Antimicrobial Resistance in Sepsis Cases Due to Escherichia coli and Klebsiella pneumoniae: Pre-Pandemic Insights from a Single Center in Southwestern Romania
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Data Collection
2.3. Inclusion Criteria
2.4. Microbiological Identification
2.5. Data Analysis
2.6. Ethical Considerations
3. Results
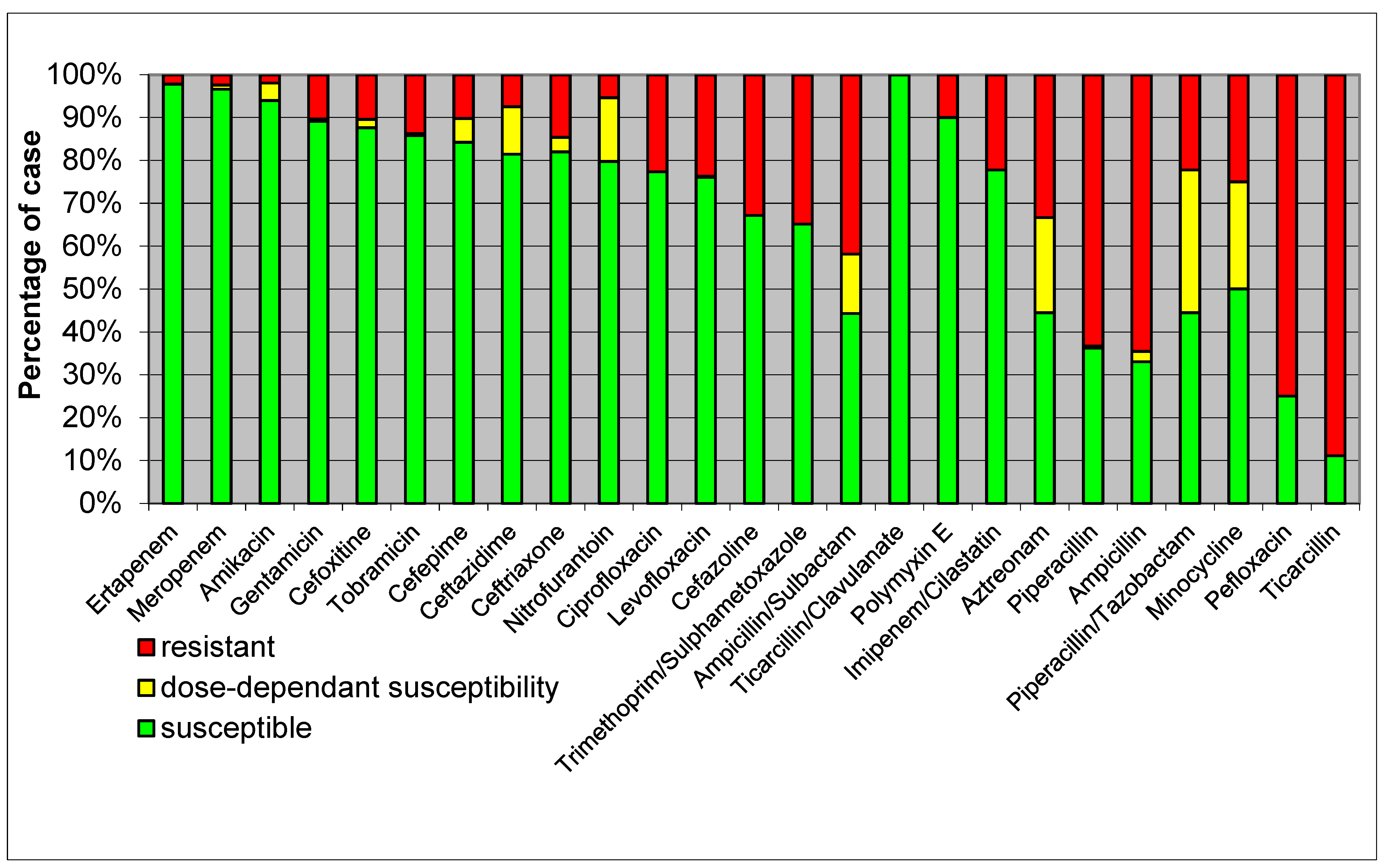
- High Resistance: E. coli isolates showed high resistance to ampicillin, reflecting common resistance patterns seen globally.
