The Effects of Prolonged Indoor Inhalation of Nature-Derived Odors on Menopausal Women
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
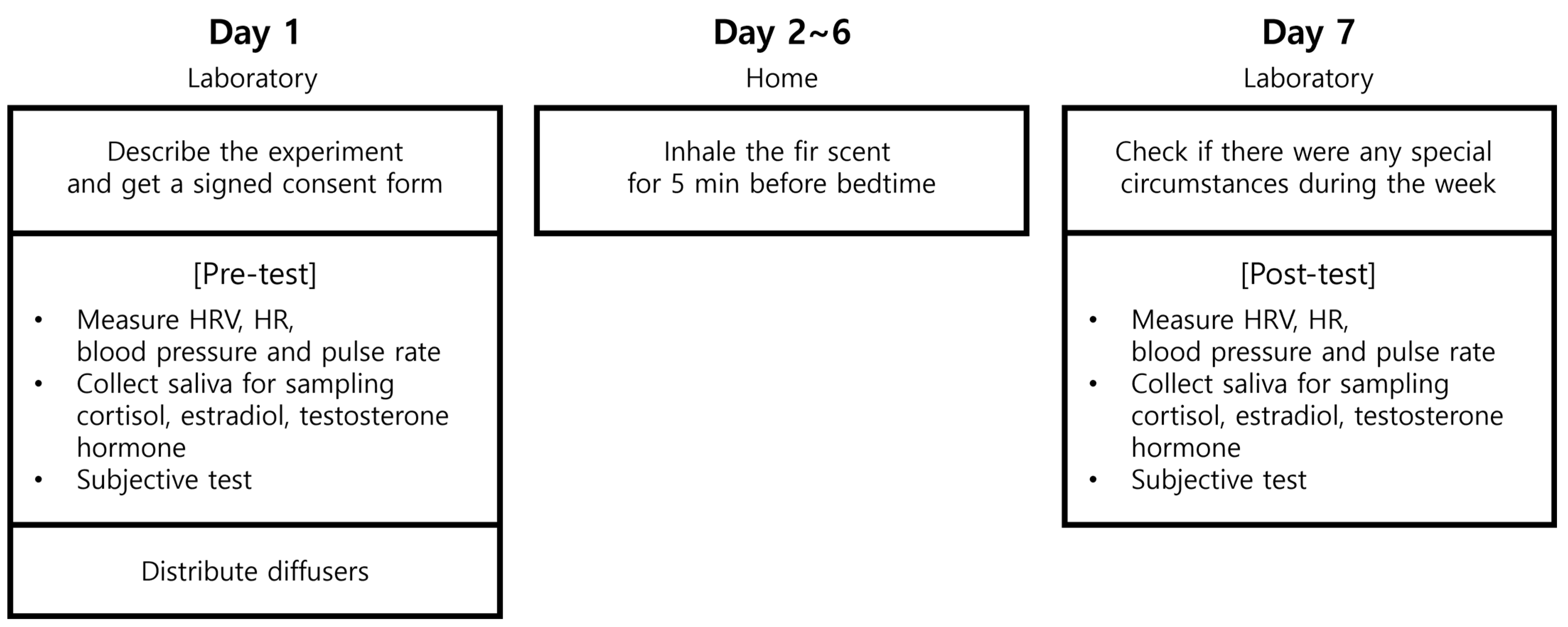
2.2. Experimental Design
2.3. Olfactory Stimulation
2.4. Physiological Measurement
2.4.1. Heart Rate Variability (HRV) and Heart Rate (HR)
