Platelet/High-Density Lipoprotein Ratio (PHR) Predicts Type 2 Diabetes in Obese Patients: A Retrospective Study
Abstract
1. Introduction
2. Methods
2.1. Study Design and Data Collection
- Obesity Class I (BMI 30 to 34.9 kg/m2)
- Obesity Class II (BMI 35 to 39.9 kg/m2)
- Obesity Class III (BMI ≥ 40 kg/m2)
2.2. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Studied Population
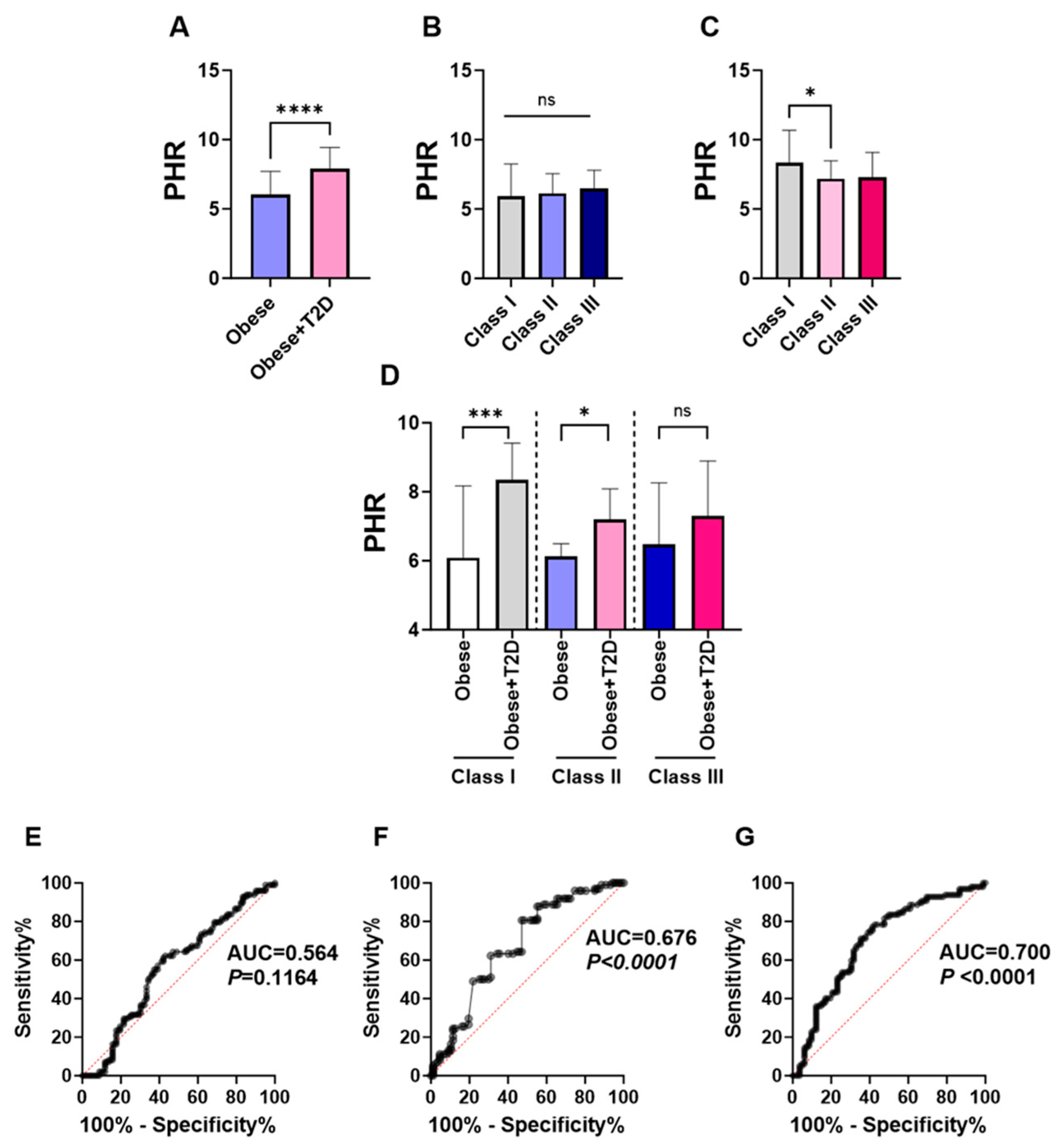
3.2. PHR Is Substantially Increased in Obese T2D Patients with a Significant Diagnostic Performance in Predicting T2D in the Studied Population
3.3. FBG and HbA1c Were Significantly Higher in High-PHR Obese Patients with T2D
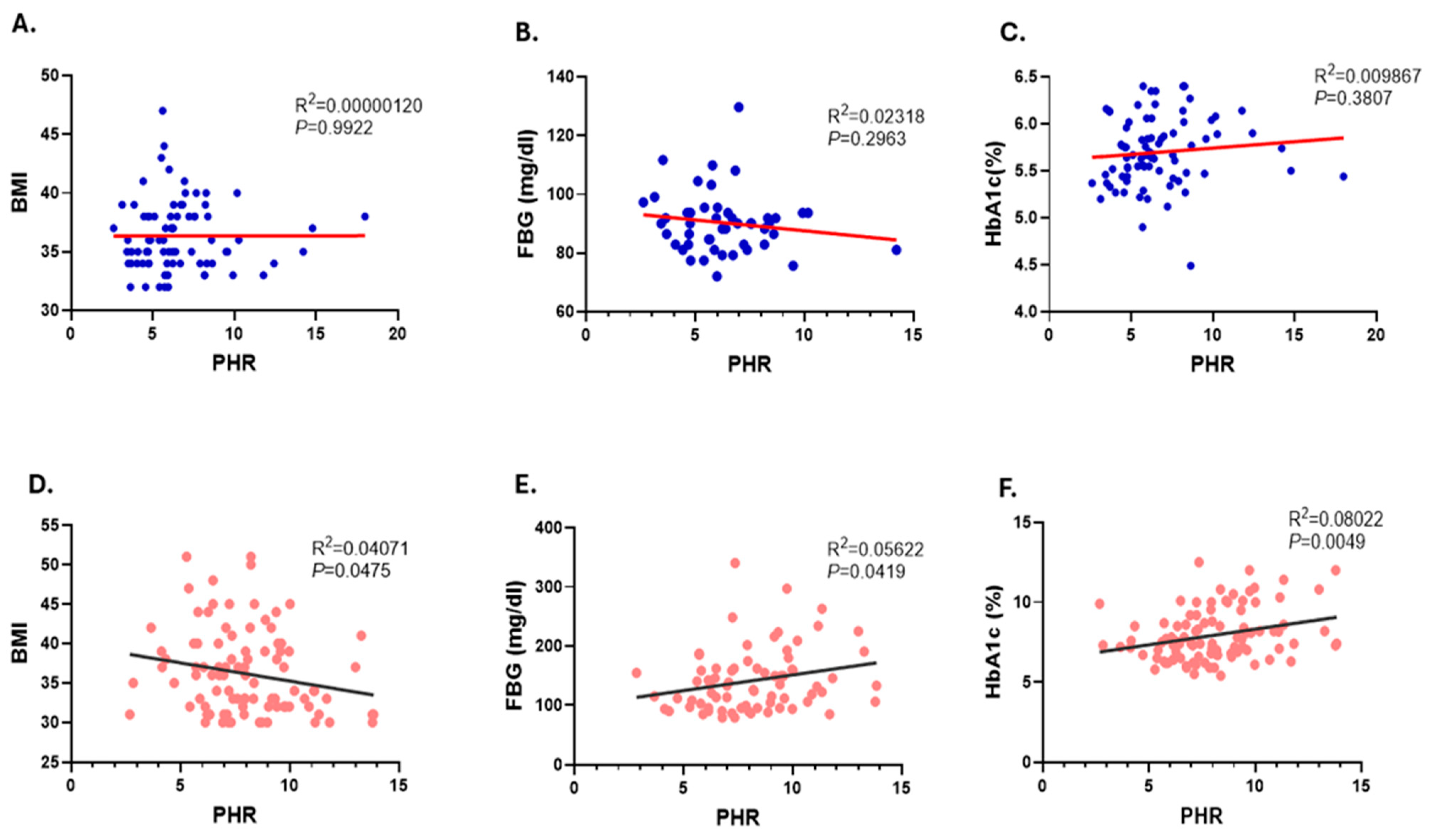
3.4. Correlation of PHR Values with BMI and Glycemic Parameters, FBG and HbA1c
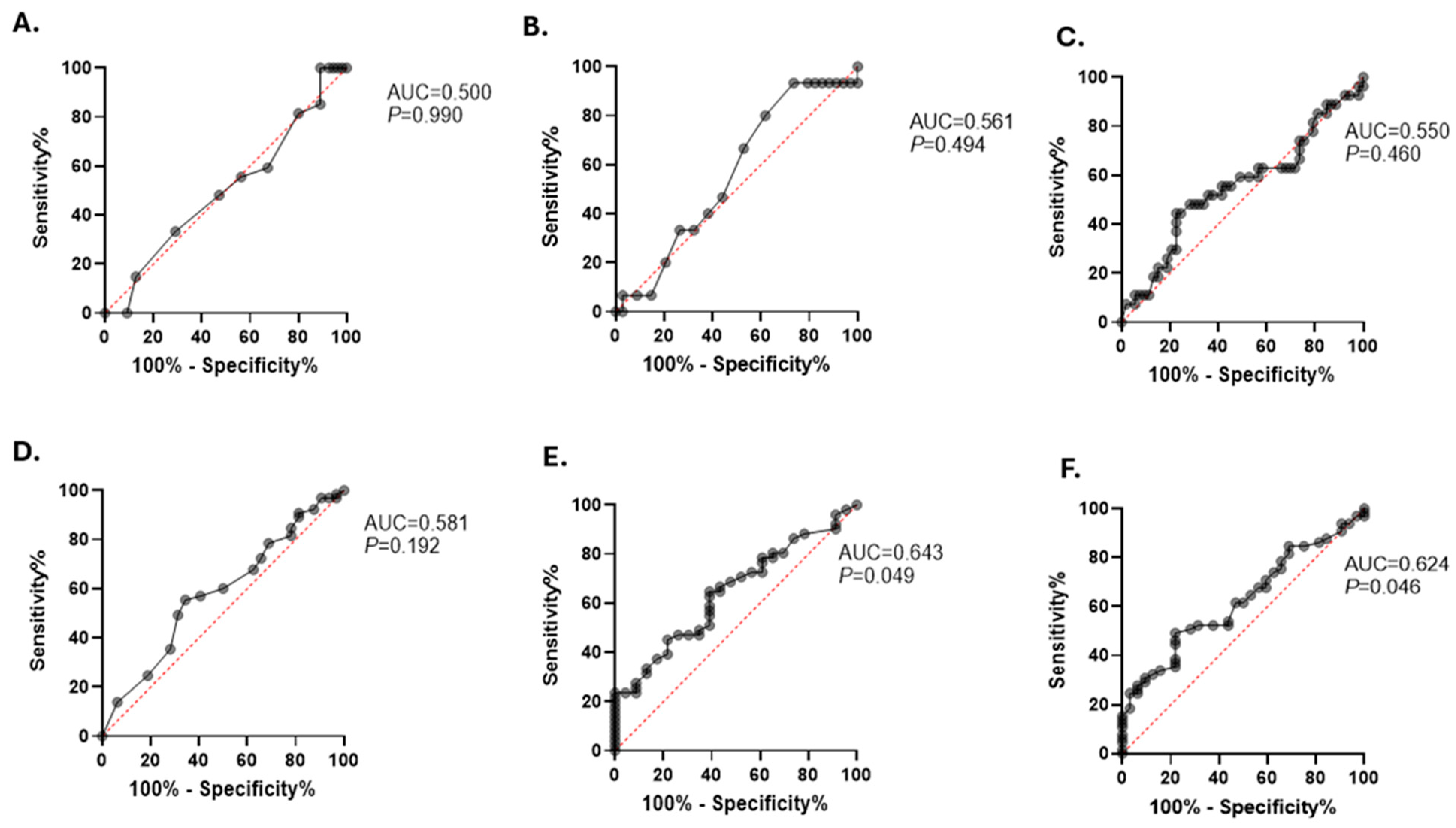
3.5. Elevated PHR Is Associated with an Increased Risk of T2D in Obese Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alqarni, S.S.M. A Review of Prevalence of Obesity in Saudi Arabia. J. Obes. Eat. Disord. 2016, 2, 1–6. [Google Scholar] [CrossRef]
- Althumiri, N.A.; Basyouni, M.H.; AlMousa, N.; AlJuwaysim, M.F.; Almubark, R.A.; BinDhim, N.F.; Alkhamaali, Z.; Alqahtani, S.A. Obesity in Saudi Arabia in 2020: Prevalence, Distribution, and Its Current Association with Various Health Conditions. Healthcare 2021, 9, 311. [Google Scholar] [CrossRef]
- Salem, V.; AlHusseini, N.; Razack, H.I.A.; Naoum, A.; Sims, O.T.; Alqahtani, S.A. Prevalence, risk factors, and interventions for obesity in Saudi Arabia: A systematic review. Obes. Rev. 2022, 23, e13448. [Google Scholar] [CrossRef]
- Malkin, J.D.; Baid, D.; Alsukait, R.F.; Alghaith, T.; Alluhidan, M.; Alabdulkarim, H.; Altowaijri, A.; Almalki, Z.S.; Herbst, C.H.; Finkelstein, E.A.; et al. The economic burden of overweight and obesity in Saudi Arabia. PLoS ONE 2022, 17, e0264993. [Google Scholar] [CrossRef]
- Ruze, R.; Liu, T.; Zou, X.; Song, J.; Chen, Y.; Xu, R.; Yin, X.; Xu, Q. Obesity and type 2 diabetes mellitus: Connections in epidemiology, pathogenesis, and treatments. Front. Endocrinol. 2023, 14, 1161521. [Google Scholar] [CrossRef]
- Pérez, M.R.; Medina-Gómez, G. Obesity, adipogenesis and insulin resistance. Endocrinol. Nutr. (Engl. Ed.) 2011, 58, 360–369. [Google Scholar] [CrossRef]
- Narayan, K.; Boyle, J.P.; Thompson, T.J.; Gregg, E.W.; Williamson, D.F. Effect of BMI on Lifetime Risk for Diabetes in the U.S. Diabetes Care 2007, 30, 1562–1566. [Google Scholar] [CrossRef]
