Demographic-, Radiographic-, and Surgery-Related Factors Do Not Affect Functional Internal Rotation Following Reverse Total Shoulder Arthroplasty: A Retrospective Comparative Study
Abstract
1. Introduction
2. Materials and Methods
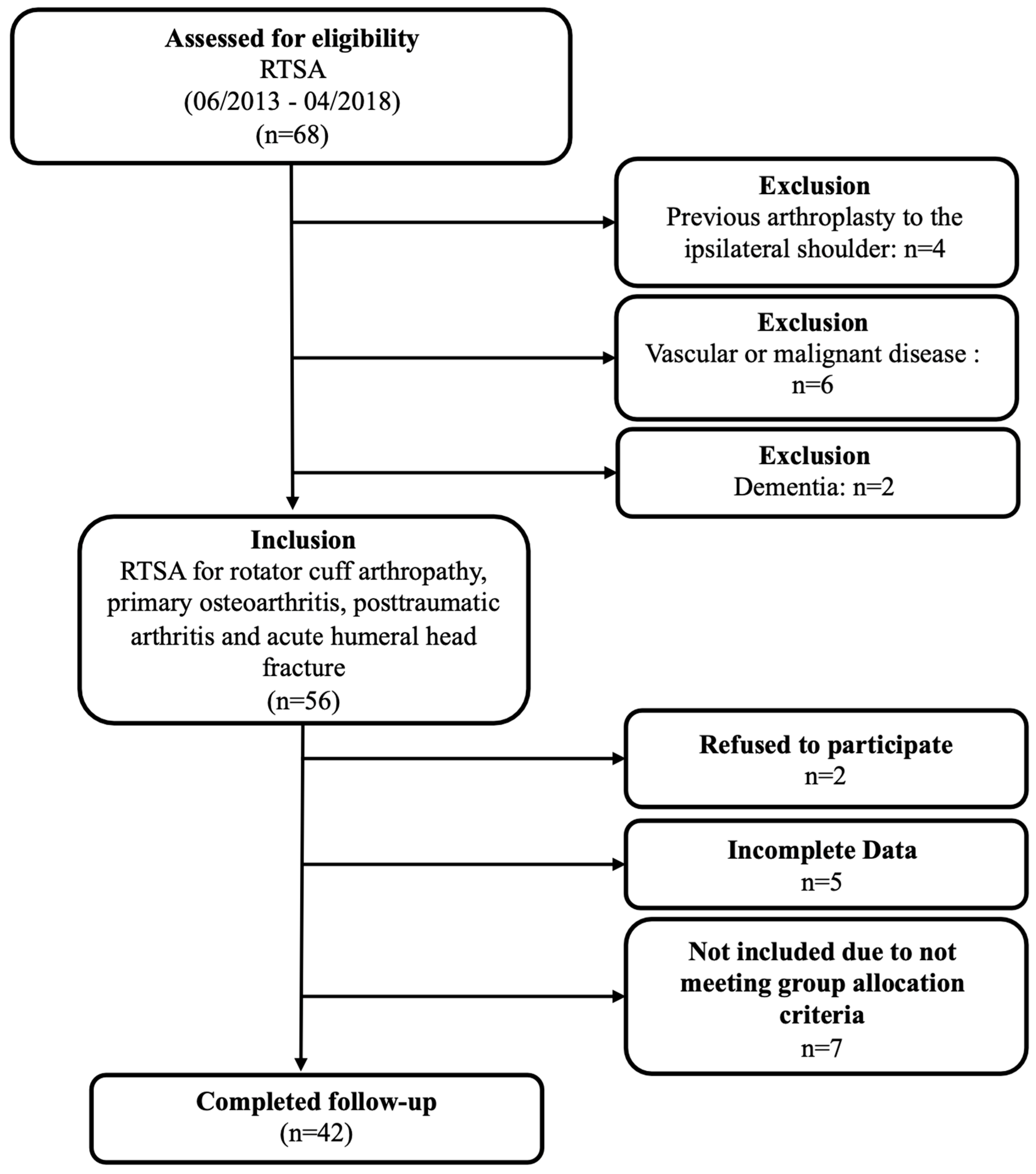
2.1. Study Population
2.2. Surgical Intervention and Postoperative Rehabilitation Protocol
2.3. Clinical Outcome Measures
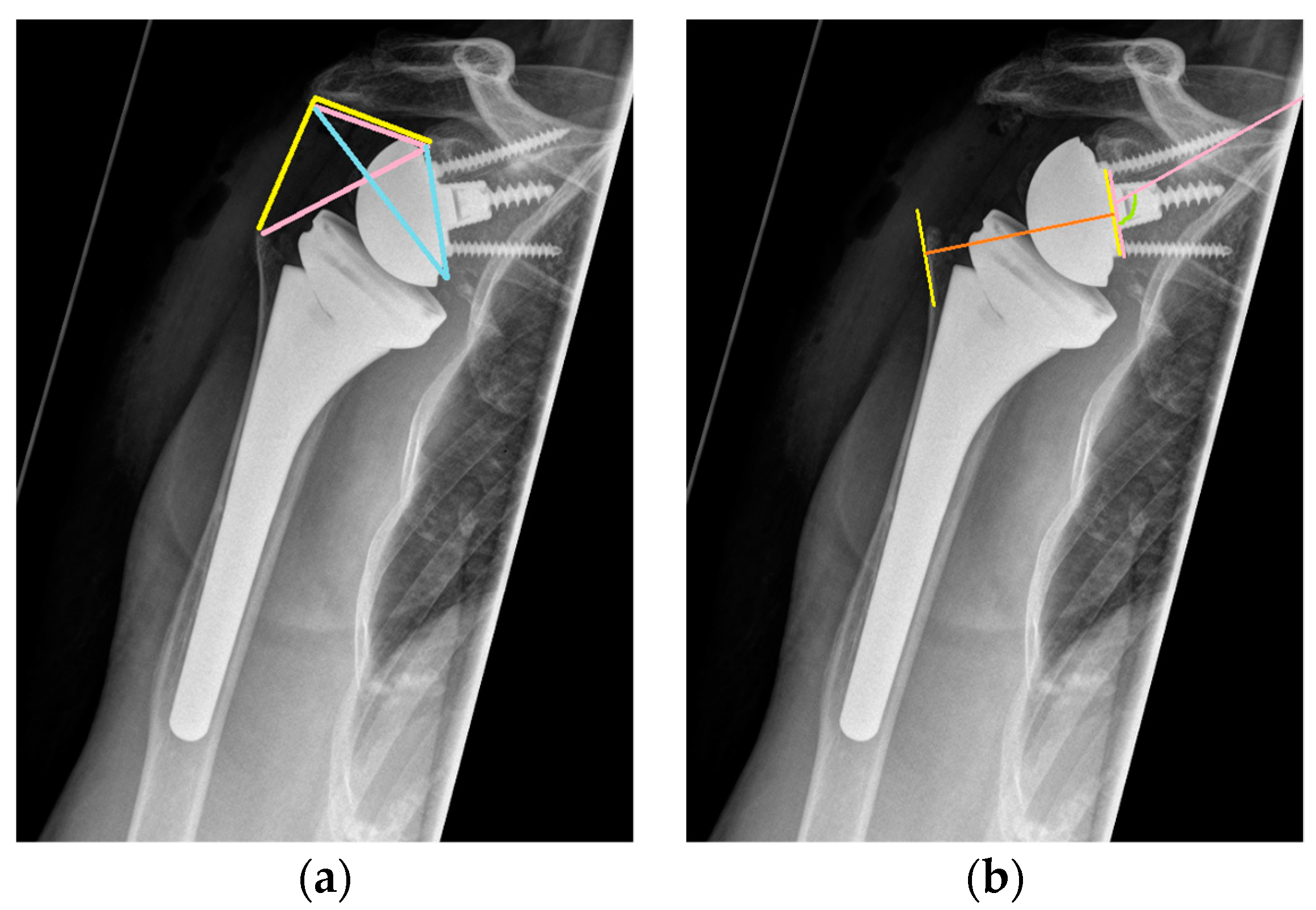
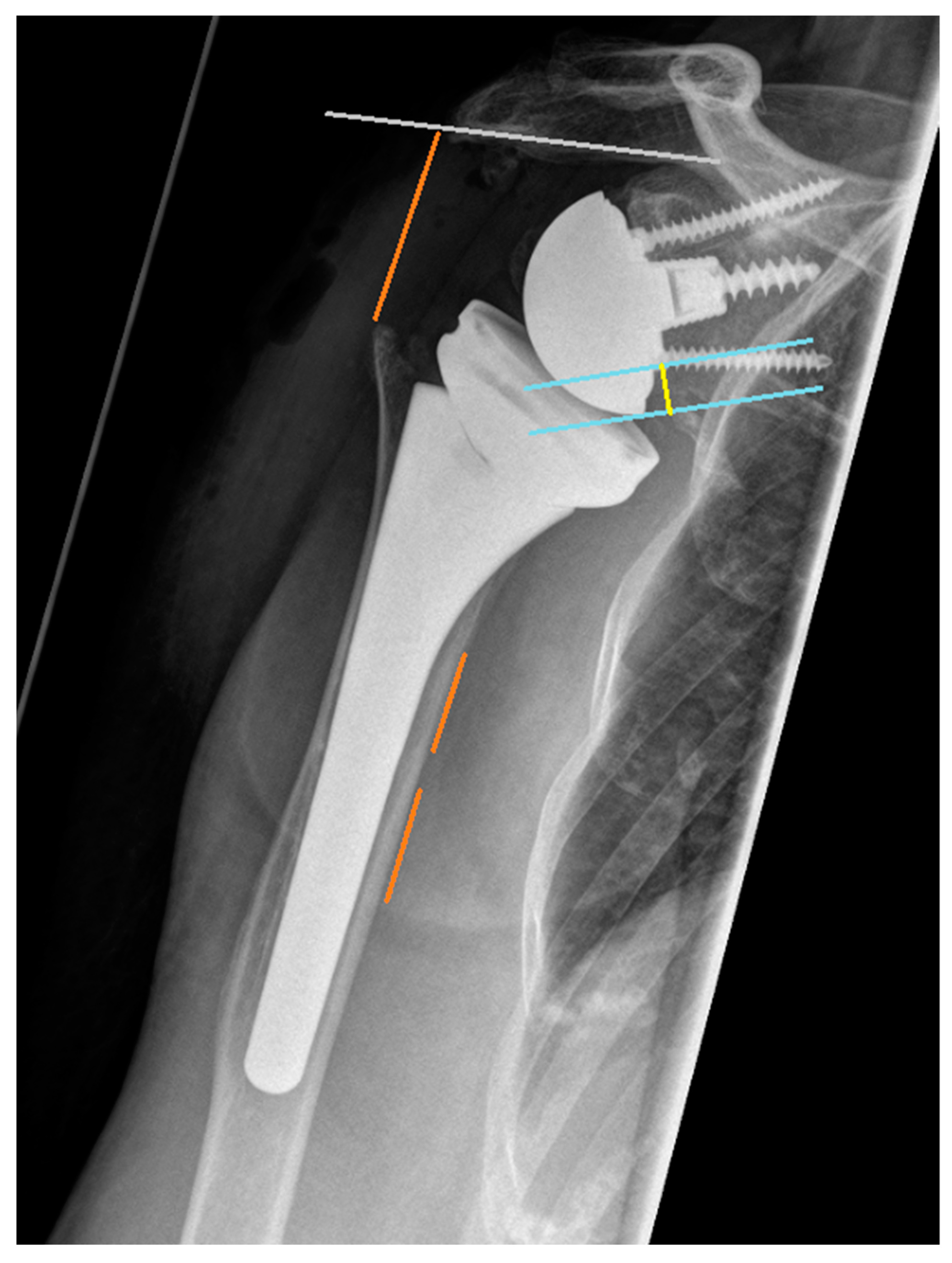
2.4. Radiographic Assessment
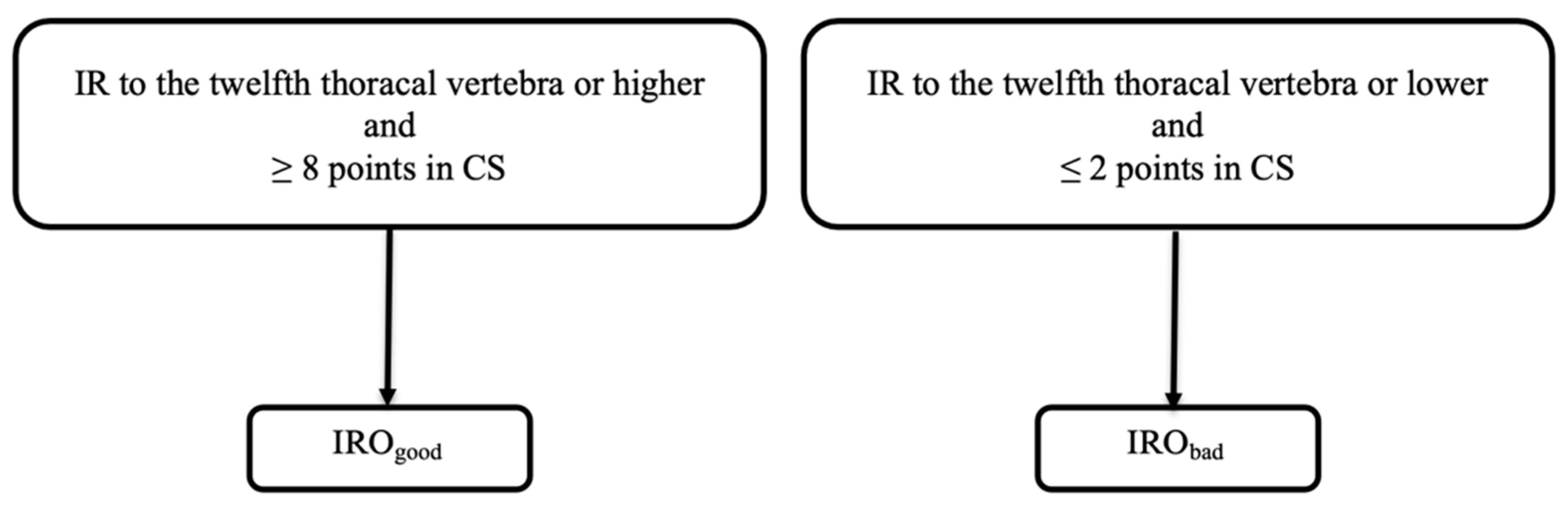
2.5. Group Allocation
2.6. Univariate Comparison
2.7. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Functional Outcomes
3.3. Radiographic Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Franceschi, F.; Giovannetti de Sanctis, E.; Gupta, A.; Athwal, G.S.; Di Giacomo, G. Reverse shoulder arthroplasty: State-of-the-art. J. ISAKOS 2023, 8, 306–317. [Google Scholar] [CrossRef] [PubMed]
- Boileau, P.; Watkinson, D.; Hatzidakis, A.M.; Hovorka, I. Neer Award 2005: The Grammont reverse shoulder prosthesis: Results in cuff tear arthritis, fracture sequelae, and revision arthroplasty. J. Shoulder Elb. Surg. 2006, 15, 527–540. [Google Scholar] [CrossRef] [PubMed]
- Flatow, E.L.; Harrison, A.K. A history of reverse total shoulder arthroplasty. Clin. Orthop. Relat. Res. 2011, 469, 2432–2439. [Google Scholar] [CrossRef]
- Henninger, H.B.; Barg, A.; Anderson, A.E.; Bachus, K.N.; Tashjian, R.Z.; Burks, R.T. Effect of deltoid tension and humeral version in reverse total shoulder arthroplasty: A biomechanical study. J. Shoulder Elb. Surg. 2012, 21, 483–490. [Google Scholar] [CrossRef]
- Terrier, A.; Scheuber, P.; Pioletti, D.P.; Farron, A. Activities of daily living with reverse prostheses: Importance of scapular compensation for functional mobility of the shoulder. J. Shoulder Elb. Surg. 2013, 22, 948–953. [Google Scholar] [CrossRef]
- Chelli, M.; Boileau, P.; Domos, P.; Clavert, P.; Berhouet, J.; Collin, P.; Walch, G.; Favard, L. Survivorship of Reverse Shoulder Arthroplasty According to Indication, Age and Gender. J. Clin. Med. 2022, 11, 2677. [Google Scholar] [CrossRef]
