Verification of Nasogastric Tube Positioning Using Ultrasound by an Intensive Care Nurse: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Setting
2.3. Participants
2.4. Variables
2.5. Data Collection
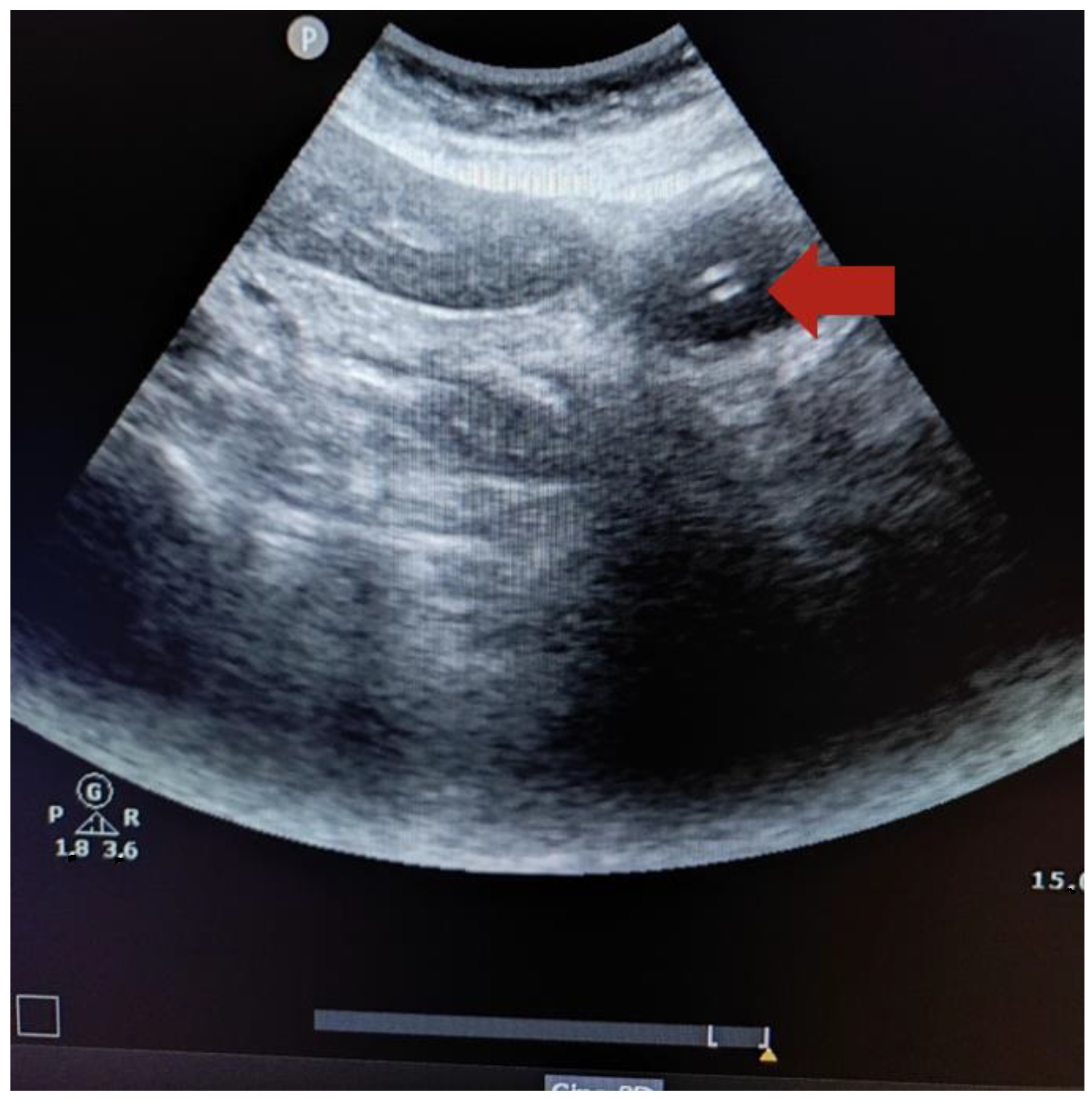
2.6. Technique to Perform the Ultrasound
2.7. Data Analysis
2.8. Ethical Considerations
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aragonés Manzanares, R.; Rincón Ferrari, M.D. Manual de Cuidados Intensivos para Enfermería; Editorial Médica Panamericana: Madrid, Spain, 2020. [Google Scholar]
- Singer, P.; Blaser, A.R.; Berger, M.M.; Calder, P.C.; Casaer, M.; Hiesmayr, M.; Mayer, K.; Montejo-Gonzalez, J.C.; Pichard, C.; Preiser, J.C.; et al. ESPEN practical and partially revised guideline: Clinical nutrition in the intensive care unit. Clin. Nutr. 2023, 42, 1671–1689. [Google Scholar] [CrossRef] [PubMed]
- Compher, C.; Bingham, A.L.; McCall, M.; Patel, J.; Rice, T.W.; Braunschweig, C.; McKeever, L. Guidelines for the provision of nutrition support therapy in the adult critically ill patient: The American Society for Parenteral and Enteral Nutrition. J. Parenter. Enter. Nutr. 2022, 46, 12–41. [Google Scholar] [CrossRef] [PubMed]
- Bechtold, M.L.; Brown, P.M.; Escuro, A.; Grenda, B.; Johnston, T.; Kozeniecki, M.; Limketkai, B.N.; Nelson, K.K.; Powers, J.; Ronan, A.; et al. When is enteral nutrition indicated? JPEN J. Parenter. Enter. Nutr. 2022, 46, 1470–1496. [Google Scholar] [CrossRef] [PubMed]
- Motta, A.P.G.; Rigobello, M.C.G.; Silveira, R.C.C.P.; Gimenes, F.R.E. Nasogastric/nasoenteric tube-related adverse events: An integrative review. Rev. Lat. Am. Enferm. 2021, 8, e3400. [Google Scholar] [CrossRef] [PubMed]
- Sparks, D.A.; Chase, D.M.; Coughlin, L.M.; Perry, E. Pulmonary complications of 9931 narrow-bore nasoenteric tubes during blind placement: A critical review. J. Parenter. Enter. Nutr. 2011, 35, 625–629. [Google Scholar] [CrossRef] [PubMed]
- Boeykens, K.; Holvoet, T.; Duysburgh, I. Nasogastric tube insertion length measurement and tip verification in adults: A narrative review. Crit. Care 2023, 27, 317. [Google Scholar] [CrossRef]