2.4.2. Blood Pressure and Pulse Rate
2.4.3. Sex Hormones (Estradiol and Testosterone)
2.4.4. Cortisol
2.5. Psychological Measurement
2.5.1. Profile of Mood State (POMS)
2.5.2. State–Trait Anxiety Inventory (STAI)
2.5.3. Menopause Rating Scale (MRS)
2.5.4. General Sleep Disturbance Scale (GSDS)
2.6. Data Analysis
3. Results
3.1. Physiological Measurement
3.1.1. Heart Rate Variability (HRV) and Heart Rate (HR)
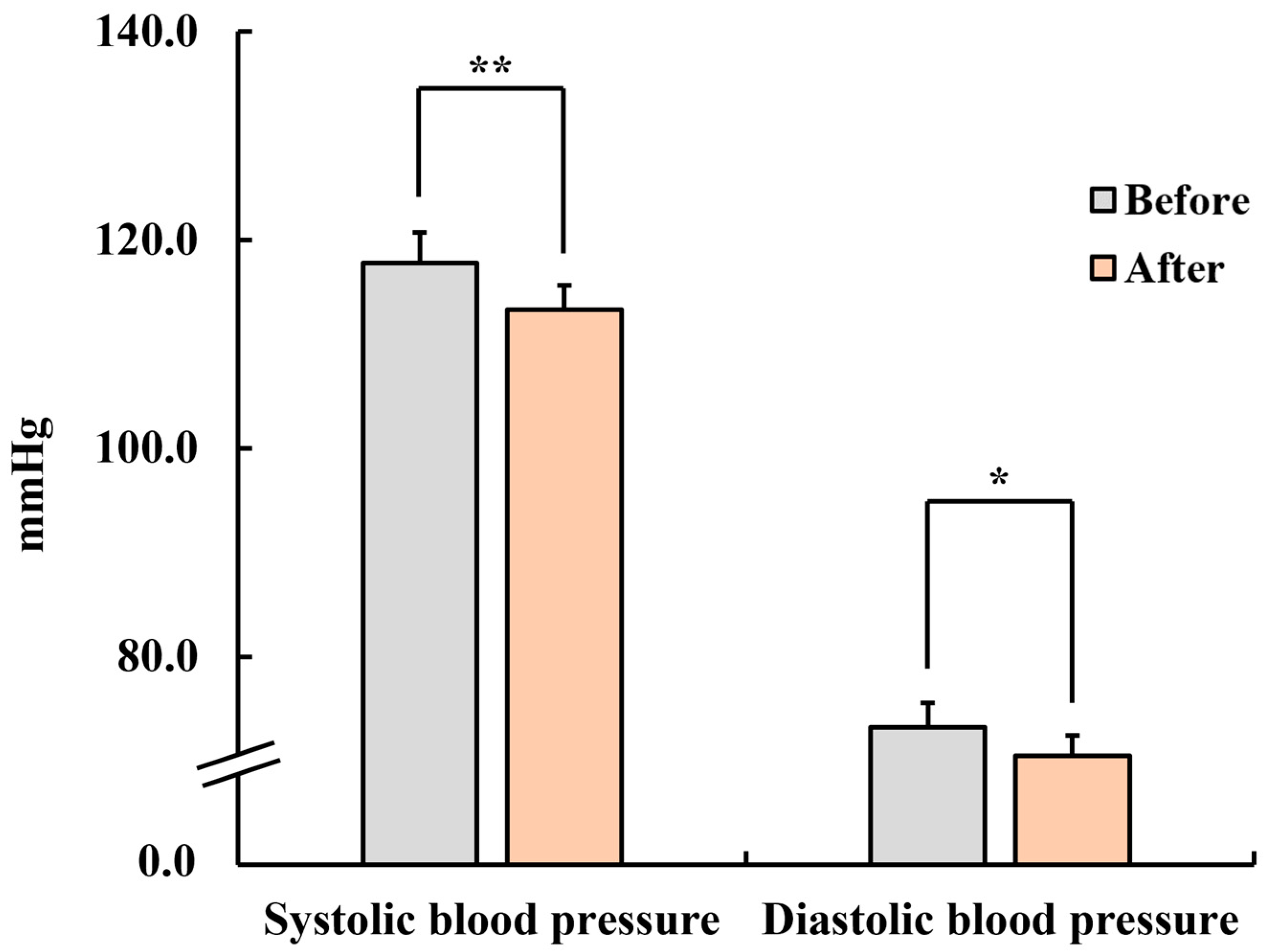
3.1.2. Blood Pressure and Pulse Rate
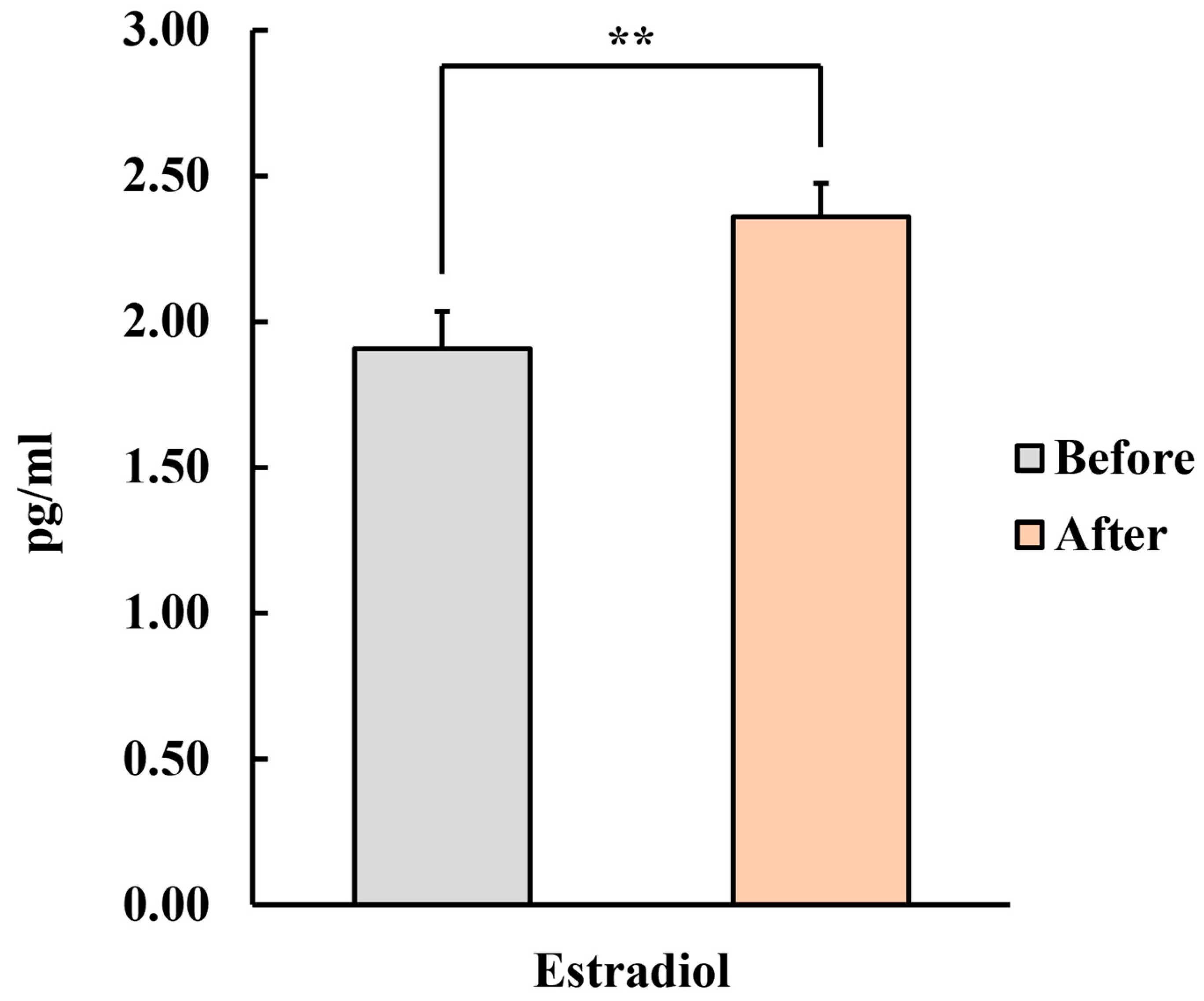
3.1.3. Sex Hormones (Estradiol and Testosterone)
3.1.4. Cortisol
3.2. Psychological Measurement
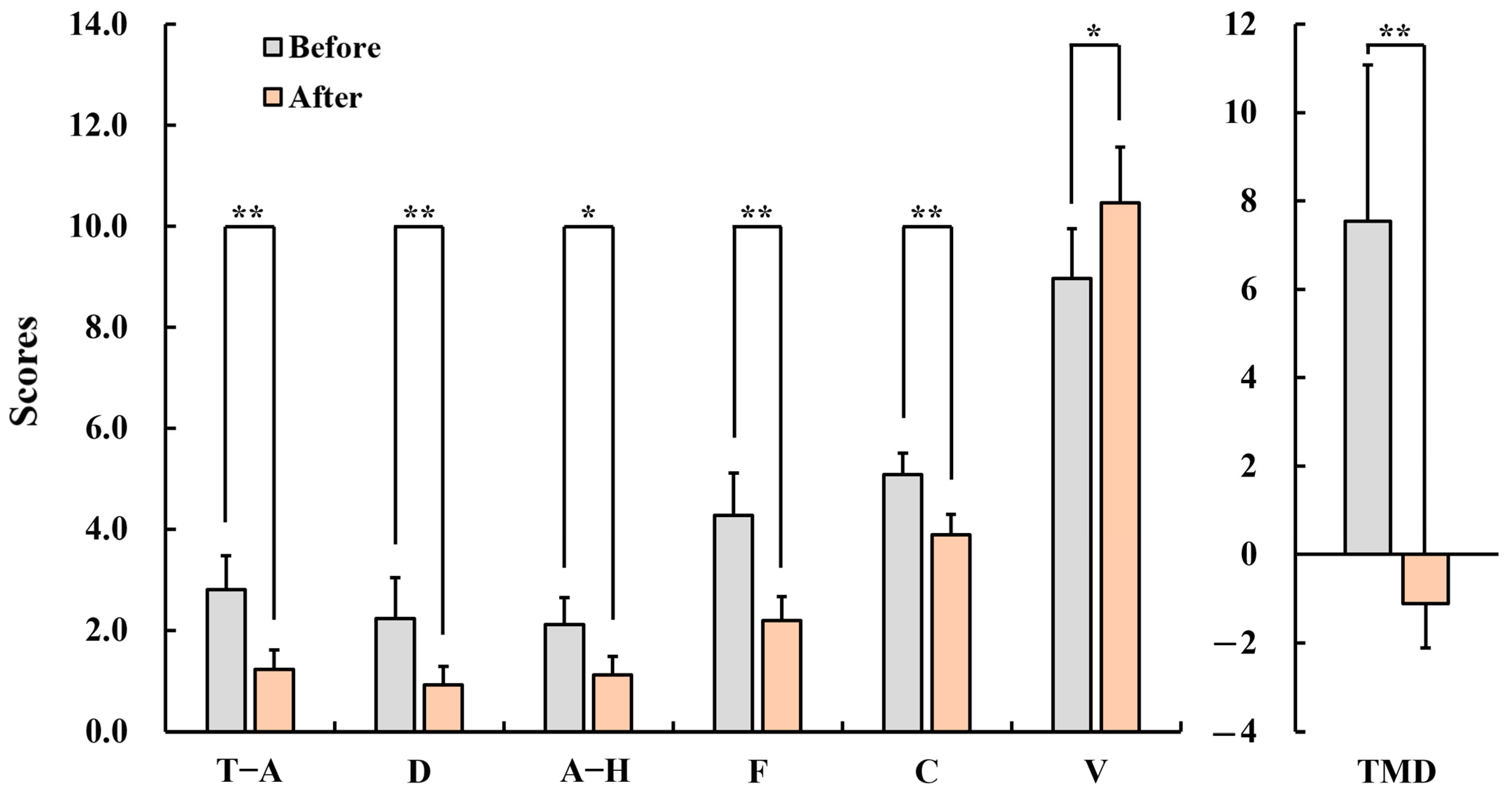