- Bacle, G.; Nove-Josserand, L.; Garaud, P.; Walch, G. Long-Term Outcomes of Reverse Total Shoulder Arthroplasty: A Follow-up of a Previous Study. J. Bone Jt. Surg. 2017, 99, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Galvin, J.W.; Kim, R.; Ment, A.; Durso, J.; Joslin, P.M.N.; Lemos, J.L.; Novikov, D.; Curry, E.J.; Alley, M.C.; Parada, S.A.; et al. Outcomes and complications of primary reverse shoulder arthroplasty with minimum of 2 years’ follow-up: A systematic review and meta-analysis. J. Shoulder Elb. Surg. 2022, 31, e534–e544. [Google Scholar] [CrossRef]
- Berhouet, J.; Kontaxis, A.; Gulotta, L.V.; Craig, E.; Warren, R.; Dines, J.; Dines, D. Effects of the humeral tray component positioning for onlay reverse shoulder arthroplasty design: A biomechanical analysis. J. Shoulder Elb. Surg. 2015, 24, 569–577. [Google Scholar] [CrossRef]
- Ernstbrunner, L.; Suter, A.; Catanzaro, S.; Rahm, S.; Gerber, C. Reverse Total Shoulder Arthroplasty for Massive, Irreparable Rotator Cuff Tears before the Age of 60 Years: Long-Term Results. J. Bone Jt. Surg. 2017, 99, 1721–1729. [Google Scholar] [CrossRef]
- Maier, M.W.; Caspers, M.; Zeifang, F.; Dreher, T.; Klotz, M.C.; Wolf, S.I.; Kasten, P. How does reverse shoulder replacement change the range of motion in activities of daily living in patients with cuff tear arthropathy? A prospective optical 3D motion analysis study. Arch. Orthop. Trauma. Surg. 2014, 134, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Jeong, H.Y.; Kim, J.D.; Ro, K.H.; Rhee, S.M.; Rhee, Y.G. Difficulty in performing activities of daily living associated with internal rotation after reverse total shoulder arthroplasty. J. Shoulder Elb. Surg. 2020, 29, 86–94. [Google Scholar] [CrossRef]
- Lauria, M.; Hastings, M.; DiPaola, M.J.; Duquin, T.R.; Ablove, R.H. Factors affecting internal rotation following total shoulder arthroplasty. JSES Rev. Rep. Tech. 2022, 2, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Gulotta, L.V.; Choi, D.; Marinello, P.; Knutson, Z.; Lipman, J.; Wright, T.; Cordasco, F.A.; Craig, E.V.; Warren, R.F. Humeral component retroversion in reverse total shoulder arthroplasty: A biomechanical study. J. Shoulder Elb. Surg. 2012, 21, 1121–1127. [Google Scholar] [CrossRef]
