Unlocking the Potential of Vessel Density and the Foveal Avascular Zone in Optical Coherence Tomography Angiography as Biomarkers in Alzheimer’s Disease
Abstract
:1. Introduction
2. Materials and Methods
3. Results
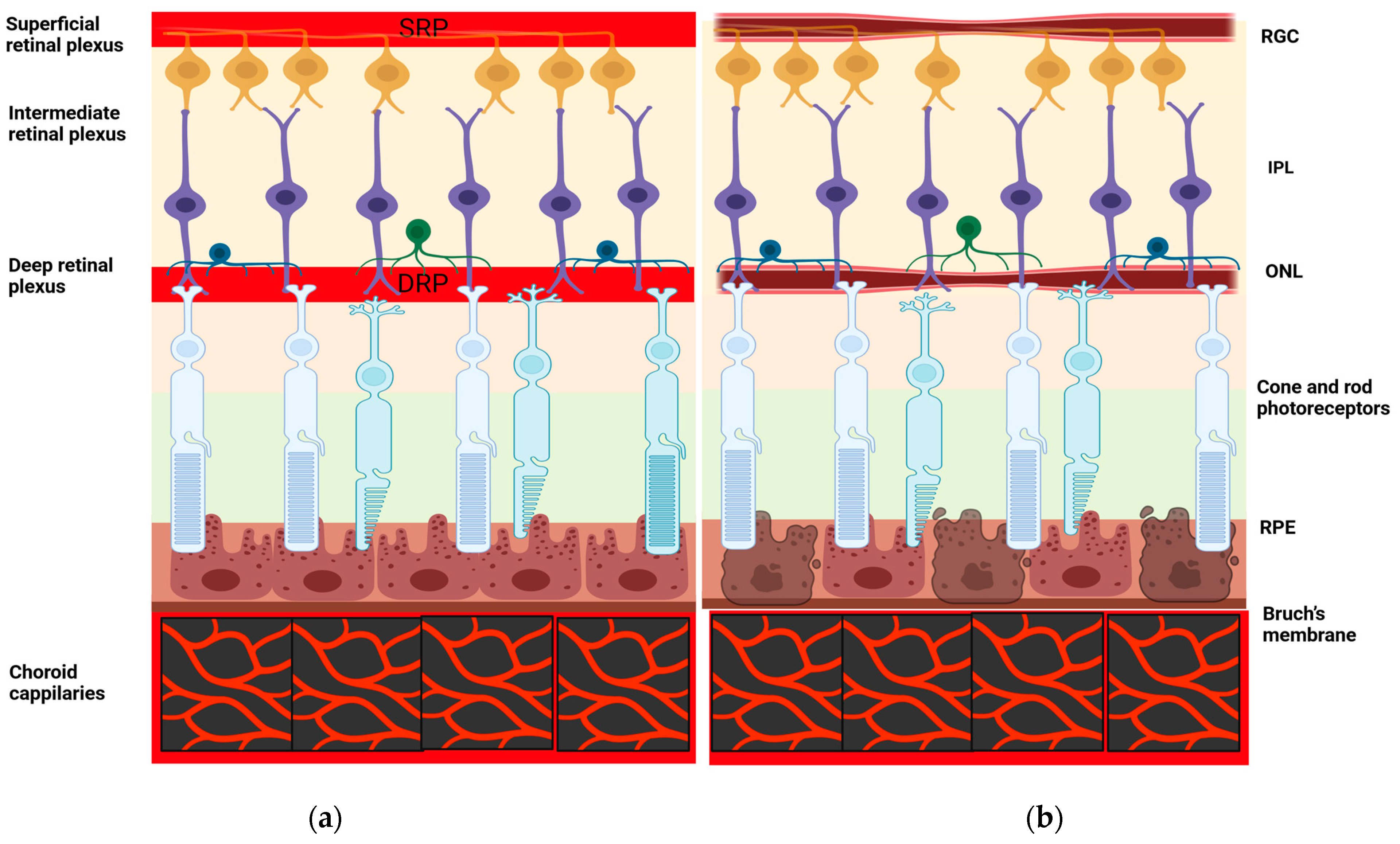
3.1. OCT-A Basic Principles
- The inner retina (from the ganglion cell layer to the inner plexiform layer);
- The middle retina (from the inner nuclear layer to the outer plexiform layer);
- The outer retina (from the outer nuclear layer to the external limiting membrane);
- The choriocapillaris;
3.2. Changes in the Density of the Superficial Retinal Layer, Deep Retinal Layer, and the Foveal Avascular Zone
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abubakar, M.B.; Sanusi, K.O.; Ugusman, A.; Mohamed, W.; Kamal, H.; Ibrahim, N.H.; Khoo, C.S.; Kumar, J. Alzheimer’s Disease: An Update and Insights into Pathophysiology. Front. Aging Neurosci. 2022, 14, 742408. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Guerrero, J.; Santiago-Balmaseda, A.; Jeronimo-Aguilar, P.; Vargas-Rodríguez, I.; Cadena-Suárez, A.R.; Sánchez-Garibay, C.; Pozo-Molina, G.; Méndez-Catalá, C.F.; Cardenas-Aguayo, M.-C.; Diaz-Cintra, S.; et al. Alzheimer’s Disease: An Updated Overview of Its Genetics. Int. J. Mol. Sci. 2023, 24, 3754. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-X.; Tian, Y.; Wang, Z.-T.; Ma, Y.-H.; Tan, L.; Yu, J.-T. The Epidemiology of Alzheimer’s Disease Modifiable Risk Factors and Prevention. J. Prev. Alzheimers Dis. 2021, 8, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Chong, F.P.; Ng, K.Y.; Koh, R.Y.; Chye, S.M. Tau Proteins and Tauopathies in Alzheimer’s Disease. Cell. Mol. Neurobiol. 2018, 38, 965–980. [Google Scholar] [CrossRef] [PubMed]
- Tahami Monfared, A.A.; Byrnes, M.J.; White, L.A.; Zhang, Q. Alzheimer’s Disease: Epidemiology and Clinical Progression. Neurol. Ther. 2022, 11, 553–569. [Google Scholar] [CrossRef] [PubMed]
- Karran, E.; Mercken, M.; Strooper, B.D. The Amyloid Cascade Hypothesis for Alzheimer’s Disease: An Appraisal for the Development of Therapeutics. Nat. Rev. Drug Discov. 2011, 10, 698–712. [Google Scholar] [CrossRef] [PubMed]
- Dubois, B.; Villain, N.; Frisoni, G.B.; Rabinovici, G.D.; Sabbagh, M.; Cappa, S.; Bejanin, A.; Bombois, S.; Epelbaum, S.; Teichmann, M.; et al. Clinical Diagnosis of Alzheimer’s Disease: Recommendations of the International Working Group. Lancet Neurol. 2021, 20, 484–496. [Google Scholar] [CrossRef]
- Gomez-Nicola, D.; Boche, D. Post-Mortem Analysis of Neuroinflammatory Changes in Human Alzheimer’s Disease. Alzheimers Res. Ther. 2015, 7, 42. [Google Scholar] [CrossRef] [PubMed]
- Porsteinsson, A.P.; Isaacson, R.S.; Knox, S.; Sabbagh, M.N.; Rubino, I. Diagnosis of Early Alzheimer’s Disease: Clinical Practice in 2021. J. Prev. Alzheimers Dis. 2021, 8, 371–386. [Google Scholar] [CrossRef]
- Li, X.; Feng, X.; Sun, X.; Hou, N.; Han, F.; Liu, Y. Global, Regional, and National Burden of Alzheimer’s Disease and Other Dementias, 1990–2019. Front. Aging Neurosci. 2022, 14, 937486. [Google Scholar] [CrossRef]
- Kerwin, D.; Abdelnour, C.; Caramelli, P.; Ogunniyi, A.; Shi, J.; Zetterberg, H.; Traber, M. Alzheimer’s Disease Diagnosis and Management: Perspectives from around the World. Alzheimers Dement. Diagn. Assess. Dis. Monit. 2022, 14, e12334. [Google Scholar] [CrossRef] [PubMed]
- Chalkias, I.-N.; Tegos, T.; Topouzis, F.; Tsolaki, M. Ocular Biomarkers and Their Role in the Early Diagnosis of Neurocognitive Disorders. Eur. J. Ophthalmol. 2021, 31, 2808–2817. [Google Scholar] [CrossRef] [PubMed]