3.2.1. Profile of Mood State (POMS)
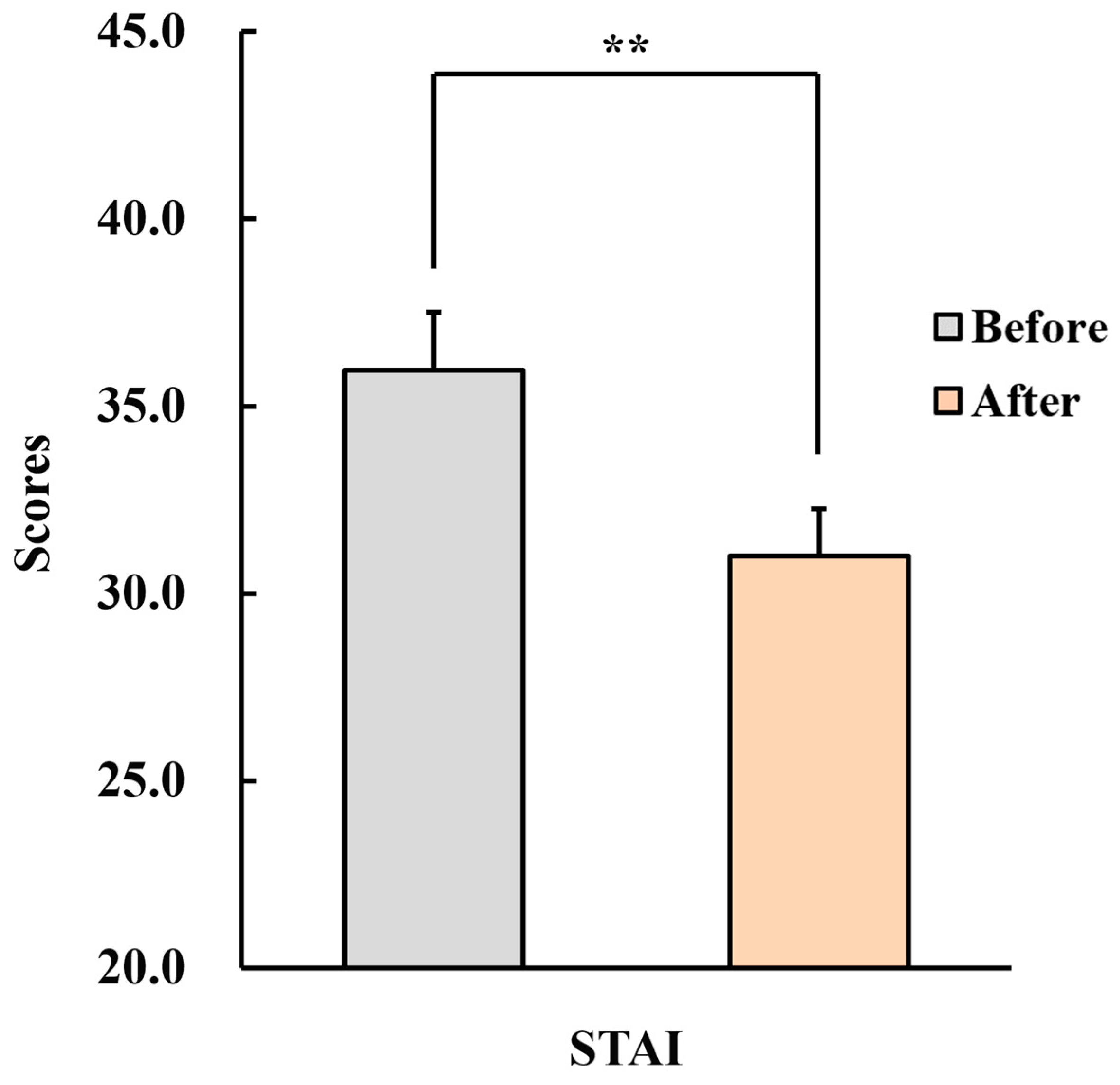
3.2.2. State–Trait Anxiety Inventory (STAI)
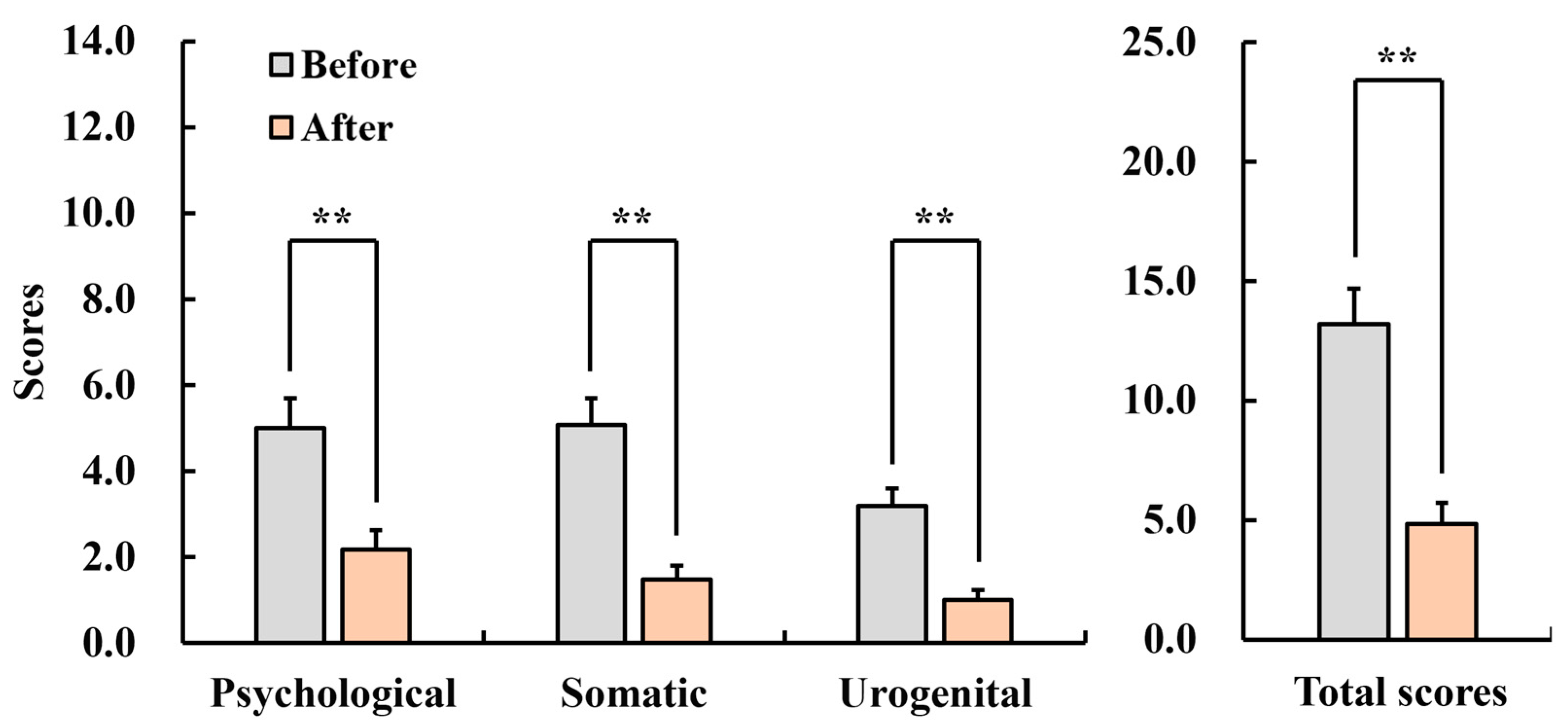
3.2.3. Menopause Rating Scale (MRS)
3.2.4. General Sleep Disturbance Scale (GSDS)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khajehzadeh, I.; Vale, B. How New Zealanders distribute their daily time between home indoors, home outdoors and out of home. Kotuitui 2017, 12, 17–31. [Google Scholar] [CrossRef]
- Seppänen, O.; Fisk, W.J.; Lei, Q.H. Room Temperature and Productivity in Office Work; Lawrence Berkeley National Lab. (LBNL): Berkeley, CA, USA, 2006.