- Jeon, B.K.; Panchal, K.A.; Ji, J.H.; Xin, Y.Z.; Park, S.R.; Kim, J.H.; Yang, S.J. Combined effect of change in humeral neck-shaft angle and retroversion on shoulder range of motion in reverse total shoulder arthroplasty—A simulation study. Clin. Biomech. 2016, 31, 12–19. [Google Scholar] [CrossRef]
- Kontaxis, A.; Chen, X.; Berhouet, J.; Choi, D.; Wright, T.; Dines, D.M.; Warren, R.F.; Gulotta, L.V. Humeral version in reverse shoulder arthroplasty affects impingement in activities of daily living. J. Shoulder Elb. Surg. 2017, 26, 1073–1082. [Google Scholar] [CrossRef]
- Keener, J.D.; Patterson, B.M.; Orvets, N.; Aleem, A.W.; Chamberlain, A.M. Optimizing reverse shoulder arthroplasty component position in the setting of advanced arthritis with posterior glenoid erosion: A computer-enhanced range of motion analysis. J. Shoulder Elb. Surg. 2018, 27, 339–349. [Google Scholar] [CrossRef]
- Ladermann, A.; Tay, E.; Collin, P.; Piotton, S.; Chiu, C.H.; Michelet, A.; Charbonnier, C. Effect of critical shoulder angle, glenoid lateralization, and humeral inclination on range of movement in reverse shoulder arthroplasty. Bone Jt. Res. 2019, 8, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Liou, W.; Yang, Y.; Petersen-Fitts, G.R.; Lombardo, D.J.; Stine, S.; Sabesan, V.J. Effect of lateralized design on muscle and joint reaction forces for reverse shoulder arthroplasty. J. Shoulder Elb. Surg. 2017, 26, 564–572. [Google Scholar] [CrossRef]
- Meisterhans, M.; Bouaicha, S.; Meyer, D.C. Posterior and inferior glenosphere position in reverse total shoulder arthroplasty supports deltoid efficiency for shoulder flexion and elevation. J. Shoulder Elb. Surg. 2019, 28, 1515–1522. [Google Scholar] [CrossRef]
- Virani, N.A.; Cabezas, A.; Gutierrez, S.; Santoni, B.G.; Otto, R.; Frankle, M. Reverse shoulder arthroplasty components and surgical techniques that restore glenohumeral motion. J. Shoulder Elb. Surg. 2013, 22, 179–187. [Google Scholar] [CrossRef]
- Langohr, G.D.; Giles, J.W.; Athwal, G.S.; Johnson, J.A. The effect of glenosphere diameter in reverse shoulder arthroplasty on muscle force, joint load, and range of motion. J. Shoulder Elb. Surg. 2015, 24, 972–979. [Google Scholar] [CrossRef] [PubMed]