- Moderate Resistance: there was moderate resistance to cephalosporins such as ceftriaxone and ceftazidime, highlighting the presence of extended-spectrum β-lactamase (ESBL)-producing strains.
- Low Resistance: the lowest resistance rates were observed for carbapenems like meropenem and imipenem, indicating their continued effectiveness, though vigilance is required due to emerging resistance.
- High Resistance: K. pneumoniae exhibited high resistance to β-lactams, including ampicillin and cefazoline, suggesting widespread β-lactamase production.
- Moderate Resistance: there was moderate resistance to quinolones such as ciprofloxacin, indicating a significant but lesser extent of resistance compared to β-lactams.
- Low Resistance: susceptibility to carbapenems like meropenem remained relatively high, although the presence of carbapenem-resistant strains is a growing concern.
4. Discussion
4.1. Study Limitations
4.2. Implications for Future Research and Practice
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, C.; Scherag, A.; Adhikari, N.K.; Hartog, C.S.; Tsaganos, T.; Schlattmann, P.; Angus, D.C.; Reinhart, K. Assessment of Global Incidence and Mortality of Hospital-treated Sepsis. Current Estimates and Limitations. Am. J. Respir. Crit. Care Med. 2016, 193, 259–272. [Google Scholar] [CrossRef]
- Cohen, J.; Vincent, J.L.; Adhikari, N.K.; Machado, F.R.; Angus, D.C.; Calandra, T.; Jaton, K.; Giulieri, S.; Delaloye, J.; Opal, S.; et al. Sepsis: A roadmap for future research. Lancet Infect. Dis. 2015, 15, 581–614. [Google Scholar] [CrossRef]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef]
- Center for Disease Control and Prevention (CDC). What Is Sepsis? Available online: https://www.cdc.gov/sepsis/about/?CDC_AAref_Val=https://www.cdc.gov/sepsis/what-is-sepsis.html (accessed on 15 October 2023).
- Bauer, M.; Gerlach, H.; Vogelmann, T.; Preissing, F.; Stiefel, J.; Adam, D. Mortality in sepsis and septic shock in Europe, North America and Australia between 2009 and 2019-results from a systematic review and meta-analysis. Crit. Care 2020, 24, 239. [Google Scholar] [CrossRef]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021, 47, 1181–1247. [Google Scholar] [CrossRef]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Livermore, D.M. Has the era of untreatable infections arrived? J. Antimicrob. Chemother. 2009, 64, i29–i36. [Google Scholar] [CrossRef] [PubMed]
- Laxminarayan, R.; Duse, A.; Wattal, C.; Zaidi, A.K.; Wertheim, H.F.; Sumpradit, N.; Vlieghe, E.; Hara, G.L.; Gould, I.M.; Goossens, H.; et al. Antibiotic resistance—The need for global solutions. Lancet Infect. Dis. 2013, 13, 1057–1098. [Google Scholar] [CrossRef]
- WHO Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics. 2017. Available online: https://remed.org/wp-content/uploads/2017/03/lobal-priority-list-of-antibiotic-resistant-bacteria-2017.pdf (accessed on 17 October 2023).
- Bassetti, M.; Garau, J. Current and future perspectives in the treatment of multidrug-resistant Gram-negative infections. J. Antimicrob. Chemother. 2021, 76, iv23–iv37. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Jubeh, B.; Karaman, R. Resistance of Gram-Negative Bacteria to Current Antibacterial Agents and Approaches to Resolve It. Molecules 2020, 25, 1340. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.; Davies, D. Origins and Evolution of Antibiotic Resistance. Microbiol. Mol. Biol. Rev. 2010, 74, 417–433. [Google Scholar] [CrossRef] [PubMed]
- Russo, T.A.; Johnson, J.R. Medical and economic impact of extraintestinal infections due to Escherichia coli: Focus on an increasingly important endemic problem. Microbes Infect. 2003, 5, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Burden of AMR Collaborative Group. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modelling analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute, Performance Standards for Antimicrobial Susceptibility Testing 2017. Available online: https://webstore.ansi.org/standards/clsi/clsim100s27 (accessed on 22 October 2023).