- Taylor, S.; Manara, A.R. X-ray checks of NG tube position: A case for guided tube placement. Br. J. Radiol. 2021, 94, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kent, K.M. Adult CCRN® Certification Review Think in Questions, Learn by Rationales, 2nd ed.; Springer Publishing Company: New York, NY, USA, 2021. [Google Scholar]
- McClave, S.A.; Dibaise, J.K.; Mullin, G.E.; Martindale, R.G. ACG clinical guideline: Nutrition therapy in the adult hospitalized patient. Am. J. Gastroenterol. 2016, 111, 315–334. [Google Scholar] [CrossRef] [PubMed]
- Metheny, N.A.; Krieger, M.M.; Healey, F.; Meert, K.L. A review of guidelines to distinguish between gastric and pulmonary placement of nasogastric tubes. Heart Lung 2019, 48, 226–235. [Google Scholar] [CrossRef]
- Bourgault, A.M.; Halm, M.A. Feeding tube placement in adults: Safe verification method for blindly inserted tubes. Am. J. Crit. Care 2009, 18, 73–76. [Google Scholar] [CrossRef]
- Judd, M. Confirming nasogastric tube placement in adults. Nursing 2020, 50, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Rowat, A.M.; Graham, C.; Dennis, M. Study to determine the likely accuracy of pH testing to confirm nasogastric tube placement. BMJ Open Gastroenterol. 2018, 5, e000211. [Google Scholar] [CrossRef] [PubMed]
- Bloom, L.; Seckel, M.A. Placement of nasogastric feeding tube and postinsertion care review. AACN Adv. Crit. Care 2022, 33, 68–84. [Google Scholar] [CrossRef] [PubMed]
- Powers, J.; Luebbehusen, M.; Aguirre, L.; Cluff, J.; David, M.A.; Holly, V.; Linford, L.; Park, N.; Brunelle, R. Improved safety and efficacy of small-bore feeding tube confirmation using an electromagnetic placement device. Nutr. Clin. Pract. 2018, 33, 268–273. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Biomedical Imaging and Bioengineering. X-rays. Available online: https://www.nibib.nih.gov/science-education/science-topics/x-rays (accessed on 21 April 2024).