- Kramer, M.; Baunker, A.; Wellmann, M.; Hurschler, C.; Smith, T. Implant impingement during internal rotation after reverse shoulder arthroplasty. The effect of implant configuration and scapula anatomy: A biomechanical study. Clin. Biomech. 2016, 33, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Tashjian, R.Z.; Burks, R.T.; Zhang, Y.; Henninger, H.B. Reverse total shoulder arthroplasty: A biomechanical evaluation of humeral and glenosphere hardware configuration. J. Shoulder Elb. Surg. 2015, 24, e68–e77. [Google Scholar] [CrossRef]
- Ladermann, A.; Denard, P.J.; Boileau, P.; Farron, A.; Deransart, P.; Terrier, A.; Ston, J.; Walch, G. Effect of humeral stem design on humeral position and range of motion in reverse shoulder arthroplasty. Int. Orthop. 2015, 39, 2205–2213. [Google Scholar] [CrossRef] [PubMed]
- Levy, J.C.; Ashukem, M.T.; Formaini, N.T. Factors predicting postoperative range of motion for anatomic total shoulder arthroplasty. J. Shoulder Elb. Surg. 2016, 25, 55–60. [Google Scholar] [CrossRef]
- Ameziane, Y.; Holschen, M.; Engel, N.M.; Schorn, D.; Witt, K.A.; Steinbeck, J. Does the subscapularis refixation affect the clinical outcome after primary reverse shoulder arthroplasty? J. Shoulder Elb. Surg. 2024, 33, 1909–1917. [Google Scholar] [CrossRef]
- Chelli, M.; Walch, G.; Azar, M.; Neyton, L.; Levigne, C.; Favard, L.; Boileau, P. Glenoid lateralization and subscapularis repair are independent predictive factors of improved internal rotation after reverse shoulder arthroplasty. Int. Orthop. 2024, 48, 127–132. [Google Scholar] [CrossRef]
- Clinker, C.; Ishikawa, H.; Presson, A.P.; Zhang, C.; Joyce, C.; Chalmers, P.N.; Tashjian, R.Z. The effect of lateralization and distalization after Grammont-style reverse total shoulder arthroplasty. J. Shoulder Elb. Surg. 2024. [Google Scholar] [CrossRef]
- Macken, A.A.; van der Poel, W.J.; Buijze, G.A.; Beckers, J.J.; Eygendaal, D.; Lafosse, L.; Lafosse, T. Reverse shoulder arthroplasty with a 155 degrees neck-shaft angle inlay implant design without reattachment of the subscapularis tendon results in satisfactory functional internal rotation and no instability: A cohort study. J. Orthop. Traumatol. 2024, 25, 10. [Google Scholar] [CrossRef]
