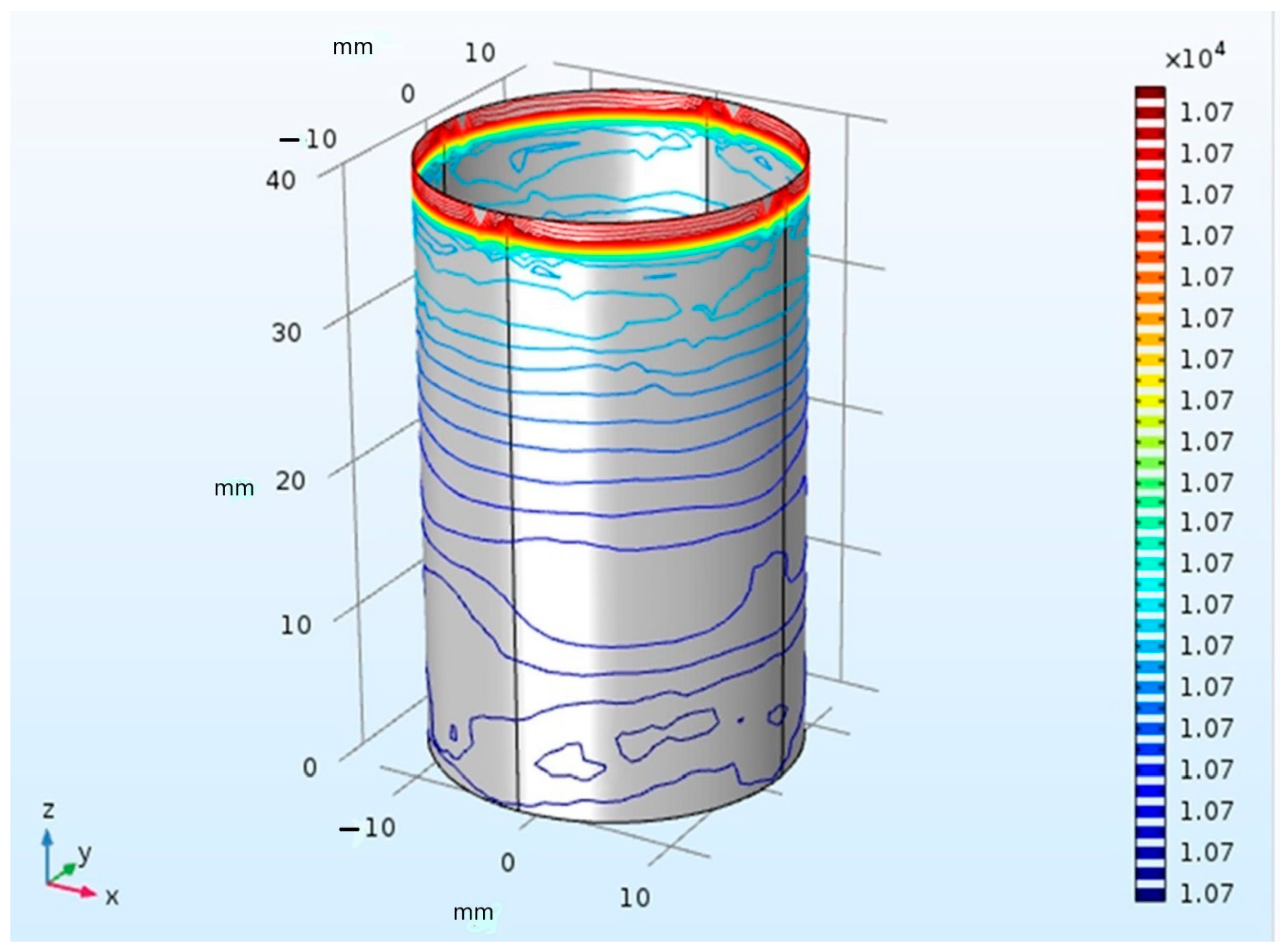
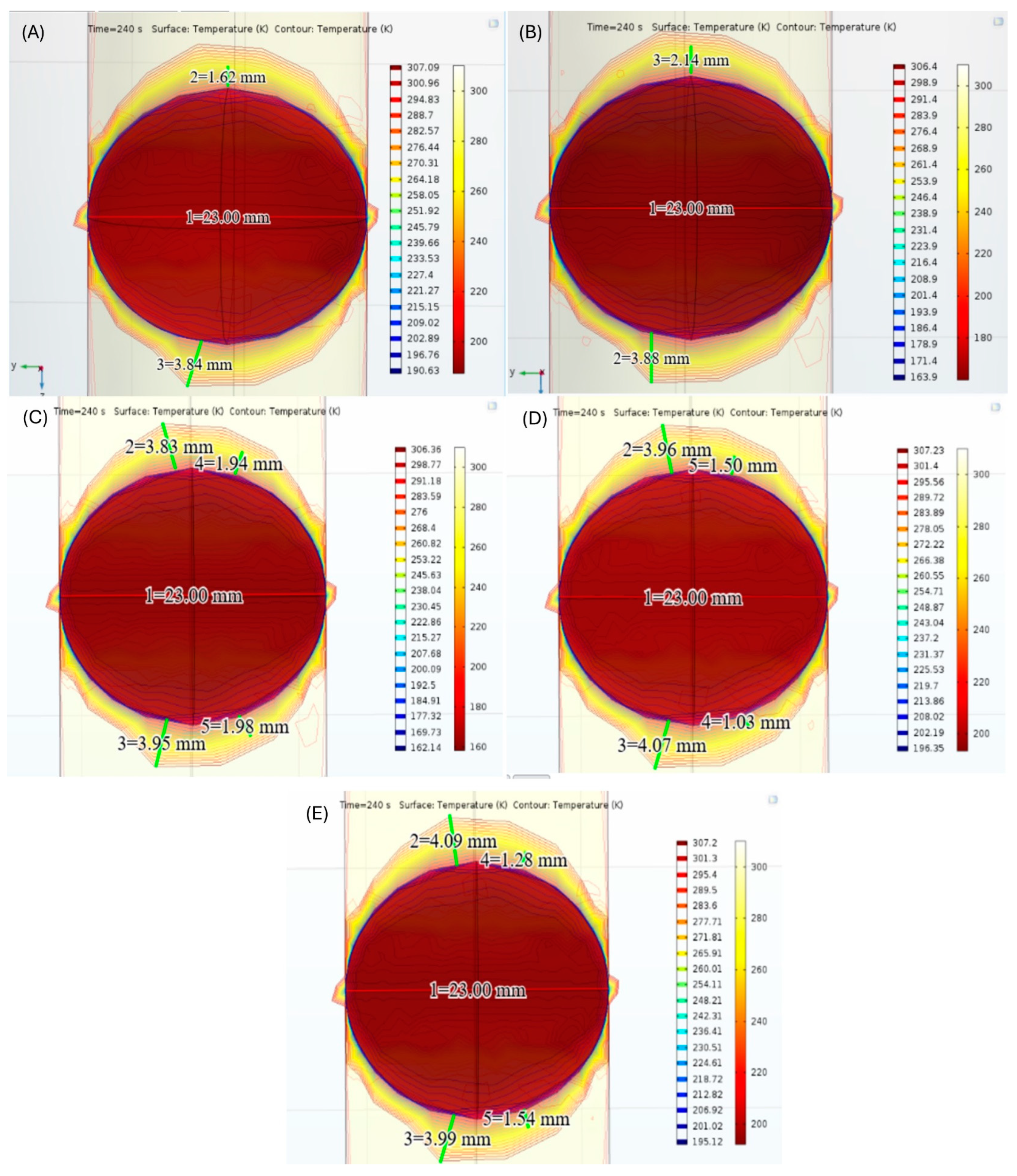
| Material | Thermal Conductivity (W/mK) | Specific Heat (J/kgK) | Tissue Density (kg/m3) | Temperature |
|---|---|---|---|---|
| Blood | 0.54 | 4000 | 1057 | 310.15 |
| Vascular wall | 1.3 | 3600 | 1200 | 310 |
| Polyurethane | 0.02 | 1500 | 1110 | 198.15 |
| Pulmonary tissue | 0.03 | 2963 | 1000 | 311 |
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 |
|---|---|---|---|---|
| TZ (0 °C) = 10 | TZ (0 °C) = 8 | TZ (0 °C) = 12 | TZ (0 °C) = 12 | TZ (0 °C) = 13 |
| TI (−30 °C) = 23 | TI (−30 °C) = 29 | TI (−30 °C) = 36 | TI (−30 °C) = 30 | TI (−30 °C) = 31 |
| TM (−60 °C) = 32 | TM (−42 °C) = 33 | TM (−41 °C) = 42 | TM (−63 °C) = 34 | TM (−43 °C) = 35 |
| TT = 240 | TT = 240 | TT = 240 | TT = 240 | TT = 240 |
| Pulmonary Vein | Ostium Diameter (mm) | Vascular Wall Thickness (mm) |
|---|---|---|
| Right superior | 18 | 2.7 |
| Right inferior | 12 | 1.8 |
| Left superior | 19 | 2.8 |
| Left inferior | 13 | 1.9 |
| Maximum Freezing Temperatures (°C) | Distance between Cryoballoon and Vascular Wall (mm) | Lesion Depth (mm) | Lesion Area (mm2) |
|---|---|---|---|