- Cockerill, F.R.; Wikler, M.A.; Alder, J.; Dudley, M.N.; Eliopoulos, G.M.; Ferraro, M.J.; Hardy, D.J.; Hecht, D.W.; Hindler, J.A.; Patel, J.B.; et al. Performance Standards for Antimicrobial Disk Susceptibility Tests, 13th ed.; Clinical and L. S. Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Weinstein, M.P.; Patel, J.B.; Burnham, C.-A.; Campeau, S.; Conville, P.S.; Doern, C.; Eliopoulos, G.M.; Galas, M.F.; Humphries, R.M.; Jenkins, S.G.; et al. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed.; Clinical and L. S. Institute: Wayne, PA, USA, 2018. [Google Scholar]
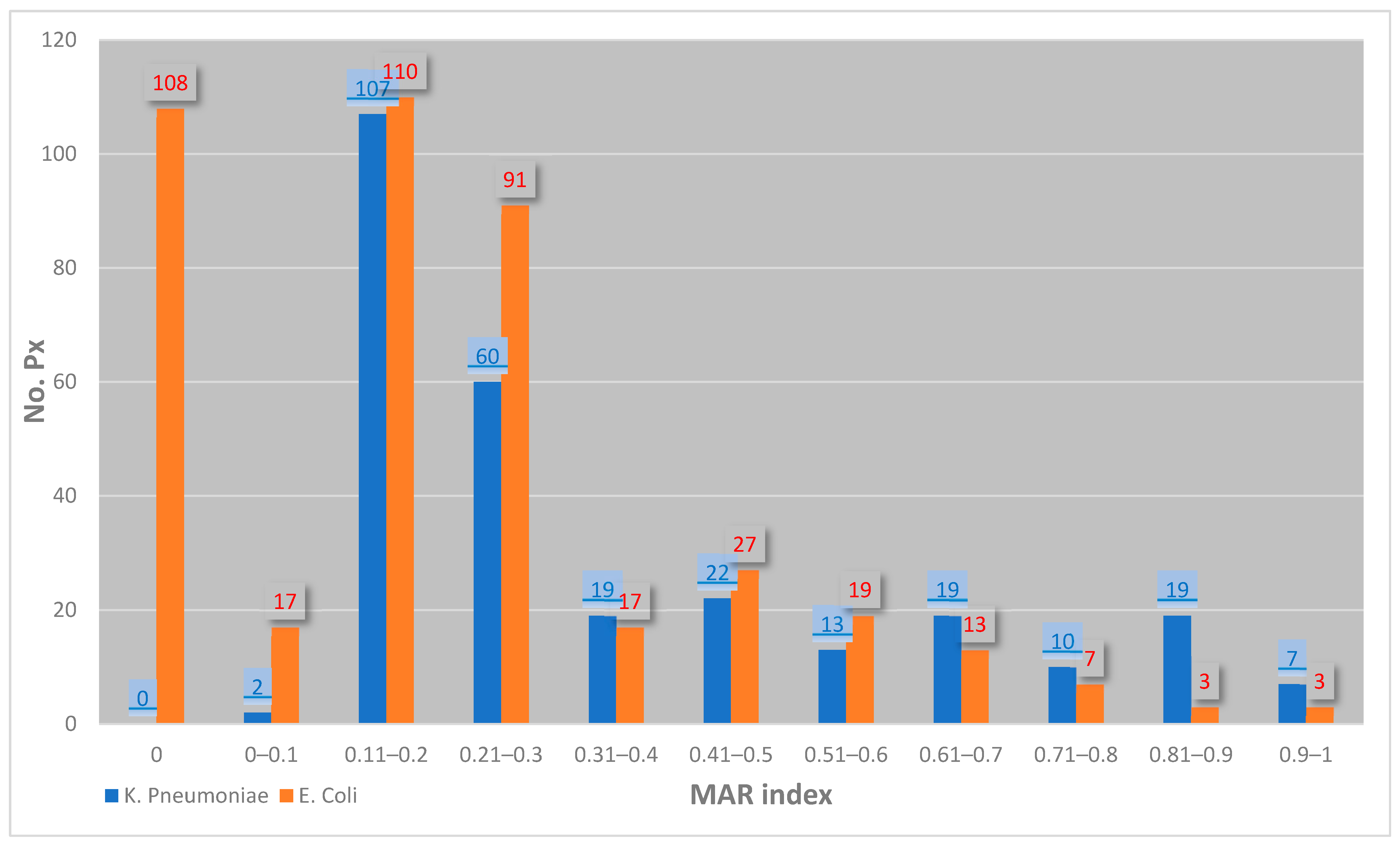
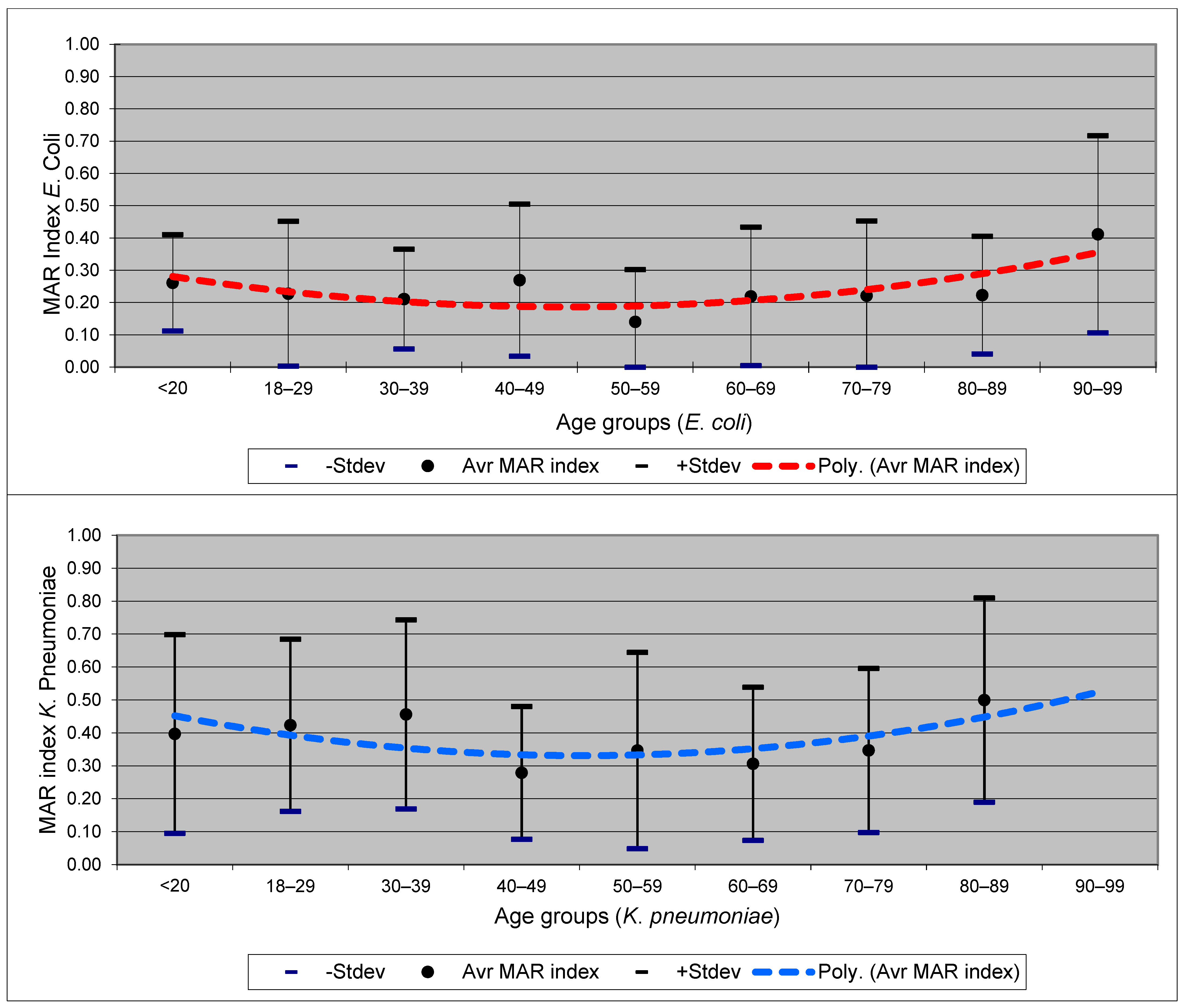
- Krumperman, P.H. Multiple antibiotic resistance indexing of Escherichia coli to identify high-risk sources of fecal contamination of foods. Appl. Environ. Microbiol. 1983, 46, 165–170. [Google Scholar] [CrossRef]
- Altman, D.G. Practical Statistics for Medical Research; Chapman and Hall/CRC: Boca Raton, FL, USA, 1990. [Google Scholar] [CrossRef]
- Mellhammar, L.; Wollter, E.; Dahlberg, J.; Donovan, B.; Olséen, C.J.; Wiking, P.O.; Rose, N.; Schwarzkopf, D.; Friedrich, M.; Fleischmann-Struzek, C.; et al. Estimating Sepsis Incidence Using Administrative Data and Clinical Medical Record Review. JAMA Netw. Open 2023, 6, e2331168. [Google Scholar] [CrossRef]