- Berisha, F.; Feke, G.T.; Trempe, C.L.; McMeel, J.W.; Schepens, C.L. Retinal Abnormalities in Early Alzheimer’s Disease. Investig. Ophthalmol. Vis. Sci. 2007, 48, 2285. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, U.; Colijn, J.M.; Ikram, M.A.; Bonnemaijer, P.W.M.; Licher, S.; Wolters, F.J.; Tiemeier, H.; Koudstaal, P.J.; Klaver, C.C.W.; Ikram, M.K. Association of Retinal Neurodegeneration on Optical Coherence Tomography with Dementia: A Population-Based Study. JAMA Neurol. 2018, 75, 1256. [Google Scholar] [CrossRef] [PubMed]
- Parisi, V.; Restuccia, R.; Fattapposta, F.; Mina, C.; Bucci, M.G.; Pierelli, F. Morphological and Functional Retinal Impairment in Alzheimer’s Disease Patients. Clin. Neurophysiol. 2001, 112, 1860–1867. [Google Scholar] [CrossRef] [PubMed]
- Coppola, G.; Di Renzo, A.; Ziccardi, L.; Martelli, F.; Fadda, A.; Manni, G.; Barboni, P.; Pierelli, F.; Sadun, A.A.; Parisi, V. Optical Coherence Tomography in Alzheimer’s Disease: A Meta-Analysis. PLoS ONE 2015, 10, e0134750. [Google Scholar] [CrossRef]
- Pillai, J.A.; Bermel, R.; Bonner-Jackson, A.; Rae-Grant, A.; Fernandez, H.; Bena, J.; Jones, S.E.; Ehlers, J.P.; Leverenz, J.B. Retinal Nerve Fiber Layer Thinning in Alzheimer’s Disease: A Case–Control Study in Comparison to Normal Aging, Parkinson’s Disease, and Non-Alzheimer’s Dementia. Am. J. Alzheimer’s Dis. Other Dement. 2016, 31, 430–436. [Google Scholar] [CrossRef]
- Song, A.; Johnson, N.; Ayala, A.; Thompson, A.C. Optical Coherence Tomography in Patients with Alzheimer’s Disease: What Can It Tell Us? Eye Brain 2021, 13, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Ries, F.; Horn, R.; Hillekamp, J.; Honisch, C.; König, M.; Solymosi, L. Differentiation of Multi-Infarct and Alzheimer Dementia by Intracranial Hemodynamic Parameters. Stroke 1993, 24, 228–235. [Google Scholar] [CrossRef]
- Snyder, H.M.; Carrillo, M.C.; Grodstein, F.; Henriksen, K.; Jeromin, A.; Lovestone, S.; Mielke, M.M.; O’Bryant, S.; Sarasa, M.; Sj⊘gren, M.; et al. Developing Novel Blood-based Biomarkers for Alzheimer’s Disease. Alzheimers Dement. 2014, 10, 109–114. [Google Scholar] [CrossRef]
- Cheung, C.Y.; Ong, Y.T.; Ikram, M.K.; Ong, S.Y.; Li, X.; Hilal, S.; Catindig, J.S.; Venketasubramanian, N.; Yap, P.; Seow, D.; et al. Microvascular Network Alterations in the Retina of Patients with Alzheimer’s Disease. Alzheimers Dement. 2014, 10, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Kesler, A.; Vakhapova, V.; Korczyn, A.D.; Naftaliev, E.; Neudorfer, M. Retinal Thickness in Patients with Mild Cognitive Impairment and Alzheimer’s Disease. Clin. Neurol. Neurosurg. 2011, 113, 523–526. [Google Scholar] [CrossRef]
- Jindal, V. Interconnection Between Brain and Retinal Neurodegenerations. Mol. Neurobiol. 2015, 51, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Feke, G.T.; Hyman, B.T.; Stern, R.A.; Pasquale, L.R. Retinal Blood Flow in Mild Cognitive Impairment and Alzheimer’s Disease. Alzheimers Dement. Diagn. Assess. Dis. Monit. 2015, 1, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Vujosevic, S.; Cunha-Vaz, J.; Figueira, J.; Löwenstein, A.; Midena, E.; Parravano, M.; Scanlon, P.H.; Simó, R.; Hernández, C.; Madeira, M.H.; et al. Standardization of Optical Coherence Tomography Angiography Imaging Biomarkers in Diabetic Retinal Disease. Ophthalmic Res. 2021, 64, 871–887. [Google Scholar] [CrossRef]
- Elnahry, A.G.; Noureldine, A.; Abdel-Kader, A.A.; Sorour, O.; Ramsey, D.J. Optical Coherence Tomography Angiography Biomarkers Predict Anatomical Response to Bevacizumab in Diabetic Macular Edema. Diabetes Metab. Syndr. Obes. Targets Ther. 2022, 15, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Hein, M.; Vukmirovic, A.; Constable, I.J.; Raja, V.; Athwal, A.; Freund, K.B.; Balaratnasingam, C. Angiographic Biomarkers Are Significant Predictors of Treatment Response to Intravitreal Aflibercept in Diabetic Macular Edema. Sci. Rep. 2023, 13, 8128. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.Y.; Park, S.E.; Lee, S.C.; Koh, H.J.; Kim, S.S.; Byeon, S.H.; Kim, M. Association Between Clinical Biomarkers and Optical Coherence Tomography Angiography Parameters in Type 2 Diabetes Mellitus. Investig. Opthalmol. Vis. Sci. 2020, 61, 4. [Google Scholar] [CrossRef]