- Longo, M.; Zatterale, F.; Naderi, J.; Parrillo, L.; Formisano, P.; Raciti, G.A.; Beguinot, F.; Miele, C. Adipose Tissue Dysfunction as Determinant of Obesity-Associated Metabolic Complications. Int. J. Mol. Sci. 2019, 20, 2358. [Google Scholar] [CrossRef]
- Klein, S.; Gastaldelli, A.; Yki-Järvinen, H.; Scherer, P.E. Why does obesity cause diabetes? Cell Metab. 2022, 34, 11–20. [Google Scholar] [CrossRef]
- Daousi, C.; Casson, I.F.; Gill, G.V.; MacFarlane, I.A.; Wilding, J.P.H.; Pinkney, J.H. Prevalence of obesity in type 2 diabetes in secondary care: Association with cardiovascular risk factors. Postgrad. Med. J. 2006, 82, 280–284. [Google Scholar] [CrossRef]
- Rohm, T.V.; Meier, D.T.; Olefsky, J.M.; Donath, M.Y. Inflammation in obesity, diabetes, and related disorders. Immunity 2022, 55, 31–55. [Google Scholar] [CrossRef] [PubMed]
- Ford, E.S. Leukocyte Count, Erythrocyte Sedimentation Rate, and Diabetes Incidence in a National Sample of US Adults. Am. J. Epidemiol. 2002, 155, 57–64. [Google Scholar] [CrossRef]
- Libby, P.; Nahrendorf, M.; Swirski, F.K. Leukocytes link local and systemic inflammation in ischemic cardiovascular disease an expanded cardiovascular continuum. J. Am. Coll. Cardiol. 2016, 67, 1091–1103. [Google Scholar] [CrossRef] [PubMed]
- Herrero-Cervera, A.; Soehnlein, O.; Kenne, E. Neutrophils in chronic inflammatory diseases. Cell. Mol. Immunol. 2022, 19, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Akboga, M.K.; Canpolat, U.; Yuksel, M.; Yayla, C.; Yilmaz, S.; Turak, O.; Ozeke, O.; Topaloglu, S.; Aras, D. Platelet to lymphocyte ratio as a novel indicator of inflammation is correlated with the severity of metabolic syndrome: A single center large-scale study. Platelets 2015, 27, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Maurer, S.; Kopp, H.-G.; Salih, H.R.; Kropp, K.N. Modulation of Immune Responses by Platelet-Derived ADAM10. Front. Immunol. 2020, 11, 44. [Google Scholar] [CrossRef]
- Rossaint, J.; Margraf, A.; Zarbock, A. Role of Platelets in Leukocyte Recruitment and Resolution of Inflammation. Front. Immunol. 2018, 9, 2712. [Google Scholar] [CrossRef]
- Van der Poll, T.; Parker, R.I. Platelet Activation and Endothelial Cell Dysfunction. Crit. Care Clin. 2020, 36, 233–253. [Google Scholar] [CrossRef]
- Qian, H.; Chen, R.; Wang, B.; Yuan, X.; Chen, S.; Liu, Y.; Shi, G. Associations of Platelet Count with Inflammation and Response to Anti-TNF-α Therapy in Patients with Ankylosing Spondylitis. Front. Pharmacol. 2020, 11, 559593. [Google Scholar] [CrossRef]