- Trecarichi, E.M.; Giuliano, G.; Cattaneo, C.; Ballanti, S.; Criscuolo, M.; Candoni, A.; Marchesi, F.; Laurino, M.; Dargenio, M.; Fanci, R.; et al. Bloodstream infections caused by Escherichia coli in onco-haematological patients: Risk factors and mortality in an Italian prospective survey. PLoS ONE 2019, 14, e0224465. [Google Scholar] [CrossRef]
- Dolin, H.H.; Papadimos, T.J.; Chen, X.; Pan, Z.K. Characterization of Pathogenic Sepsis Etiologies and Patient Profiles: A Novel Approach to Triage and Treatment. Microbiol. Insights 2019, 12, 1178636118825081. [Google Scholar] [CrossRef]
- Yan, H.; Guo, L.; Pang, Y.; Liu, F.; Liu, T.; Gao, M. Clinical characteristics and predictive model of pulmonary tuberculosis patients with pulmonary fungal coinfection. BMC Pulm. Med. 2023, 23, 56. [Google Scholar] [CrossRef]
- Galvin, J.; Tiberi, S.; Akkerman, O.; Kerstjens, H.A.M.; Kunst, H.; Kurhasani, X.; Ambrosino, N.; Migliori, G.B. Pulmonary tuberculosis in intensive care setting, with a focus on the use of severity scores, a multinational collaborative systematic review. Pulmonology 2022, 28, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Niederman, M.S.; Baron, R.M.; Bouadma, L.; Calandra, T.; Daneman, N.; DeWaele, J.; Kollef, M.H.; Lipman, J.; Nair, G.B. Initial antimicrobial management of sepsis. Crit. Care 2021, 25, 307. [Google Scholar] [CrossRef] [PubMed]