- Rhee, S.M.; Lee, J.W.; Lee, J.U.; Kim, C.H.; Kim, S.Y.; Ham, H.J.; Kantanavar, R.; Rhee, Y.G. Subscapularis Repair in Reverse Total Shoulder Arthroplasty: Differences in Outcomes Based on Preoperative Quality of Subscapularis Tendon. Indian J. Orthop. 2024, 58, 747–754. [Google Scholar] [CrossRef]
- Werner, B.C.; Lederman, E.; Gobezie, R.; Denard, P.J. Glenoid lateralization influences active internal rotation after reverse shoulder arthroplasty. J. Shoulder Elb. Surg. 2021, 30, 2498–2505. [Google Scholar] [CrossRef]
- Neyton, L.; Nigues, A.; McBride, A.P.; Giovannetti de Sanctis, E. Neck shaft angle in reverse shoulder arthroplasty: 135 vs. 145 degrees at minimum 2-year follow-up. J. Shoulder Elb. Surg. 2023, 32, 1486–1493. [Google Scholar] [CrossRef] [PubMed]
- Constant, C.R.; Gerber, C.; Emery, R.J.; Sojbjerg, J.O.; Gohlke, F.; Boileau, P. A review of the Constant score: Modifications and guidelines for its use. J. Shoulder Elb. Surg. 2008, 17, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Constant, C.R.; Murley, A.H. A clinical method of functional assessment of the shoulder. Clin. Orthop. Relat. Res. 1987, 214, 160–164. [Google Scholar] [CrossRef]
- Boutsiadis, A.; Lenoir, H.; Denard, P.J.; Panisset, J.C.; Brossard, P.; Delsol, P.; Guichard, F.; Barth, J. The lateralization and distalization shoulder angles are important determinants of clinical outcomes in reverse shoulder arthroplasty. J. Shoulder Elb. Surg. 2018, 27, 1226–1234. [Google Scholar] [CrossRef] [PubMed]
- Moor, B.K.; Bouaicha, S.; Rothenfluh, D.A.; Sukthankar, A.; Gerber, C. Is there an association between the individual anatomy of the scapula and the development of rotator cuff tears or osteoarthritis of the glenohumeral joint?: A radiological study of the critical shoulder angle. Bone Jt. J. 2013, 95-B, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Maurer, A.; Fucentese, S.F.; Pfirrmann, C.W.; Wirth, S.H.; Djahangiri, A.; Jost, B.; Gerber, C. Assessment of glenoid inclination on routine clinical radiographs and computed tomography examinations of the shoulder. J. Shoulder Elb. Surg. 2012, 21, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.S.; Rhee, Y.G. Factors associated with poor active anterior elevation after reverse total shoulder arthroplasty. J. Shoulder Elb. Surg. 2018, 27, 786–793. [Google Scholar] [CrossRef]