- Gibson, P.R.; Iser, J. Inflammatory bowel disease. Aust. Fam. Physician 2005, 34, 233–237. [Google Scholar] [CrossRef]
- Li, L.; Xu, P.; Zhang, Z.; Zhou, X.; Chen, C.; Lu, C. Platelets can reflect the severity of Crohn’s disease without the effect of anemia. Clinics 2020, 75, e1596. [Google Scholar] [CrossRef]
- Milovanovic, M.; Nilsson, E.; Järemo, P. Relationships between platelets and inflammatory markers in rheumatoid arthritis. Clin. Chim. Acta 2004, 343, 237–240. [Google Scholar] [CrossRef]
- Jung, J.-H.; Soh, M.-S.; Ahn, Y.-H.; Um, Y.-J.; Jung, J.-Y.; Suh, C.-H.; Kim, H.-A. Thrombocytopenia in Systemic Lupus Erythematosus. Medicine 2016, 95, e2818. [Google Scholar] [CrossRef]
- Angiolillo, D.J.; Fernandez-Ortiz, A.; Bernardo, E.; Ramírez, C.; Sabaté, M.; Jimenez-Quevedo, P.; Hernández, R.; Moreno, R.; Escaned, J.; Alfonso, F.; et al. Platelet Function Profiles in Patients With Type 2 Diabetes and Coronary Artery Disease on Combined Aspirin and Clopidogrel Treatment. Diabetes 2005, 54, 2430–2435. [Google Scholar] [CrossRef]
- Neergaard-Petersen, S.; Hvas, A.-M.; Grove, E.L.; Larsen, S.B.; Gregersen, S.; Kristensen, S.D. The Influence of Haemoglobin A1c Levels on Platelet Aggregation and Platelet Turnover in Patients with Coronary Artery Disease Treated with Aspirin. PLoS ONE 2015, 10, e0132629. [Google Scholar] [CrossRef]
- De Pergola, G.; Giagulli, V.; Guastamacchia, E.; Bartolomeo, N.; Tatoli, R.; Lampignano, L.; Silvestris, F.; Triggiani, V. Platelet number is positively and independently associated with glycated hemoglobin in non-diabetic overweight and obese subjects. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Barter, P.J.; Nicholls, S.; Rye, K.-A.; Anantharamaiah, G.; Navab, M.; Fogelman, A.M. Antiinflammatory Properties of HDL. Circ. Res. 2004, 95, 764–772. [Google Scholar] [CrossRef]
- Zhang, S.; Ritter, L.R.; Ibragimov, A.I. Foam cell formation in atherosclerosis: HDL and macrophage reverse cholesterol transport. Discret. Contin. Dyn. Syst. Ser. S 2013, 2013, 825–835. [Google Scholar] [CrossRef]
- Brites, F.; Martin, M.; Guillas, I.; Kontush, A. Antioxidative activity of high-density lipoprotein (HDL): Mechanistic insights into potential clinical benefit. BBA Clin. 2017, 8, 66–77. [Google Scholar] [CrossRef]
- Denimal, D.; Monier, S.; Bouillet, B.; Vergès, B.; Duvillard, L. High-Density Lipoprotein Alterations in Type 2 Diabetes and Obesity. Metabolites 2023, 13, 253. [Google Scholar] [CrossRef]