- Gauer, R.; Forbes, D.; Boyer, N. Sepsis: Diagnosis and Management. Am. Fam. Physician 2020, 101, 409–418. [Google Scholar] [PubMed]
- de Kraker, M.E.A.; Wolkewitz, M.; Davey, P.G.; Koller, W.; Berger, J.; Nagler, J.; Icket, C.; Kalenic, S.; Horvatic, J.; Seifert, H.; et al. Burden of antimicrobial resistance in European hospitals: Excess mortality and length of hospital stay associated with bloodstream infections due to Escherichia coli resistant to third-generation cephalosporins. J. Antimicrob. Chemother. 2011, 66, 398–407. [Google Scholar] [CrossRef]
- ECDC Surveillance of Antimicrobial Resistance in Europe 2018. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/surveillance-antimicrobial-resistance-Europe-2018.pdf (accessed on 15 October 2023).
- Popescu, G.A.; Şerban, R.; Niculcea, A. 2019 CARMIN-ROM 2017 Consumul de Antibiotice, Rezistenţa Microbiană şi Infecţiile Asociate Asistenţei Medicale (Nosocomiale) în România—2017. Available online: www.cnscbt.ro/index.php/analiza-date-supraveghere/infectii-nosocomiale-1/1309-consumul-de-antibiotice-rezistenta-microbiana-si-infectii-asociate-asistentei-medicale-nosocomiale-in-romania-2017/file (accessed on 22 October 2023).