- Berthold, D.P.; Morikawa, D.; Muench, L.N.; Baldino, J.B.; Cote, M.P.; Creighton, R.A.; Denard, P.J.; Gobezie, R.; Lederman, E.; Romeo, A.A.; et al. Negligible Correlation between Radiographic Measurements and Clinical Outcomes in Patients Following Primary Reverse Total Shoulder Arthroplasty. J. Clin. Med. 2021, 10, 809. [Google Scholar] [CrossRef]
- Jang, Y.H.; Lee, J.H.; Kim, S.H. Effect of scapular notching on clinical outcomes after reverse total shoulder arthroplasty. Bone Jt. J. 2020, 102-B, 1438–1445. [Google Scholar] [CrossRef]
- Hochreiter, B.; Hasler, A.; Hasler, J.; Kriechling, P.; Borbas, P.; Gerber, C. Factors influencing functional internal rotation after reverse total shoulder arthroplasty. JSES Int. 2021, 5, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Eichinger, J.K.; Rao, M.V.; Lin, J.J.; Goodloe, J.B.; Kothandaraman, V.; Barfield, W.R.; Parada, S.A.; Roche, C.; Friedman, R.J. The effect of body mass index on internal rotation and function following anatomic and reverse total shoulder arthroplasty. J. Shoulder Elb. Surg. 2021, 30, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Muller, A.M.; Born, M.; Jung, C.; Flury, M.; Kolling, C.; Schwyzer, H.K.; Audige, L. Glenosphere size in reverse shoulder arthroplasty: Is larger better for external rotation and abduction strength? J. Shoulder Elb. Surg. 2018, 27, 44–52. [Google Scholar] [CrossRef]
- Verdano, M.A.; Aliani, D.; Galavotti, C.; Maroun, C.; Vaienti, E.; Ceccarelli, F. Grammont versus lateralizing reverse shoulder arthroplasty for proximal humerus fracture: Functional and radiographic outcomes. Musculoskelet. Surg. 2018, 102, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Longo, U.G.; Gulotta, L.V.; De Salvatore, S.; Berton, A.; Piergentili, I.; Bandini, B.; Lalli, A.; Denaro, V. The Role of Humeral Neck-Shaft Angle in Reverse Total Shoulder Arthroplasty: 155° versus <155°—A Systematic Review. J. Clin. Med. 2022, 11, 3641. [Google Scholar] [CrossRef] [PubMed]