- Barter, P.J. The Causes and Consequences of Low Levels of High Density Lipoproteins in Patients with Diabetes. Diabetes Metab. J. 2011, 35, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xu, Y.; Xu, Y. The association of the platelet/high-density lipoprotein cholesterol ratio with self-reported stroke and cardiovascular mortality: A population-based observational study. Lipids Health Dis. 2024, 23, 121. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Jia, C.; Xu, X.; He, Y.; Xie, Y.; Zhou, Y.; Lu, H.; Liu, J.; Chen, J.; Liu, Y. Impact of Platelet-to-HDL-Cholesterol Ratio on Long-Term Mortality in Coronary Artery Disease Patients with or Without Type 2 Diabetes: Insights from a Chinese Multicenter Cohort. J. Inflamm. Res. 2024, 17, 2731–2744. [Google Scholar] [CrossRef]
- Jialal, I.; Jialal, G.; Adams-Huet, B. The platelet to high density lipoprotein -cholesterol ratio is a valid biomarker of nascent metabolic syndrome. Diabetes/Metab. Res. Rev. 2020, 37, e3403. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee; ElSayed, N.A.; Aleppo, G.; Bannuru, R.R.; Bruemmer, D.; Collins, B.S.; Ekhlaspour, L.; Gaglia, J.L.; Hilliard, M.E.; Johnson, E.L.; et al. 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes—2024. Diabetes Care 2023, 47, S20–S42. [Google Scholar] [CrossRef]
- Weir, C.B.; Jan, A. BMI Classification Percentile and Cut Off Points; StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Robert, A.A.; Al Dawish, M.A. The Worrying Trend of Diabetes Mellitus in Saudi Arabia: An Urgent Call to Action. Curr. Diabetes Rev. 2019, 16, 204–210. [Google Scholar] [CrossRef]
- Farhangi, M.A.; Keshavarz, S.-A.; Eshraghian, M.; Ostadrahimi, A.; Saboor-Yaraghi, A.-A. White Blood Cell Count in Women: Relation to Inflammatory Biomarkers, Haematological Profiles, Visceral Adiposity, and Other Cardiovascular Risk Factors. J. Health Popul. Nutr. 2013, 31, 58–64. [Google Scholar] [CrossRef]
- Han, S.; Gan, D.; Wang, G.; Ru, Y.; Huang, C.; Lin, J.; Zhang, L.; Meng, Z.; Zhu, S. Associations of Platelet Indices with Body Fat Mass and Fat Distribution. Obesity 2018, 26, 1637–1643. [Google Scholar] [CrossRef]
- Samocha-Bonet, D.; Justo, D.; Rogowski, O.; Saar, N.; Abu-Abeid, S.; Shenkerman, G.; Shapira, I.; Berliner, S.; Tomer, A. Platelet Counts and Platelet Activation Markers in Obese Subjects. Mediat. Inflamm. 2008, 2008, 834153. [Google Scholar] [CrossRef]
- Coban, E.; Ozdogan, M.; Yazicioglu, G.; Akcit, F. The mean platelet volume in patients with obesity. Int. J. Clin. Pract. 2005, 59, 981–982. [Google Scholar] [CrossRef]