| −60 | 3.88 | 4.28 | 77.28 |
| −42 | 3.84 | 3.24 | 58.50 |
| −41 | 4.07 | 3.00 | 54.17 |
| −61 | 3.95 | 3.96 | 71.50 |
| −43 | 4.09 | 3.08 | 55.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivera, S.I.; Bernal, C.P.; Martínez-Peláez, R.; Robledo-Nolasco, R.; De León-Larios, G.; Félix, V.G.; Ostos, R.; Maestre, G.E.; Melgarejo, J.D.; Mena, L.J. Computer Simulation of Catheter Cryoablation for Pulmonary Vein Isolation. Healthcare 2024, 12, 1508. https://doi.org/10.3390/healthcare12151508
Rivera SI, Bernal CP, Martínez-Peláez R, Robledo-Nolasco R, De León-Larios G, Félix VG, Ostos R, Maestre GE, Melgarejo JD, Mena LJ. Computer Simulation of Catheter Cryoablation for Pulmonary Vein Isolation. Healthcare. 2024; 12(15):1508. https://doi.org/10.3390/healthcare12151508
Chicago/Turabian StyleRivera, Solange I., Clara P. Bernal, Rafael Martínez-Peláez, Rogelio Robledo-Nolasco, Gerardo De León-Larios, Vanessa G. Félix, Rodolfo Ostos, Gladys E. Maestre, Jesús D. Melgarejo, and Luis J. Mena. 2024. "Computer Simulation of Catheter Cryoablation for Pulmonary Vein Isolation" Healthcare 12, no. 15: 1508. https://doi.org/10.3390/healthcare12151508