- Salehi, M.A.; Rezagholi, F.; Mohammadi, S.; Zakavi, S.S.; Jahanshahi, A.; Gouravani, M.; Yazdanpanah, G.; Seddon, I.; Jabbehdari, S.; Singh, R.P. Optical Coherence Tomography Angiography Measurements in Parkinson’s Disease: A Systematic Review and Meta-Analysis. Eye 2023, 37, 3145–3156. [Google Scholar] [CrossRef]
- Vautier, A.; Lebreton, A.L.; Codron, P.; Awada, Z.; Gohier, P.; Cassereau, J. Retinal Vessels as a Window on Amyotrophic Lateral Sclerosis Pathophysiology: A Systematic Review. Rev. Neurol. 2023, 179, 548–562. [Google Scholar] [CrossRef]
- Chalkias, I.-N.; Bakirtzis, C.; Pirounides, D.; Boziki, M.K.; Grigoriadis, N. Optical Coherence Tomography and Optical Coherence Tomography with Angiography in Multiple Sclerosis. Healthcare 2022, 10, 1386. [Google Scholar] [CrossRef]
- O’Bryhim, B.E.; Lin, J.B.; Van Stavern, G.P.; Apte, R.S. OCT Angiography Findings in Preclinical Alzheimer’s Disease: 3-Year Follow-Up. Ophthalmology 2021, 128, 1489–1491. [Google Scholar] [CrossRef] [PubMed]
- Bulut, M.; Kurtuluş, F.; Gözkaya, O.; Erol, M.K.; Cengiz, A.; Akıdan, M.; Yaman, A. Evaluation of Optical Coherence Tomography Angiographic Findings in Alzheimer’s Type Dementia. Br. J. Ophthalmol. 2018, 102, 233–237. [Google Scholar] [CrossRef]
- Yoon, S.P.; Grewal, D.S.; Thompson, A.C.; Polascik, B.W.; Dunn, C.; Burke, J.R.; Fekrat, S. Retinal Microvascular and Neurodegenerative Changes in Alzheimer’s Disease and Mild Cognitive Impairment Compared with Control Participants. Ophthalmol. Retina 2019, 3, 489–499. [Google Scholar] [CrossRef]
- Lahme, L.; Esser, E.L.; Mihailovic, N.; Schubert, F.; Lauermann, J.; Johnen, A.; Eter, N.; Duning, T.; Alnawaiseh, M. Evaluation of Ocular Perfusion in Alzheimer’s Disease Using Optical Coherence Tomography Angiography. J. Alzheimers Dis. 2018, 66, 1745–1752. [Google Scholar] [CrossRef] [PubMed]
- Zabel, P.; Kaluzny, J.J.; Wilkosc-Debczynska, M.; Gebska-Toloczko, M.; Suwala, K.; Zabel, K.; Zaron, A.; Kucharski, R.; Araszkiewicz, A. Comparison of Retinal Microvasculature in Patients with Alzheimer’s Disease and Primary Open-Angle Glaucoma by Optical Coherence Tomography Angiography. Investig. Opthalmol. Vis. Sci. 2019, 60, 3447. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, X.; Azhati, G.; Li, T.; Xu, G.; Liu, F. Retinal Microvascular Attenuation in Mental Cognitive Impairment and Alzheimer’s Disease by Optical Coherence Tomography Angiography. Acta Ophthalmol. 2020, 98, 781–787. [Google Scholar] [CrossRef]
- Chua, J.; Hu, Q.; Ke, M.; Tan, B.; Hong, J.; Yao, X.; Hilal, S.; Venketasubramanian, N.; Garhöfer, G.; Cheung, C.Y.; et al. Retinal Microvasculature Dysfunction Is Associated with Alzheimer’s Disease and Mild Cognitive Impairment. Alzheimers Res. Ther. 2020, 12, 161. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, Q.; Tao, R.; Lu, H.; Xiao, Z.; Zheng, L.; Ding, D.; Ding, S.; Ma, Y.; Lu, Z.; et al. Decreased Retinal Vascular Density in Alzheimer’s Disease (AD) and Mild Cognitive Impairment (MCI): An Optical Coherence Tomography Angiography (OCTA) Study. Front. Aging Neurosci. 2021, 12, 572484. [Google Scholar] [CrossRef]
- Xie, J.; Yi, Q.; Wu, Y.; Zheng, Y.; Liu, Y.; Macerollo, A.; Fu, H.; Xu, Y.; Zhang, J.; Behera, A.; et al. Deep Segmentation of OCTA for Evaluation and Association of Changes of Retinal Microvasculature with Alzheimer’s Disease and Mild Cognitive Impairment. Br. J. Ophthalmol. 2023, 108, 423–439. [Google Scholar] [CrossRef]