- Vazzana, N.; Ranalli, P.; Cuccurullo, C.; Davì, G. Diabetes mellitus and thrombosis. Thromb. Res. 2011, 129, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Gordon, L.; Ragoobirsingh, D.; Morrison, E.Y.S.A.; Choo-Kang, E.; McGrowder, D.; Martorell, E. Lipid Profile of Type 2 Diabetic and Hypertensive Patients in the Jamaican Population. J. Lab. Physicians 2010, 2, 025–030. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.-C.; Hayashi, T.; Fujimoto, W.Y.; Kahn, S.E.; Leonetti, D.L.; McNeely, M.J.; Boyko, E.J. Differential Association Between HDL Subclasses and the Development of Type 2 Diabetes in a Prospective Study of Japanese Americans. Diabetes Care 2015, 38, 2100–2105. [Google Scholar] [CrossRef] [PubMed]
- Tabara, Y.; Arai, H.; Hirao, Y.; Takahashi, Y.; Setoh, K.; Kawaguchi, T.; Kosugi, S.; Ito, Y.; Nakayama, T.; Matsuda, F. Different inverse association of large high-density lipoprotein subclasses with exacerbation of insulin resistance and incidence of type 2 diabetes: The Nagahama study. Diabetes Res. Clin. Pract. 2017, 127, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Hu, Z.; Yang, S.; Sun, L.; Yu, Z.; Wang, G. Role of Adaptive and Innate Immunity in Type 2 Diabetes Mellitus. J. Diabetes Res. 2018, 2018, 7457269. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.H.; Bergmeier, W. Sugar makes neutrophils RAGE: Linking diabetes-associated hyperglycemia to thrombocytosis and platelet reactivity. J. Clin. Investig. 2017, 127, 2040–2043. [Google Scholar] [CrossRef] [PubMed]
- Keating, F.K.; Sobel, B.E.; Schneider, D.J. Effects of increased concentrations of glucose on platelet reactivity in healthy subjects and in patients with and without diabetes mellitus. Am. J. Cardiol. 2003, 92, 1362–1365. [Google Scholar] [CrossRef] [PubMed]
- Kodiatte, T.A.; Manikyam, U.K.; Rao, S.B.; Jagadish, T.M.; Reddy, M.; Lingaiah, H.K.M.; Lakshmaiah, V. Mean Platelet Volume in Type 2 Diabetes Mellitus. J. Lab. Physicians 2012, 4, 005–009. [Google Scholar] [CrossRef] [PubMed]
- Spectre, G.; Östenson, C.-G.; Li, N.; Hjemdahl, P. Postprandial Platelet Activation Is Related to Postprandial Plasma Insulin Rather Than Glucose in Patients with Type 2 Diabetes. Diabetes 2012, 61, 2380–2384. [Google Scholar] [CrossRef]
- Ferreira, I.A.; Eybrechts, K.L.; Mocking, A.I.M.; Kroner, C.; Akkerman, J.-W.N. IRS-1 Mediates Inhibition of Ca2+ Mobilization by Insulin via the Inhibitory G-protein Gi. J. Biol. Chem. 2004, 279, 3254–3264. [Google Scholar] [CrossRef]