- Rol, M.; Favard, L.; Berhouet, J.; la Société d’orthopédie de l’Ouest (SOO). Factors associated with internal rotation outcomes after reverse shoulder arthroplasty. Orthop. Traumatol. Surg. Res. 2019, 105, 1515–1519. [Google Scholar] [CrossRef]
- Werner, B.S.; Chaoui, J.; Walch, G. Glenosphere design affects range of movement and risk of friction-type scapular impingement in reverse shoulder arthroplasty. Bone Jt. J. 2018, 100-B, 1182–1186. [Google Scholar] [CrossRef]
| IRObad | IROgood | p-Value | |
|---|---|---|---|
| Number of included patients, n | 17 | 25 | |
| Age at surgery (years) | 73.7 ± 5 | 72 ± 6.1 | 0.337 n.s. |
| BMI (kg/m2) | 28.7 (9.1) * | 24.8 (5.6) * | 0.086 n.s. |
| Follow-up (months) | 42.2 (28) * | 47 (35) * | 0.356 n.s |
| Sex | |||
| Male, n (%) | 9 (52.9) * | 11 (44) * | 0.112 n.s. |
| Female, n (%) | 8 (47.1) * | 14 (56) * | 0.059 n.s. |
| Postoperative Scores | |||
| VAS Shoulder (points) | 1 (0.5) * | 1 (0) * | 0.630 n.s. |
| SST (points) | 8 (2.5) * | 10 (4) * | 0.037 n.s. |
| ASES (points) | 85 (23) * | 90 (73) * | 0.076 n.s. |
| CS (points) | 62.6 ± 11.6 | 70.8 ± 11.1 | 0.026 n.s. |
| IRObad | IROgood | p-Value | |
|---|---|---|---|
| Postoperative Functional Assessment | |||
| ROM active (°) | |||
| Forward Flexion | 130.6 ± 24.8 | 140.2 ± 18.5 | 0.158 n.s. |
| Abduction | 120 (40.) * | 120 (50) * | 0.284 n.s. |
| External Rotation | 27.4 ± 17 | 29.8 ± 18.3 | 0.662 n.s. |
| ROM passive (°) | |||
| Forward Flexion | 90 (0) * | 90 (0) * | 0.694 n.s. |
| Abduction | 85 (10) * | 90 (7.5) * | 0.093 n.s. |
| External Rotation | 28.2 ± 18.2 | 35.4 ± 21.2 | 0.262 n.s. |
| Abduction strength (N) | 3.6 ± 2.1 | 3.7 ± 19 | 0.862 n.s. |
| IRObad | IROgood | p-Value | |
|---|---|---|---|
| Radiographic Assessment | |||
| Preoperatively | |||
| LSA | 98.2 (20.2) * | 101.4 (8.8) * | 0.969 n.s. |
| DSA | 15.7 ± 5.8 | 16.5 ± 8.3 | 0.727 n.s. |
| CSA | 31.8 ± 4.6 | 34.4 ± 5.6 | 0.118 n.s. |
| AHD | 7.3 ± 4.7 | 6.4 ± 7.3 | 0.476 n.s. |
| Medialization | 40.4 ± 10.3 | 41.8 ± 7.4 | 0.613 n.s. |