- Poggio, G.A.; Mariano, J.; Gipar, L.A.; Ucar, M.E. La ecografía primero: ¿Por qué, cómo y cuándo? Rev. Argent. Radiol. 2017, 81, 192–203. [Google Scholar] [CrossRef]
- Atalay, Y.O.; Aydin, R.; Ertugrul, O.; Gul, S.B.; Polat, A.V.; Paksu, M.S. Does bedside sonography effectively identify nasogastric tube placements in pediatric critical care patients? Nutr. Clin. Pract. 2016, 31, 805–809. [Google Scholar] [CrossRef] [PubMed]
- Chenaitia, H.; Brun, P.M.; Querellou, E.; Leyral, J.; Bessereau, J.; Aimé, C.; Bouaziz, R.; Georges, A.; Louis, F.; WINFOCUS Group France. Ultrasound to confirm gastric tube placement in prehospital management. Resuscitation 2012, 83, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Tai, P.; Lau, W.S.; Chan, P.Y.; Ng, S.Y.; Lam, Y.C.; Mak, H.T.; Mak, Y.T. Nurse performed ultrasonography in confirming the position of nasogastric tube in the emergency department: A prospective single group diagnostic. Hong Kong J. Emerg. Med. 2016, 23, 340–349. [Google Scholar] [CrossRef]
- Kim, H.M.; So, B.H.; Jeong, W.J.; Choi, S.M.; Park, K.N. The effectiveness of ultrasonography in verifying the placement of a nasogastric tube in patients with low consciousness at an emergency center. Scand. J. Trauma Resusc. Emerg. Med. 2012, 20, 38. [Google Scholar] [CrossRef]
- Mumoli, N.; Vitale, J.; Pagnamenta, A.; Mastroiacovo, D.; Cei, M.; Pomero, F.; Giorgi-Pierfranceschi, M.; Giuntini, L.; Porta, C.; Capra, R.; et al. Bedside abdominal ultrasound in evaluating nasogastric tube placement: A multicenter, prospective, cohort study. Chest 2021, 159, 2366–2372. [Google Scholar] [CrossRef]
- McMullen, C.D.; Anstey, C.; Garrett, P.; Moore, J. Nasogastric tube placement under sonographic observation: A comparison study of ultrasound and chest radiography in mechanically ventilated patients. Aust. Crit. Care 2022, 35, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Zatelli, M.; Vezzali, N. 4-Point ultrasonography to confirm the correct position of the nasogastric tube in 114 critically ill patients. J. Ultrasound 2017, 20, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Brotfain, E.; Erblat, A.; Luft, P.; Elir, A.; Gruenbaum, B.F.; Livshiz-Riven, I.; Koyfman, A.; Fridrich, D.; Koyfman, L.; Friger, M.; et al. Nurse-performed ultrasound assessment of gastric residual volume and enteral nasogastric tube placement in the general intensive care unit. Intensive Crit. Care Nurs. 2022, 69, 103183. [Google Scholar] [CrossRef] [PubMed]
- Tsujimoto, Y.; Kataoka, Y.; Banno, M.; Anan, K.; Shiroshita, A.; Jujo, S. Ultrasonography for confirmation of gastric tube placement. Cochrane Database Syst. Rev. 2024, 7, CD012083. [Google Scholar] [CrossRef] [PubMed]
- Pita Fernández, S.; Pértegas Díaz, S. Guía: Pruebas diagnósticas: Sensibilidad y especificidad. Cad. Aten. Primaria 2003, 10, 120–124. Available online: https://www.fisterra.com/formacion/metodologia-investigacion/pruebas-diagnosticas-sensibilidad-especificidad/ (accessed on 6 May 2024).
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef] [PubMed]
- Brahee, D.D.; Ogedegbe, C.; Hassler, C.; Nyirenda, T.; Hazelwood, V.; Morchel, H.; Patel, R.S.; Feldman, J. Body mass index and abdominal ultrasound image quality: A pilot survey of sonographers. J. Diagn. Med. Sonogr. 2013, 29, 66–72. [Google Scholar] [CrossRef]
- Comay, Y.; Quint, E.; Bichovsky, Y.; Koyfman, L.; Osyntsov, A.; Acker, A.; Brotfain, E. Case Series: Nasogastric (NG) Feeding Tube Misplacement in Critically Ill Tracheostomized Patients. Case Rep. Clin. Med. 2020, 9, 399–407. [Google Scholar] [CrossRef]
- Wang, P.C.; Tseng, G.Y.; Yang, H.B.; Chou, K.C.; Chen, C.H. Inadvertent tracheobronchial placement of feeding tube in a mechanically ventilated patient. J. Chin. Med. Assoc. 2008, 71, 365–367. [Google Scholar] [CrossRef]
- Brun, P.; Chenaitia, H.; Lablanche, C.; Pradel, A. 2-Point Ultrasonography to confirm correct position of the gastric tube in prehospital setting. Mil. Med. 2014, 179, 959–963. [Google Scholar] [CrossRef]
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Duan, M.; Chen, X.; Qin, X.; Liang, Q.; Dong, W.; Zhang, Y.; Lin, J. A review of location methods of nasogastric tube in critically ill patients. Open J. Nurs. 2020, 10, 943–951. [Google Scholar] [CrossRef]
- Piton, G.; Parel, R.; Delabrousse, E.; Capellier, G. Echography for nasogastric tube placement verification. Eur. J. Clin. Nutr. 2017, 71, 669–670. [Google Scholar] [CrossRef] [PubMed]
- Mak, M.Y.; Tam, G. Ultrasonography for nasogastric tube placement verification: An additional reference. Br. J. Community Nurs. 2020, 25, 328–334. [Google Scholar] [CrossRef]
- American Association of Critical Care Nurses. AACN practice alert: Initial and ongoing verification of feeding tube placement in adults. Crit. Care Nurse 2017, 37, 100. Available online: https://www.aacn.org/clinical-resources/practice-alerts/initial-and-ongoing-verification-of-feeding-tube-placement-in-adults (accessed on 25 April 2024).