- Querques, G.; Borrelli, E.; Sacconi, R.; De Vitis, L.; Leocani, L.; Santangelo, R.; Magnani, G.; Comi, G.; Bandello, F. Functional and Morphological Changes of the Retinal Vessels in Alzheimer’s Disease and Mild Cognitive Impairment. Sci. Rep. 2019, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Den Haan, J.; Van De Kreeke, J.A.; Van Berckel, B.N.; Barkhof, F.; Teunissen, C.E.; Scheltens, P.; Verbraak, F.D.; Bouwman, F.H. Is Retinal Vasculature a Biomarker in Amyloid Proven Alzheimer’s Disease? Alzheimers Dement. Diagn. Assess. Dis. Monit. 2019, 11, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Foulsham, W.; Chien, J.; Lenis, T.L.; Papakostas, T.D. Optical Coherence Tomography Angiography: Clinical Utility and Future Directions. J. Vitreoretin. Dis. 2022, 6, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Kraker, J.A.; Omoba, B.S.; Cava, J.A.; Gilat Schmidt, T.; Chui, T.Y.; Rosen, R.B.; Kim, J.E.; Carroll, J.; Linderman, R.E. Assessing the Influence of OCT-A Device and Scan Size on Retinal Vascular Metrics. Transl. Vis. Sci. Technol. 2020, 9, 7. [Google Scholar] [CrossRef]
- Savastano, M.C.; Rispoli, M.; Lumbroso, B.; Di Antonio, L.; Mastropasqua, L.; Virgili, G.; Savastano, A.; Bacherini, D.; Rizzo, S. Fluorescein Angiography versus Optical Coherence Tomography Angiography: FA vs OCTA Italian Study. Eur. J. Ophthalmol. 2021, 31, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Lipson, B.K.; Yannuzzi, L.A. Complications of intravenous fluorescein injections. Int. Ophthalmol. Clin. 1989, 29, 200–205. [Google Scholar] [CrossRef] [PubMed]
- De Carlo, T.E.; Romano, A.; Waheed, N.K.; Duker, J.S. A Review of Optical Coherence Tomography Angiography (OCTA). Int. J. Retin. Vitr. 2015, 1, 5. [Google Scholar] [CrossRef] [PubMed]
- Greig, E.C.; Duker, J.S.; Waheed, N.K. A Practical Guide to Optical Coherence Tomography Angiography Interpretation. Int. J. Retin. Vitr. 2020, 6, 55. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Swanson, E.A.; Lin, C.P.; Schuman, J.S.; Stinson, W.G.; Chang, W.; Hee, M.R.; Flotte, T.; Gregory, K.; Puliafito, C.A.; et al. Optical Coherence Tomography. Science 1991, 254, 1178–1181. [Google Scholar] [CrossRef]
- Choi, W.J. Imaging Motion: A Comprehensive Review of Optical Coherence Tomography Angiography. In Advanced Imaging and Bio Techniques for Convergence Science; Kim, J.K., Kim, J.K., Pack, C.-G., Eds.; Advances in Experimental Medicine and Biology; Springer: Singapore, 2021; Volume 1310, pp. 343–365. ISBN 978-981-336-063-1. [Google Scholar]
- Spaide, R.F.; Fujimoto, J.G.; Waheed, N.K.; Sadda, S.R.; Staurenghi, G. Optical Coherence Tomography Angiography. Prog. Retin. Eye Res. 2018, 64, 1–55. [Google Scholar] [CrossRef]
- Kashani, A.H.; Chen, C.-L.; Gahm, J.K.; Zheng, F.; Richter, G.M.; Rosenfeld, P.J.; Shi, Y.; Wang, R.K. Optical Coherence Tomography Angiography: A Comprehensive Review of Current Methods and Clinical Applications. Prog. Retin. Eye Res. 2017, 60, 66–100. [Google Scholar] [CrossRef]
- Kim, D.Y.; Fingler, J.; Zawadzki, R.J.; Park, S.S.; Morse, L.S.; Schwartz, D.M.; Fraser, S.E.; Werner, J.S. Optical Imaging of the Chorioretinal Vasculature in the Living Human Eye. Proc. Natl. Acad. Sci. USA 2013, 110, 14354–14359. [Google Scholar] [CrossRef]
- Koutsiaris, A.G.; Batis, V.; Liakopoulou, G.; Tachmitzi, S.V.; Detorakis, E.T.; Tsironi, E.E. Optical Coherence Tomography Angiography (OCTA) of the Eye: A Review on Basic Principles, Advantages, Disadvantages and Device Specifications. Clin. Hemorheol. Microcirc. 2023, 83, 247–271. [Google Scholar] [CrossRef]