- Ferreira, I.A.; Mocking, A.I.; Feijge, M.A.; Gorter, G.; van Haeften, T.W.; Heemskerk, J.W.; Akkerman, J.-W.N. Platelet Inhibition by Insulin Is Absent in Type 2 Diabetes Mellitus. Arter. Thromb. Vasc. Biol. 2006, 26, 417–422. [Google Scholar] [CrossRef]
- Monnier, L.; Mas, E.; Ginet, C.; Michel, F.; Villon, L.; Cristol, J.-P.; Colette, C. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA 2006, 295, 1681–1687. [Google Scholar] [CrossRef] [PubMed]
- Sampson, M.J.; Gopaul, N.; Davies, I.R.; Hughes, D.A.; Carrier, M.J. Plasma F2 isoprostanes: Direct evidence of increased free radical damage during acute hyperglycemia in type 2 diabetes. Diabetes Care 2002, 25, 537–541. [Google Scholar] [CrossRef]
- Femlak, M.; Gluba-Brzózka, A.; Ciałkowska-Rysz, A.; Rysz, J. The role and function of HDL in patients with diabetes mellitus and the related cardiovascular risk. Lipids Health Dis. 2017, 16, 207. [Google Scholar] [CrossRef]
- Lam, J.K.; Matsubara, S.; Mihara, K.; Zheng, X.-L.; Mooradian, A.D.; Wong, N.C.W. Insulin Induction of Apolipoprotein AI, Role of Sp1. Biochemistry 2003, 42, 2680–2690. [Google Scholar] [CrossRef] [PubMed]
- Taskinen, M.-R. LDL-cholesterol, HDL-cholesterol or triglycerides—Which is the culprit? Diabetes Res. Clin. Pract. 2003, 61, S19–S26. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Gao, X.; Yao, Z.; Xu, Y. Low apoA-I is associated with insulin resistance in patients with impaired glucose tolerance: A cross-sectional study. Lipids Health Dis. 2017, 16, 69. [Google Scholar] [CrossRef]
- Kashyap, S.R.; Osme, A.; Ilchenko, S.; Golizeh, M.; Lee, K.; Wang, S.; Bena, J.; Previs, S.F.; Smith, J.D.; Kasumov, T. Glycation Reduces the Stability of ApoAI and Increases HDL Dysfunction in Diet-Controlled Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2018, 103, 388–396. [Google Scholar] [CrossRef]
- Han, C.Y.; Tang, C.; Guevara, M.E.; Wei, H.; Wietecha, T.; Shao, B.; Subramanian, S.; Omer, M.; Wang, S.; O’Brien, K.D.; et al. Serum amyloid A impairs the antiinflammatory properties of HDL. J. Clin. Investig. 2016, 126, 266–281. [Google Scholar] [CrossRef]
- Mao, J.Y.; Sun, J.T.; Yang, K.; Shen, W.F.; Lu, L.; Zhang, R.Y.; Tong, X.; Liu, Y. Serum amyloid A enrichment impairs the anti-inflammatory ability of HDL from diabetic nephropathy patients. J. Diabetes Complicat. 2017, 31, 1538–1543. [Google Scholar] [CrossRef]
- Jayaraman, S.; Haupt, C.; Gursky, O. Paradoxical effects of SAA on lipoprotein oxidation suggest a new antioxidant function for SAA. J. Lipid Res. 2016, 57, 2138–2149. [Google Scholar] [CrossRef]