| Characteristics of the participating critically ill patients | Median | Interquartile Range | ||
| Age (years) | 65 | 24 | ||
| Weight (kg) | 75 | 23 | ||
| Height (cm) | 174 | 15 | ||
| Body mass index (kg/m2) | 26.17 | 7.4 | ||
| Reason for admission | Frequency (n) | Percentage (%) | ||
| Neurological disease | 12 | 52.2% | ||
| Heart surgery | 4 | 17.4% | ||
| Septic shock | 2 | 8.7% | ||
| Cardiorespiratory arrest | 2 | 8.7% | ||
| Autolytic attempt | 2 | 8.7% | ||
| Respiratory disease | 1 | 4.4% | ||
| Characteristics of the nasogastric tubes placed | Medical indication | Enteral nutrition | 18 | 78.3% |
| Gastric emptying | 4 | 17.4% | ||
| Gastric lavage | 1 | 4.4% | ||
| Number of lumens | Single-lumen tube | 18 | 78.3% | |
| Double-lumen tube | 5 | 22.7% | ||
| Type | Polyurethane feeding tube | 13 | 56.5% | |
| PVC Salem™ tube | 9 | 39.1% | ||
| PVC esophageal balloon | 1 | 4.4% | ||
| Caliber | 12-French gauge | 13 | 56.5% | |
| 14-French gauge | 10 | 43.5% |
| Ultrasound | X-ray | Total Ultrasoundn (%) | Sensitivity | Specificity | Positive Predictive Value | Negative Predictive Value | |||
|---|---|---|---|---|---|---|---|---|---|
| Correct Positioning (n) | Incorrect Positioning (n) | ||||||||
| Intensive care nurse | Direct visualization | Yes | 7 | 0 | 7 (30.4%) | 35% | 100% | 100% | 19% |
| No | 13 | 3 | 16 (69.6%) | ||||||
| Indirect visualization | Yes | 17 | 0 | 17 (73.9%) | 85% | 100% | 100% | 50% | |
| No | 3 | 3 | 6 (26.1) | ||||||
| Intensive care physician | Direct visualization | Yes | 7 | 0 | 7 (30.4%) | 35% | 100% | 100% | 19% |
| No | 13 | 3 | 16 (69.6%) | ||||||
| Indirect visualization | Yes | 18 | 0 | 18 (78.3%) | 86% | 100% | 100% | 60% | |
| No | 2 | 3 | 5 (21.7%) | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robles-González, M.; Arrogante, O.; Sánchez Giralt, J.A.; Ortuño-Soriano, I.; Zaragoza-García, I. Verification of Nasogastric Tube Positioning Using Ultrasound by an Intensive Care Nurse: A Pilot Study. Healthcare 2024, 12, 1618. https://doi.org/10.3390/healthcare12161618
Robles-González M, Arrogante O, Sánchez Giralt JA, Ortuño-Soriano I, Zaragoza-García I. Verification of Nasogastric Tube Positioning Using Ultrasound by an Intensive Care Nurse: A Pilot Study. Healthcare. 2024; 12(16):1618. https://doi.org/10.3390/healthcare12161618
Chicago/Turabian StyleRobles-González, María, Oscar Arrogante, Juan Antonio Sánchez Giralt, Ismael Ortuño-Soriano, and Ignacio Zaragoza-García. 2024. "Verification of Nasogastric Tube Positioning Using Ultrasound by an Intensive Care Nurse: A Pilot Study" Healthcare 12, no. 16: 1618. https://doi.org/10.3390/healthcare12161618
APA StyleRobles-González, M., Arrogante, O., Sánchez Giralt, J. A., Ortuño-Soriano, I., & Zaragoza-García, I. (2024). Verification of Nasogastric Tube Positioning Using Ultrasound by an Intensive Care Nurse: A Pilot Study. Healthcare, 12(16), 1618. https://doi.org/10.3390/healthcare12161618