- Hagag, A.; Gao, S.; Jia, Y.; Huang, D. Optical Coherence Tomography Angiography: Technical Principles and Clinical Applications in Ophthalmology. Taiwan J. Ophthalmol. 2017, 7, 115. [Google Scholar] [CrossRef]
- Ting, D.S.; Cheung, G.C.; Lim, L.S.; Yeo, I.Y. Comparison of Swept Source Optical Coherence Tomography and Spectral Domain Optical Coherence Tomography in Polypoidal Choroidal Vasculopathy. Clin. Experiment. Ophthalmol. 2015, 43, 815–819. [Google Scholar] [CrossRef]
- Cicinelli, M.V.; Cavalleri, M.; Consorte, A.C.; Rabiolo, A.; Sacconi, R.; Bandello, F.; Querques, G. Swept-source and spectral domain optical coherence tomography angiography versus dye angiography in the measurement of type 1 neovascularization. Retina 2020, 40, 499–506. [Google Scholar] [CrossRef]
- Munk, M.R.; Giannakaki-Zimmermann, H.; Berger, L.; Huf, W.; Ebneter, A.; Wolf, S.; Zinkernagel, M.S. OCT-Angiography: A Qualitative and Quantitative Comparison of 4 OCT-A Devices. PLoS ONE 2017, 12, e0177059. [Google Scholar] [CrossRef]
- Agemy, S.A.; Scripsema, N.K.; Shah, C.M.; Chui, T.; Garcia, P.M.; Lee, J.G.; Gentile, R.C.; Hsiao, Y.-S.; Zhou, Q.; Ko, T.; et al. Retinal vascular perfusion density mapping using optical coherence tomography angiography in normals and diabetic retinopathy patients. Retina 2015, 35, 2353–2363. [Google Scholar] [CrossRef]
- Huang, D.; Jia, Y.; Gao, S.S.; Lumbroso, B.; Rispoli, M. Optical Coherence Tomography Angiography Using the Optovue Device. In Developments in Ophthalmology; Bandello, F., Souied, E.H., Querques, G., Eds.; S. Karger AG: Basel, Switzerland, 2016; Volume 56, pp. 6–12. ISBN 978-3-318-05829-1. [Google Scholar]
- Rosenfeld, P.J.; Durbin, M.K.; Roisman, L.; Zheng, F.; Miller, A.; Robbins, G.; Schaal, K.B.; Gregori, G. ZEISS AngioplexTM Spectral Domain Optical Coherence Tomography Angiography: Technical Aspects. In Developments in Ophthalmology; Bandello, F., Souied, E.H., Querques, G., Eds.; S. Karger AG: Basel, Switzerland, 2016; Volume 56, pp. 18–29. ISBN 978-3-318-05829-1. [Google Scholar]
- Kim, A.Y.; Rodger, D.C.; Shahidzadeh, A.; Chu, Z.; Koulisis, N.; Burkemper, B.; Jiang, X.; Pepple, K.L.; Wang, R.K.; Puliafito, C.A.; et al. Quantifying Retinal Microvascular Changes in Uveitis Using Spectral-Domain Optical Coherence Tomography Angiography. Am. J. Ophthalmol. 2016, 171, 101–112. [Google Scholar] [CrossRef]
- Lavia, C.; Bonnin, S.; Maule, M.; Erginay, A.; Tadayoni, R.; Gaudric, A. Vessel density of superficial, intermediate, and deep capillary plexuses using optical coherence tomography angiography. Retina 2019, 39, 247–258. [Google Scholar] [CrossRef]
- Tsokolas, G.; Tsaousis, K.T.; Diakonis, V.F.; Matsou, A.; Tyradellis, S. Optical Coherence Tomography Angiography in Neurodegenerative Diseases: A Review. Eye Brain 2020, 12, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Panigrahi, P.K. Foveal Avascular Zone Area Measurement Using OCT Angiography. Photodiagnosis Photodyn. Ther. 2023, 43, 103717. [Google Scholar] [CrossRef] [PubMed]
- Anvari, P.; Najafi, A.; Mirshahi, R.; Sardarinia, M.; Ashrafkhorasani, M.; Kazemi, P.; Aghai, G.; Habibi, A.; Falavarjani, K.G. Comparison of Superficial and Deep Foveal Avascular Zone Area in Healthy Subjects Using Two Spectral Domain Optical Coherence Tomography Angiography Devices. J. Ophthalmic Vis. Res. 2020, 15, 517. [Google Scholar] [CrossRef] [PubMed]
- Dorr, A.; Sahota, B.; Chinta, L.V.; Brown, M.E.; Lai, A.Y.; Ma, K.; Hawkes, C.A.; McLaurin, J.; Stefanovic, B. Amyloid-β-Dependent Compromise of Microvascular Structure and Function in a Model of Alzheimer’s Disease. Brain 2012, 135, 3039–3050. [Google Scholar] [CrossRef] [PubMed]