- Animaw, W.; Seyoum, Y. Increasing prevalence of diabetes mellitus in a developing country and its related factors. PLoS ONE 2017, 12, e0187670. [Google Scholar] [CrossRef] [PubMed]
- Hills, A.P.; Arena, R.; Khunti, K.; Yajnik, C.S.; Jayawardena, R.; Henry, C.J.; Street, S.J.; Soares, M.J.; Misra, A. Epidemiology and determinants of type 2 diabetes in south Asia. Lancet Diabetes Endocrinol. 2018, 6, 966–978. [Google Scholar] [CrossRef]
- Ng, M.C.Y. Genetics of Type 2 Diabetes in African Americans. Curr. Diabetes Rep. 2015, 15, 74. [Google Scholar] [CrossRef] [PubMed]

| Variables | All Subjects (n = 203) | Obese (n = 103) | Obese + T2D (n = 100) | p-Value |
|---|---|---|---|---|
| Demographics | ||||
| Age (years), | 48 (35–58) | 37 (29–46) | 58 (51–65) | <0.0001 |
| Sex (female), n (%) | 141 (69.4%) | 73 (70.8%) | 68 (68.0%) | - |
| BMI (kg/m2), | 35 (33–38) | 36 (34–38) | 35 (32–40) | 0.4322 |
| Lab Parameters | ||||
| RBC count (×1012/μL) | 4.88 (4.49–5.31) | 4.90(4.53–5.41) | 4.80 (4.40–5.10) | 0.0676 |
| Hb (g/dL) | 13.0 (12.00–14.70) | 13.2 (11.98–14.73) | 13.0 (12–14.25) | <0.0001 |
| Hct (L/L) | 27.70 (25.60–29.00) | 27.40 (25.15–29.25) | 28.0 (26.0–29.0) | 0.2346 |
| WBC (×109/μL), | 7.5 (6.0–7.0) | 7.1 (5.5–8.3) | 8.1 (6.6–9.4) | 0.0002 |
| Neutrophils (×103/μL) | 3.77 (2.76–5.00) | 3.49 (2.29–4.61) | 4.00 (3.07–5.22) | 0.0065 |
| Lymphocytes (×103/μL) | 2.75 (2.20–3.32) | 2.62 (2.16–3.12) | 3.00 (2.29–3.80) | 0.0085 |
| Monocytes (×103/μL) | 0.55 (0.44–0.68) | 0.54 (0.40–0.65) | 0.57 (0.50–0.74) | 0.0080 |
| Basophils (×103/μL) | 0.05 (0.03–0.07) | 0.04 (0.03–0.06) | 0.05 (0.04–0.07) | 0.0035 |
| Eosinophils (×103/μL) | 0.18 (0.11–0.28) | 0.18 (0.11–0.25) | 0.20 (0.11–0.32) | 0.1343 |
| ALT (U/L) | 17 (13–26) | 16 (12–25) | 18 (14–26) | 0.1152 |
| PLT count (×106/mL) | 312.0 (265.3–369.8) | 299.0 (255.5–363.3) | 327.5 (272.3–378.3) | 0.1166 |
| FBG (mg/dL) | 97.30 (90.1–140.1) | 90.09 (82.9–93.7) | 131.5 (97.3–162.2) | <0.0001 |
| HbA1c (%) | 6.2 (5.7–7.4) | 5.7 (5.5–5.9) | 7.4 (6.7–8.7) | <0.0001 |
| TC (mg/dL) | 177.9 (147.7–211.3) | 196.8 (172.1–227.8) | 154.7 (135.3–185.6) | <0.0001 |
| TG (mg/dL) | 115.1 (88.6–156.8) | 103.6 (77.1–150.6) | 124.0 (88.6–159.4) | 0.0393 |
| HDL (mg/dL) | 43.7 (38.5–52.4) | 47.6 (39.8–59.6) | 40.8 (36.9–46.4) | <0.0001 |
| LDL (mg/dL) | 108.9 (85.1–136.8) | 127.2 (106.1–152.1) | 92.8 (69.6–116.0) | <0.0001 |
| PHR | 7.06 (5.71–8.785) | 6.05 (4.75–7.72) | 7.91 (6.39–9.44) | <0.0001 |