- Colenberg, S.; Jylhä, T.; Arkesteijn, M. The relationship between interior office space and employee health and well-being—A literature review. Build. Res. Inf. 2021, 49, 352–366. [Google Scholar] [CrossRef]
- Chen, H.C.; Wang, C.H.; Chen, K.S.; Chang, T.L. Analysis and construction of stress relief model for healthy indoor environments. Qual. Quant. 2014, 48, 2053–2067. [Google Scholar] [CrossRef]
- Liu, G.; Zou, J.; Qiao, M.; Zhu, H.; Yang, Y.; Guan, H.; Hu, S. Stress recovery at home: Effects of the indoor visual and auditory stimuli in buildings. Build. Environ. 2023, 244, 110752. [Google Scholar]
- Park, S.A.; Song, C.; Choi, J.Y.; Son, K.C.; Miyazaki, Y. Foliage plants cause physiological and psychological relaxation as evidenced by measurements of prefrontal cortex activity and profile of mood states. HortScience 2016, 51, 1308–1312. [Google Scholar] [CrossRef]
- Ikei, H.; Song, C.; Igarashi, M.; Namekawa, T.; Miyazaki, Y. Physiological and psychological relaxing effects of visual stimulation with foliage plants in high school students. Int. J. Environ. Res. Public Health 2014, 28, 111–116. [Google Scholar] [CrossRef]
- Park, S.H.; Mattson, R.H. Ornamental Indoor Plants in Hospital Rooms Enhanced Health Outcomes of Patients Recovering from Surgery. J. Altern. Complement. Med. 2009, 15, 975–980. [Google Scholar] [PubMed]
- Song, C.; Ikei, H.; Nara, M.; Takayama, D.; Miyazaki, Y. Physiological effects of viewing bonsai in elderly patients undergoing rehabilitation. Int. J. Environ. Res. Public Health 2018, 15, 2635. [Google Scholar] [CrossRef]
- Matsubara, E.; Kawai, S. VOCs emitted from Japanese cedar (Cryptomeria japonica) interior walls induce physiological relaxation. Build. Environ. 2014, 72, 125–130. [Google Scholar] [CrossRef]
- Choi, N.; Yamanaka, T.; Takemura, A.; Kobayashi, T.; Eto, A.; Hirano, M. Impact of indoor aroma on students’ mood and learning performance. Build. Environ. 2022, 223, 109490. [Google Scholar] [CrossRef]
- Ikei, H.; Song, C.; Miyazaki, Y. Physiological effects of touching the wood of hinoki cypress (Chamaecyparis obtusa) with the soles of the feet. Int. J. Environ. Res. Public Health 2018, 15, 2135. [Google Scholar] [CrossRef]
- Kim, C.; Song, C. Physiological and Psychological Relaxation Effects of Fir Essential Oil on University Students. Int. J. Environ. Res. Public Health 2022, 19, 5063. [Google Scholar] [CrossRef]
- Hamdamian, S.; Nazarpour, S.; Simbar, M.; Hajian, S.; Mojab, F.; Talebi, A. Effects of aromatherapy with Rosa damascena on nulliparous women’s pain and anxiety of labor during first stage of labor. J. Integr. Med. 2018, 16, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Karimzadeh, Z.; Azizzadeh Forouzi, M.; Tajadini, H.; Ahmadinejad, M.; Roy, C.; Dehghan, M. Effects of lavender and Citrus aurantium on pain of conscious intensive care unit patients: A parallel randomized placebo-controlled trial. J. Integr. Med. 2021, 19, 333–339. [Google Scholar] [CrossRef]
- Tungsukruthai, P.; Nootim, P.; Worakunphanich, W.; Tabtong, N. Efficacy and safety of herbal steam bath in allergic rhinitis: A randomized controlled trial. J. Integr. Med. 2018, 16, 39–44. [Google Scholar] [CrossRef]
- Carmichael, S.T.; Clugnet, M.C.; Price, J.L. Central olfactory connections in the macaque monkey. J. Comp. Neurol. 1994, 346, 403–434. [Google Scholar] [CrossRef]