- Patton, N.; Aslam, T.; MacGillivray, T.; Pattie, A.; Deary, I.J.; Dhillon, B. Retinal Vascular Image Analysis as a Potential Screening Tool for Cerebrovascular Disease: A Rationale Based on Homology between Cerebral and Retinal Microvasculatures. J. Anat. 2005, 206, 319–348. [Google Scholar] [CrossRef] [PubMed]
- Olafsdottir, O.B.; Saevarsdottir, H.S.; Hardarson, S.H.; Hannesdottir, K.H.; Traustadottir, V.D.; Karlsson, R.A.; Einarsdottir, A.B.; Jonsdottir, K.D.; Stefansson, E.; Snaedal, J. Retinal Oxygen Metabolism in Patients with Mild Cognitive Impairment. Alzheimers Dement. Diagn. Assess. Dis. Monit. 2018, 10, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Samara, W.A.; Say, E.A.T.; Khoo, C.T.L.; Higgins, T.P.; Magrath, G.; Ferenczy, S.; Shields, C.L. Correlation of foveal avascular zone size with foveal morphology in normal eyes using optical coherence tomography angiography. Retina 2015, 35, 2188–2195. [Google Scholar] [CrossRef]
- O’Bryhim, B.E.; Apte, R.S.; Kung, N.; Coble, D.; Van Stavern, G.P. Association of Preclinical Alzheimer Disease with Optical Coherence Tomographic Angiography Findings. JAMA Ophthalmol. 2018, 136, 1242. [Google Scholar] [CrossRef]
- La Morgia, C.; Di Vito, L.; Carelli, V.; Carbonelli, M. Patterns of Retinal Ganglion Cell Damage in Neurodegenerative Disorders: Parvocellular vs Magnocellular Degeneration in Optical Coherence Tomography Studies. Front. Neurol. 2017, 8, 710. [Google Scholar] [CrossRef]
- López-de-Eguileta, A.; López-García, S.; Lage, C.; Pozueta, A.; García-Martínez, M.; Kazimierczak, M.; Bravo, M.; Irure, J.; López-Hoyos, M.; Muñoz-Cacho, P.; et al. The Retinal Ganglion Cell Layer Reflects Neurodegenerative Changes in Cognitively Unimpaired Individuals. Alzheimers Res. Ther. 2022, 14, 57. [Google Scholar] [CrossRef]
- Tsai, J.; Asanad, S.; Whiting, M.; Zhang, X.; Magder, L.; Saeedi, O. Repeatability and Comparability of Retinal Blood Vessel Caliber Measurements by OCTA. Vision 2023, 7, 48. [Google Scholar] [CrossRef]
- Lei, J.; Pei, C.; Wen, C.; Abdelfattah, N.S. Repeatability and Reproducibility of Quantification of Superficial Peri-Papillary Capillaries by Four Different Optical Coherence Tomography Angiography Devices. Sci. Rep. 2018, 8, 17866. [Google Scholar] [CrossRef] [PubMed]
- Corvi, F.; Pellegrini, M.; Erba, S.; Cozzi, M.; Staurenghi, G.; Giani, A. Reproducibility of Vessel Density, Fractal Dimension, and Foveal Avascular Zone Using 7 Different Optical Coherence Tomography Angiography Devices. Am. J. Ophthalmol. 2018, 186, 25–31. [Google Scholar] [CrossRef] [PubMed]
- De Vitis, L.A.; Benatti, L.; Tomasso, L.; Baldin, G.; Carnevali, A.; Querques, L.; Querques, G.; Bandello, F. Comparison of the Performance of Two Different Spectral-Domain Optical Coherence Tomography Angiography Devices in Clinical Practice. Ophthalmic Res. 2016, 56, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Salobrar-Garcia, E.; Méndez-Hernández, C.; Hoz, R.D.; Ramírez, A.I.; López-Cuenca, I.; Fernández-Albarral, J.A.; Rojas, P.; Wang, S.; García-Feijoo, J.; Gil, P.; et al. Ocular Vascular Changes in Mild Alzheimer’s Disease Patients: Foveal Avascular Zone, Choroidal Thickness, and ONH Hemoglobin Analysis. J. Pers. Med. 2020, 10, 231. [Google Scholar] [CrossRef]
- Zheng, F.; Deng, X.; Zhang, Q.; He, J.; Ye, P.; Liu, S.; Li, P.; Zhou, J.; Fang, X. Advances in Swept-Source Optical Coherence Tomography and Optical Coherence Tomography Angiography. Adv. Ophthalmol. Pract. Res. 2023, 3, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.R.; Roisman, L.; Zhang, Q.; Zheng, F.; Rafael De Oliveira Dias, J.; Yehoshua, Z.; Schaal, K.B.; Feuer, W.; Gregori, G.; Chu, Z.; et al. Comparison Between Spectral-Domain and Swept-Source Optical Coherence Tomography Angiographic Imaging of Choroidal Neovascularization. Investig. Opthalmol. Vis. Sci. 2017, 58, 1499. [Google Scholar] [CrossRef] [PubMed]