| Comorbidities | ||||
| Hypertension, n (%) | 60 (29.5%) | 13 (12.6%) | 47 (47%) | - |
| Hypothyroidism, n (%) | 18 (8.8%) | 16 (15.5%) | 2 (2%) | - |
| DLD, n (%) | 96 (47.2%) | 19 (18.4%) | 77 (77%) | - |
| Smoking, n (%) | 22 (10.8%) | 17 (16.5%) | 5 (5%) | - |
| Medications | - | |||
| Metformin, n (%) | 104 (51.2%) | 11 (10.6%) | 93 (93%) | - |
| GLP-1 agonist, n (%) | 57 (28%) | 31 (30%) | 26 (26%) | - |
| SGL2 inhibitor, n (%) | 34 (16.7%) | 0 (0%) | 34 (34%) | - |
| Parameter | Non-Diabetic Obese | Diabetic Obese |
|---|---|---|
| N-PHR | 67.07% | 29.90% |
| H-PHR | 32.93% | 70.10% |
| Score | 95% CI | Z Statistic | Significance Level | |
|---|---|---|---|---|
| RR | ||||
| Total subjects | 2.13 | 1.52 to 2.97 | 4.42 | p < 0.0001 |
| Obesity class I | 2.12 | 1.23 to 3.67 | 2.71 | p = 0.0067 |
| Obesity class II | 1.6 | 0.86 to 2.97 | 1.49 | p = 0.1345 |
| Obesity class III | 0.58 | 0.41 to 0.81 | 3.12 | p = 0.0018 |
| OR | ||||
| Total subjects | 4.77 | 2.53 to 8.99 | 4.83 | p < 0.0001 |
| Obesity class I | 5.81 | 2.01 to 16.78 | 3.25 | p = 0.0011 |
| Obesity class II | 5.62 | 1.87 to 16.83 | 3.08 | p = 0.002 |
| Obesity class III | 0.15 | 0.0074 to 3.16 | 1.21 | p = 0.225 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshuweishi, Y.; Abudawood, A.; Alfayez, D.; Almufarrih, A.A.; Alanazi, F.; Alshuweishi, F.A.; Almuqrin, A.M. Platelet/High-Density Lipoprotein Ratio (PHR) Predicts Type 2 Diabetes in Obese Patients: A Retrospective Study. Healthcare 2024, 12, 1540. https://doi.org/10.3390/healthcare12151540
Alshuweishi Y, Abudawood A, Alfayez D, Almufarrih AA, Alanazi F, Alshuweishi FA, Almuqrin AM. Platelet/High-Density Lipoprotein Ratio (PHR) Predicts Type 2 Diabetes in Obese Patients: A Retrospective Study. Healthcare. 2024; 12(15):1540. https://doi.org/10.3390/healthcare12151540
Chicago/Turabian StyleAlshuweishi, Yazeed, Arwa Abudawood, Dalal Alfayez, Abdulmalik A. Almufarrih, Fuad Alanazi, Fahd A. Alshuweishi, and Abdulaziz M. Almuqrin. 2024. "Platelet/High-Density Lipoprotein Ratio (PHR) Predicts Type 2 Diabetes in Obese Patients: A Retrospective Study" Healthcare 12, no. 15: 1540. https://doi.org/10.3390/healthcare12151540
APA StyleAlshuweishi, Y., Abudawood, A., Alfayez, D., Almufarrih, A. A., Alanazi, F., Alshuweishi, F. A., & Almuqrin, A. M. (2024). Platelet/High-Density Lipoprotein Ratio (PHR) Predicts Type 2 Diabetes in Obese Patients: A Retrospective Study. Healthcare, 12(15), 1540. https://doi.org/10.3390/healthcare12151540