- Poo, C.; Agarwal, G.; Bonacchi, N.; Mainen, Z. Spatial maps in piriform cortex during olfactory navigation. Nature 2022, 601, 595–599. [Google Scholar] [CrossRef]
- Ehrlichman, H.; Halpern, J.N. Affect and Memory: Effects of Pleasant and Unpleasant Odors on Retrieval of Happy and Unhappy Memories. J. Pers. Soc. Psychol. 1988, 55, 769–779. [Google Scholar] [CrossRef]
- Kobayashi, H.; Ishibashi, K.; Noguchi, H. Heart Rate Variability; An Index for Monitoring and Analyzing Human Autonomic Activities. Appl. Hum. Sci. 1999, 18, 53–59. [Google Scholar]
- Janig, W.; Habler, H.J. Specificity in the organization of the autonomic nervous system: A basis for precise neural regulation of homeostatic and protective body functions. Prog. Brain Res. 2000, 122, 351–367. [Google Scholar] [CrossRef] [PubMed]
- Kuo, T.B.J.; Lin, T.; Yang, C.C.H.; Li, C.; Chen, C.F.; Chou, P. Effect of aging on gender differences in neural control of heart rate. Heart Circ Physiol. 1999, 46, 2233–2239. [Google Scholar] [CrossRef]
- Cacioppo, J.T.; Berntson, G.G.; Binkley, P.F.; Quigley, K.S.; Uchino, B.N.; Fieldstone, A. Autonomic_cardiac_control_II_Noninvasive. Psychophysiology 1994, 31, 586–598. [Google Scholar] [PubMed]
- Beevers, G.; Lip, G.Y.; O’Brien, E. ABC of hypertension Blood pressure measurement Part I-Sphygmomanometry: Factors common to all techniques Methods of blood pressure measurement. BMJ 2001, 332, 981–985. [Google Scholar] [CrossRef]
- Cha, J.; Sun, X.; Dey, S.K. Mechanisms of implantation: Strategies for successful pregnancy. Nat. Med. 2012, 18, 1754–1767. [Google Scholar] [CrossRef]
- Barbieri, R.L. The endocrinology of the menstrual cycle. Methods Mol. Biol. 2014, 1154, 145–169. [Google Scholar] [CrossRef] [PubMed]
- Devoto, L.; Henríquez, S.; Kohen, P.; Strauss, J.F. The significance of estradiol metabolites in human corpus luteum physiology. Steroids 2017, 123, 50–54. [Google Scholar] [CrossRef]
- Lang, T.F. The Bone-Muscle Relationship in Men and Women. J. Osteoporos. 2011, 2011, 1–4. [Google Scholar] [CrossRef]
- Vingren, J.L.; Kraemer, W.J.; Ratamess, N.A.; Anderson, J.M.; Volek, J.S.; Maresh, C.M. Testosterone Physiology in Resistance Exercise and Training the Up-Stream Regulatory Elements. Sports Med. 2010, 40, 1037–1053. [Google Scholar]
- Michaud, K.; Matheson, K.; Kelly, O.; Anisman, H. Impact of stressors in a natural context on release of cortisol in healthy adult humans: A meta-analysis. Stress 2008, 11, 177–197. [Google Scholar] [CrossRef]
- Edwards, S.; Clow, A.; Evans, P.; Hucklebridge, F. Exploration of the awakening cortisol response in relation to diurnal cortisol secretory activity. Life Sci. 2001, 68, 2093–2103. [Google Scholar]
- Poll, E.M.; Kreitschmann-Andermahr, I.; Langejuergen, Y.; Stanzel, S.; Gilsbach, J.M.; Gressner, A.; Yagmur, E. Saliva collection method affects predictability of serum cortisol. Clin. Chim. Acta 2007, 382, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Spielberg, C.D. State-Trait Anxiety Inventory for Adults; Consulting Psychologists Press: Palo Alto, CA, USA, 1983. [Google Scholar]
- Berlin Center for Epidemiology and Health Research. Menopause Rating Scale. Available online: https://zeg-berlin.de/expertise/diagnostics-tools/menopause-rating-scale/development/ (accessed on 23 August 2023).