| Distalization | 11.9 ± 6.3 | 14.1 ± 7.1 | 0.301 n.s. |
| Lateralization | 49.7 (13) * | 49.9 (13.9) * | 0.505 n.s. |
| Glenoid inclination | 102.4 ± 6.4 | 103.1 ± 7.3 | 0.746 n.s. |
| Glenoid version | 83.4 (15.4) * | 86 (7) * | 0.595 n.s. |
| Glenoid overhang | 3.4 ± 2.7 | 4.2 ± 2.9 | 0.373 n.s. |
| Postoperatively | |||
| LSA | 85.5 ± 8.1 | 88.9 ± 9.3 | 0.155 n.s. |
| DSA | 46.7 ± 8 | 42.8 ± 11.2 | 0.214 n.s. |
| Medialization | 18.7 ± 6.6 | 18.8 ± 19.8 | 0.969 n.s. |
| Distalization | 36.9 ± 7 | 36 ± 6.6 | 0.343 n.s. |
| Lateralization | 51.5 ± 5.8 | 52.4 ± 5.7 | 0.878 n.s. |
| Glenoid inclination | 95.4 ± 9.3 | 96 ± 9.1 | 0.749 n.s. |
| Glenoid overhang | 4.4 ± 3.4 | 5.8 ± 3.7 | 0.373 n.s. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hochberger, F.; Siebler, J.; Rupp, M.-C.; Scheiderer, B.; Siebenlist, S.; Geyer, S. Demographic-, Radiographic-, and Surgery-Related Factors Do Not Affect Functional Internal Rotation Following Reverse Total Shoulder Arthroplasty: A Retrospective Comparative Study. Healthcare 2024, 12, 1695. https://doi.org/10.3390/healthcare12171695
Hochberger F, Siebler J, Rupp M-C, Scheiderer B, Siebenlist S, Geyer S. Demographic-, Radiographic-, and Surgery-Related Factors Do Not Affect Functional Internal Rotation Following Reverse Total Shoulder Arthroplasty: A Retrospective Comparative Study. Healthcare. 2024; 12(17):1695. https://doi.org/10.3390/healthcare12171695
Chicago/Turabian StyleHochberger, Felix, Jakob Siebler, Marco-Christopher Rupp, Bastian Scheiderer, Sebastian Siebenlist, and Stephanie Geyer. 2024. "Demographic-, Radiographic-, and Surgery-Related Factors Do Not Affect Functional Internal Rotation Following Reverse Total Shoulder Arthroplasty: A Retrospective Comparative Study" Healthcare 12, no. 17: 1695. https://doi.org/10.3390/healthcare12171695
APA StyleHochberger, F., Siebler, J., Rupp, M.-C., Scheiderer, B., Siebenlist, S., & Geyer, S. (2024). Demographic-, Radiographic-, and Surgery-Related Factors Do Not Affect Functional Internal Rotation Following Reverse Total Shoulder Arthroplasty: A Retrospective Comparative Study. Healthcare, 12(17), 1695. https://doi.org/10.3390/healthcare12171695