- Mae, E.-S.A.; Zhong, L.L.; Shen, C.; Yang, Y.; Tian, G.B. Colistin and its role in the Era of antibiotic resistance: An extended review (2000–2019). Emerg. Microbes Infect. 2020, 9, 868–885. [Google Scholar]
- Munita, J.M.; Arias, C.A. Mechanisms of Antibiotic Resistance. Microbiol. Spectr. 2016, 4, 464–473. [Google Scholar] [CrossRef]
- Naqvi, S.A.R.; Drlica, K. Fluoroquinolones as imaging agents for bacterial infection. Dalton Trans. 2017, 46, 14452–14460. [Google Scholar] [CrossRef]
- Pitout, J.D. Extraintestinal Pathogenic Escherichia coli: A Combination of Virulence with Antibiotic Resistance. Front. Microbiol. 2012, 3, 9. [Google Scholar] [CrossRef]
- Podschun, R.; Ullmann, U. Klebsiella spp. as nosocomial pathogens: Epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin. Microbiol. Rev. 1998, 11, 589–603. [Google Scholar] [CrossRef]
- Buehrle, D.J.; Decker, B.K.; Wagener, M.M.; Adalja, A.; Singh, N.; McEllistrem, M.C.; Nguyen, M.H.; Clancy, C.J. Antibiotic Consumption and Stewardship at a Hospital outside of an Early Coronavirus Disease 2019 Epicenter. Antimicrob. Agents Chemother. 2020, 64, e01011-20. [Google Scholar] [CrossRef]
- Guarino, M.; Perna, B.; Cesaro, A.E.; Maritati, M.; Spampinato, M.D.; Contini, C.; De Giorgio, R. Update on Sepsis and Septic Shock in Adult Patients: Management in the Emergency Department. J. Clin. Med. 2023, 12, 3188. [Google Scholar] [CrossRef] [PubMed]
- Holmes, A.H.; Moore, L.S.; Sundsfjord, A.; Steinbakk, M.; Regmi, S.; Karkey, A.; Guerin, P.J.; Piddock, L.J. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet 2016, 387, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, J.G.; Gilbert, D.N.; Spellberg, B. Seven Ways to Preserve the Miracle of Antibiotics. Clin. Infect. Dis. 2013, 56, 1445–1450. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | E. coli (n = 415) | K. pneumoniae (n = 278) | p-Value |
|---|---|---|---|
| Environment | |||
| 198 (47.71%) | 134 (48.20%) | |
| 217 (52.29%) | 144 (51.80%) | |
| Age (years) | |||
| 53.03 ± 19.31 | 56.51 ± 15.16 | 0.0117 |
| 56 (18–94) | 59 (16–88) | |
| Sex | |||
| 133 (32.05%) | 155 (55.76%) | |
| 282 (67.95%) | 123 (44.24%) | |
| 415 | 278 | p < 0.001 |
| Specimen | E. coli Cases | % | K. pneumoniae Cases | % | p |
|---|---|---|---|---|---|
| Sputum | 57 | 13.73 | 175 | 62.95 | <0.0001 |
| Bronchial aspirate | 0 | 0.00 | 4 | 1.44 | N/A |
| Pus from wound | 1 | 0.24 | 6 | 2.16 | N/A |
| Blood | 29 | 6.99 | 9 | 3.24 | 0.0128 |
| Urine | 323 | 77.83 | 79 | 28.42 | <0.0001 |
| Feces | 2 | 0.48 | 0 | 0.00 | N/A |
| Vaginal secretion | 2 | 0.48 | 2 | 0.72 | N/A |
| Pericardial fluid | 1 | 0.24 | 0 | 0.00 | N/A |
| Cerebrospinal fluid | 0 | 0.00 | 1 | 0.36 | N/A |
| Peritoneal fluid | 0 | 0.00 | 1 | 0.36 | N/A |
| Bile | 0 | 0.00 | 1 | 0.36 | N/A |
| Total | 415 | 278 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giubelan, L.-I.; Neacșu, A.I.; Rotaru-Zavaleanu, A.D.; Osiac, E. Antimicrobial Resistance in Sepsis Cases Due to Escherichia coli and Klebsiella pneumoniae: Pre-Pandemic Insights from a Single Center in Southwestern Romania. Healthcare 2024, 12, 1713. https://doi.org/10.3390/healthcare12171713
Giubelan L-I, Neacșu AI, Rotaru-Zavaleanu AD, Osiac E. Antimicrobial Resistance in Sepsis Cases Due to Escherichia coli and Klebsiella pneumoniae: Pre-Pandemic Insights from a Single Center in Southwestern Romania. Healthcare. 2024; 12(17):1713. https://doi.org/10.3390/healthcare12171713
Chicago/Turabian StyleGiubelan, Lucian-Ion, Alexandru Ionuț Neacșu, Alexandra Daniela Rotaru-Zavaleanu, and Eugen Osiac. 2024. "Antimicrobial Resistance in Sepsis Cases Due to Escherichia coli and Klebsiella pneumoniae: Pre-Pandemic Insights from a Single Center in Southwestern Romania" Healthcare 12, no. 17: 1713. https://doi.org/10.3390/healthcare12171713