- Choi, H.J.; Kim, S.J.; Kim, B.J.; Kim, I.J. Korean Versions of Self-reported Sleep Questionnaires for Research and Practice on Sleep Disturbance. Korean J. Rehabil. Nurs. 2012, 15, 1–10. [Google Scholar] [CrossRef]
- Ju, M.S.; Lee, S.; Bae, I.; Hur, M.H.; Seong, K.; Lee, M.S. Effects of aroma massage on home blood pressure, ambulatory blood pressure, and sleep quality in middle-aged women with hypertension. Evid. Based Complement. Alternat. Med. 2013, 2013, 403251. [Google Scholar] [CrossRef] [PubMed]
- Fukui, H.; Toyoshima, K.; Komaki, R. Psychological and neuroendocrinological effects of odor of saffron (Crocus sativus). Phytomedicine 2011, 18, 726–730. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, P.; Hosseininik, M.; Afrasiabifar, A.; Sadeghi, H.; Hosseininik, A.; Tabatabaei, S.M.; Hosseini, N. The effect of Fennel seed powder on estradiol levels, menopausal symptoms, and sexual desire in postmenopausal women. Menopause 2020, 27, 1281–1286. [Google Scholar] [CrossRef]
- Watanabe, E.; Kuchta, K.; Kimura, M.; Rauwald, H.W.; Kamei, T.; Imanishi, J. Effects of Bergamot (Citrus bergamia (Risso) Wright & Arn.) Essential Oil Aromatherapy on Mood States, Parasympathetic Nervous System Activity, and Salivary Cortisol Levels in 41 Healthy Females. Complement. Med. Res. 2015, 22, 43–49. [Google Scholar] [CrossRef]
- Hwang, E.; Shin, S. The effects of aromatherapy on sleep improvement: A systematic literature review and meta-analysis. J. Altern. Complement. Med. 2015, 21, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Andini, S.; Husni, E.; Aini, E.; Kasiati, K.; Kaur, K. Effect of Fennel Aromatherapy (Foeniculum vulgare) on Decreasing Menopause Symptom Levels in Menopausal Women in Tunjung Village Bangkalan Regency Indonesia. Int. J. Adv. Health Sci. Technol. 2022, 2, 238–246. [Google Scholar] [CrossRef]
- Dick, S.; DeWitt, D.; Anawalt, B. Postmenopausal hormone replacement therapy and major clinical outcomes: A focus on cardiovascular disease, osteoporosis, dementia, and breast cancer. Am. J. Manag. Care 2002, 8, 95–104. [Google Scholar] [PubMed]
- Tankó, L.B.; Christiansen, C.; Cox, D.A.; Geiger, M.J.; McNabb, M.A.; Cummings, S.R. Relationship Between Osteoporosis and Cardiovascular Disease in Postmenopausal Women. J. Bone Miner. Res. 2005, 20, 1912–1920. [Google Scholar] [CrossRef]
- Greenblum, C.A.; Rowe, M.A.; Neff, D.F.; Greenblum, J.S. Midlife women: Symptoms associated with menopausal transition and early postmenopause and quality of life. Menopause 2013, 20, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Bureau of Labor Statistics, United States Department of Labor. NEW RELEASE; EMPLOYMENT CHARACTERISTICS OF FAMILIES 2022. Available online: https://www.bls.gov/news.release/famee.nr0.htm (accessed on 13 November 2023).
- Kim, C.; Lee, G.; Song, C. The Effect of Short-term Inhalation of Fir Essential Oil on Autonomic Nervous Activity in Middle-aged Women. Explore 2023, 19, 820–826. [Google Scholar] [CrossRef] [PubMed]

| Compound | Detected Concentration (mg/kg) | Relative Area (%) |
|---|---|---|
| alpha-Pinene | 226.8 | 22.68 |
| Camphene | 100.5 | 10.05 |
| 3-Carene | 99.6 | 9.96 |
| Limonene | 88.1 | 8.81 |
| p-Cymene | 37.8 | 3.78 |
| Camphor | 36.6 | 3.66 |
| gamma-Terpinene | 31.6 | 3.16 |
| (−)-beta-Pinene | 28.4 | 2.84 |
| alpha-Terpinene | 13.8 | 1.38 |
| alpha-Terpinolene | 10.9 | 1.09 |
| alpha-Terpineol | 10.8 | 1.08 |
| beta-Myrcene | 10.3 | 1.03 |
| Eucalyptol | 9.5 | 0.95 |
| (−)-trans-Caryophyllene | 2.3 | 0.23 |
| (−)-Bornyl acetate | 1.0 | 0.10 |
| Sum (%) | 69.62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, C.; Lee, G.; Song, C. The Effects of Prolonged Indoor Inhalation of Nature-Derived Odors on Menopausal Women. Healthcare 2024, 12, 1667. https://doi.org/10.3390/healthcare12161667
Kim C, Lee G, Song C. The Effects of Prolonged Indoor Inhalation of Nature-Derived Odors on Menopausal Women. Healthcare. 2024; 12(16):1667. https://doi.org/10.3390/healthcare12161667
Chicago/Turabian StyleKim, Choyun, Gayoung Lee, and Chorong Song. 2024. "The Effects of Prolonged Indoor Inhalation of Nature-Derived Odors on Menopausal Women" Healthcare 12, no. 16: 1667. https://doi.org/10.3390/healthcare12161667