- Chalkias, E.; Chalkias, I.-N.; Bakirtzis, C.; Messinis, L.; Nasios, G.; Ioannidis, P.; Pirounides, D. Differentiating Degenerative from Vascular Dementia with the Help of Optical Coherence Tomography Angiography Biomarkers. Healthcare 2022, 10, 539. [Google Scholar] [CrossRef]
- Rifai, O.M.; McGrory, S.; Robbins, C.B.; Grewal, D.S.; Liu, A.; Fekrat, S.; MacGillivray, T.J. The Application of Optical Coherence Tomography Angiography in Alzheimer’s Disease: A Systematic Review. Alzheimers Dement. Diagn. Assess. Dis. Monit. 2021, 13, e12149. [Google Scholar] [CrossRef]
- Linderman, R.E.; Muthiah, M.N.; Omoba, S.B.; Litts, K.M.; Tarima, S.; Visotcky, A.; Kim, J.E.; Carroll, J. Variability of Foveal Avascular Zone Metrics Derived from Optical Coherence Tomography Angiography Images. Transl. Vis. Sci. Technol. 2018, 7, 20. [Google Scholar] [CrossRef]
- Verma, A.; Magesan, K.; Amose, T.; Alagorie, A.R.; Gnanaraj, R.; Sadda, S.R.; Sen, P. Age-Related Assessment of Foveal Avascular Zone and Surrounding Capillary Networks with Swept Source Optical Coherence Tomography Angiography in Healthy Eyes. Eye 2022, 36, 1857–1864. [Google Scholar] [CrossRef] [PubMed]
- Abay, R.N.; Akdeniz, G.Ş.; Katipoğlu, Z.; Kerimoğlu, H. Normative Data Assessment of Age-Related Changes in Macular and Optic Nerve Head Vessel Density Using Optical Coherence Tomography Angiography. Photodiagn. Photodyn. Ther. 2022, 37, 102624. [Google Scholar] [CrossRef]
- You, Q.S.; Chan, J.C.H.; Ng, A.L.K.; Choy, B.K.N.; Shih, K.C.; Cheung, J.J.C.; Wong, J.K.W.; Shum, J.W.H.; Ni, M.Y.; Lai, J.S.M.; et al. Macular Vessel Density Measured with Optical Coherence Tomography Angiography and Its Associations in a Large Population-Based Study. Investig. Opthalmol. Vis. Sci. 2019, 60, 4830. [Google Scholar] [CrossRef]
- Duits, F.H.; Wesenhagen, K.E.J.; Ekblad, L.; Wolters, E.; Willemse, E.A.J.; Scheltens, P.; Van Der Flier, W.M.; Teunissen, C.E.; Visser, P.J.; Tijms, B.M. Four Subgroups Based on Tau Levels in Alzheimer’s Disease Observed in Two Independent Cohorts. Alzheimers Res. Ther. 2021, 13, 2. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, A.; Morizane, Y.; Hosokawa, M.; Kimura, S.; Shiode, Y.; Hirano, M.; Doi, S.; Toshima, S.; Takahashi, K.; Hosogi, M.; et al. Factors Affecting Foveal Avascular Zone in Healthy Eyes: An Examination Using Swept-Source Optical Coherence Tomography Angiography. PLoS ONE 2017, 12, e0188572. [Google Scholar] [CrossRef] [PubMed]
- Ghassemi, F.; Mirshahi, R.; Bazvand, F.; Fadakar, K.; Faghihi, H.; Sabour, S. The Quantitative Measurements of Foveal Avascular Zone Using Optical Coherence Tomography Angiography in Normal Volunteers. J. Curr. Ophthalmol. 2017, 29, 293–299. [Google Scholar] [CrossRef]
- 2021 Alzheimer’s Disease Facts and Figures. Alzheimers Dement. 2021, 17, 327–406. [CrossRef]
- Zhang, J.; Tang, F.Y.; Cheung, C.Y.; Chen, H. Different Effect of Media Opacity on Vessel Density Measured by Different Optical Coherence Tomography Angiography Algorithms. Transl. Vis. Sci. Technol. 2020, 9, 19. [Google Scholar] [CrossRef]
- Yoshikawa, Y.; Shoji, T.; Kanno, J.; Ibuki, H.; Ozaki, K.; Ishii, H.; Inami, H.; Shinoda, K. Examination of Age-Related Retinal Vascular Changes in the Macula Using Optical Coherence Tomography Angiography of the Eyes After Cataract Surgery. Clin. Ophthalmol. 2021, 15, 3687–3695. [Google Scholar] [CrossRef]
- Garrity, S.T.; Iafe, N.A.; Phasukkijwatana, N.; Chen, X.; Sarraf, D. Quantitative Analysis of Three Distinct Retinal Capillary Plexuses in Healthy Eyes Using Optical Coherence Tomography Angiography. Investig. Opthalmol. Vis. Sci. 2017, 58, 5548. [Google Scholar] [CrossRef]
- Taylor, T.R.P.; Menten, M.J.; Rueckert, D.; Sivaprasad, S.; Lotery, A.J. The Role of the Retinal Vasculature in Age-Related Macular Degeneration: A Spotlight on OCTA. Eye 2024, 38, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Coscas, F.; Sellam, A.; Glacet-Bernard, A.; Jung, C.; Goudot, M.; Miere, A.; Souied, E.H. Normative Data for Vascular Density in Superficial and Deep Capillary Plexuses of Healthy Adults Assessed by Optical Coherence Tomography Angiography. Investig. Opthalmol. Vis. Sci. 2016, 57, OCT211. [Google Scholar] [CrossRef] [PubMed]
| Device | Optovue | Zeiss |
|---|---|---|
| Segmentation for Superficial Vessel Complex | A: 3 µm beneath ILM B: 15 µm beneath IPL | Upper 60% → ILM up to outer boundary of IPL [58] |
| Segmentation for Deep Vessel Complex | A: 15 µm beneath IPL B: 70 µm beneath OPL | Lower 40% → Inner boundary of IPL up to the outer boundary of OPL [58] |
| Authors | Participants | OCT-A Device | Segmentation Software | Scan Area mm2 | Parameters | SVC | DVC |
|---|---|---|---|---|---|---|---|
| O’Bryhim et al. [32] | 14 AD 16 control | RTVue XR Avanti | AngioVue | 8 × 8 | FAZ | FAZ ↑ | FAZ ↑ |
| Bulut et al. [33] | 26 AD 26 control | RTVue XR 100–2 | AngioVue | 6 × 6 | VD, FAZ | VD ↓ in AD FAZ ↑ in AD | Not analyzed |
| Yoon et al. [34] | 39 AD 133 control | Cirrus HD- 5000 | AngioPlex | 3 × 3 6 × 6 | VD, FAZ | VD ↓ in AD, no significant difference in FAZ | Not analyzed |
| Lahme et al. [35] | 36 AD 38 control | RTVue XR Avanti | AngioVue | 3 × 3 | VD | VD ↓ in AD, no significant difference in FAZ | No significant difference in VD and FAZ |
| Zabel et al. [36] | 27 AD 27 control | RTVue XR Avanti | AngioVue | 6 × 6 | VD, FAZ | No significant difference | VD ↓ in AD FA ↑ in AD |
| Wu et al. [37] | 18 AD 21 control | RTVue XR Avanti | AngioVue | 6 × 6 | VD, FAZ | VD ↓ in AD in one sector FA ↑ in AD | VD ↓ in AD FA ↑ in AD |
| Chua et al. [38] | 24 AD | Cirrus HD- 5000 | AngioPlex | 3 × 3 | VD, FAZ | VD ↓ in AD, no significant difference in FAZ | VD ↓ in AD No significant difference in FAZ |
| Wang et al. [39] | 62 AD 49 control | RTVue XR Avanti | AngioVue | 3 × 3 | VD, FAZ | VD ↓in AD, no difference in FAZ | No significant difference |
| Xie et al. [40] | 55 AD 62 control | RTVue XR Avanti | AngioVue | 3 × 3 6 × 6 | VD, FAZ, other microvascular changes (e.g., vascular bifurcations, tortuosity, etc.) | No significant difference | No significant difference |
| Querques et al. [41] | 12 AD 32 control | Cirrus HD- 5000 | AngioPlex | 3 × 3 6 × 6 | Perfusion density | No significant difference | No significant difference |
| den Haan et al. [42] | 48 AD 38 control | Cirrus HD- 5000 | AngioPlex | 6 × 6 | VD, FAZ | No significant difference | No significant difference |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vagiakis, I.; Bakirtzis, C.; Andravizou, A.; Pirounides, D. Unlocking the Potential of Vessel Density and the Foveal Avascular Zone in Optical Coherence Tomography Angiography as Biomarkers in Alzheimer’s Disease. Healthcare 2024, 12, 1589. https://doi.org/10.3390/healthcare12161589
Vagiakis I, Bakirtzis C, Andravizou A, Pirounides D. Unlocking the Potential of Vessel Density and the Foveal Avascular Zone in Optical Coherence Tomography Angiography as Biomarkers in Alzheimer’s Disease. Healthcare. 2024; 12(16):1589. https://doi.org/10.3390/healthcare12161589
Chicago/Turabian StyleVagiakis, Iordanis, Christos Bakirtzis, Athina Andravizou, and Demetrios Pirounides. 2024. "Unlocking the Potential of Vessel Density and the Foveal Avascular Zone in Optical Coherence Tomography Angiography as Biomarkers in Alzheimer’s Disease" Healthcare 12, no. 16: 1589. https://doi.org/10.3390/healthcare12161589






