Comparison of Endoscopic and Microscopic Surgery for the Treatment of Acquired Cholesteatoma by EAONO/JOS Staging
Abstract
1. Introduction
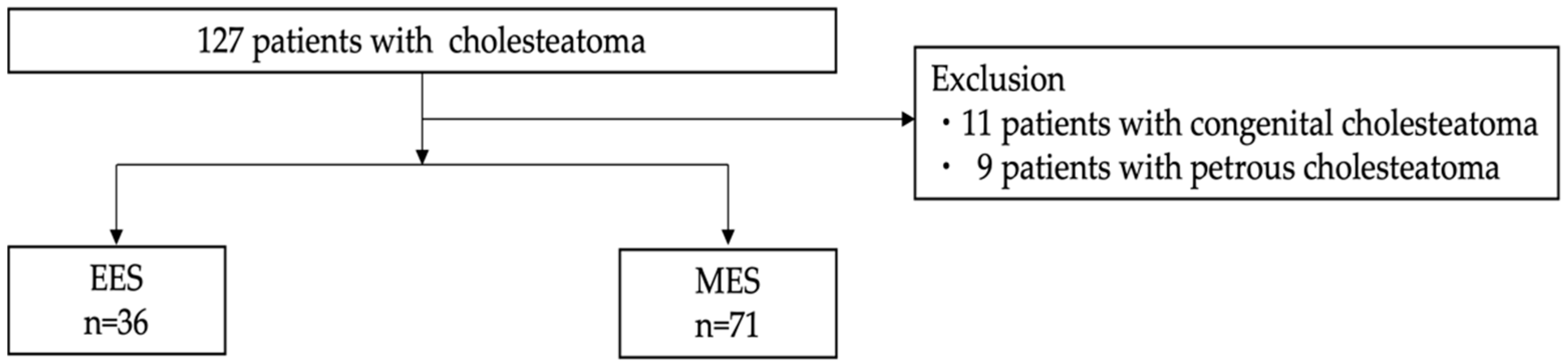
2. Materials and Methods
2.1. Study Designs
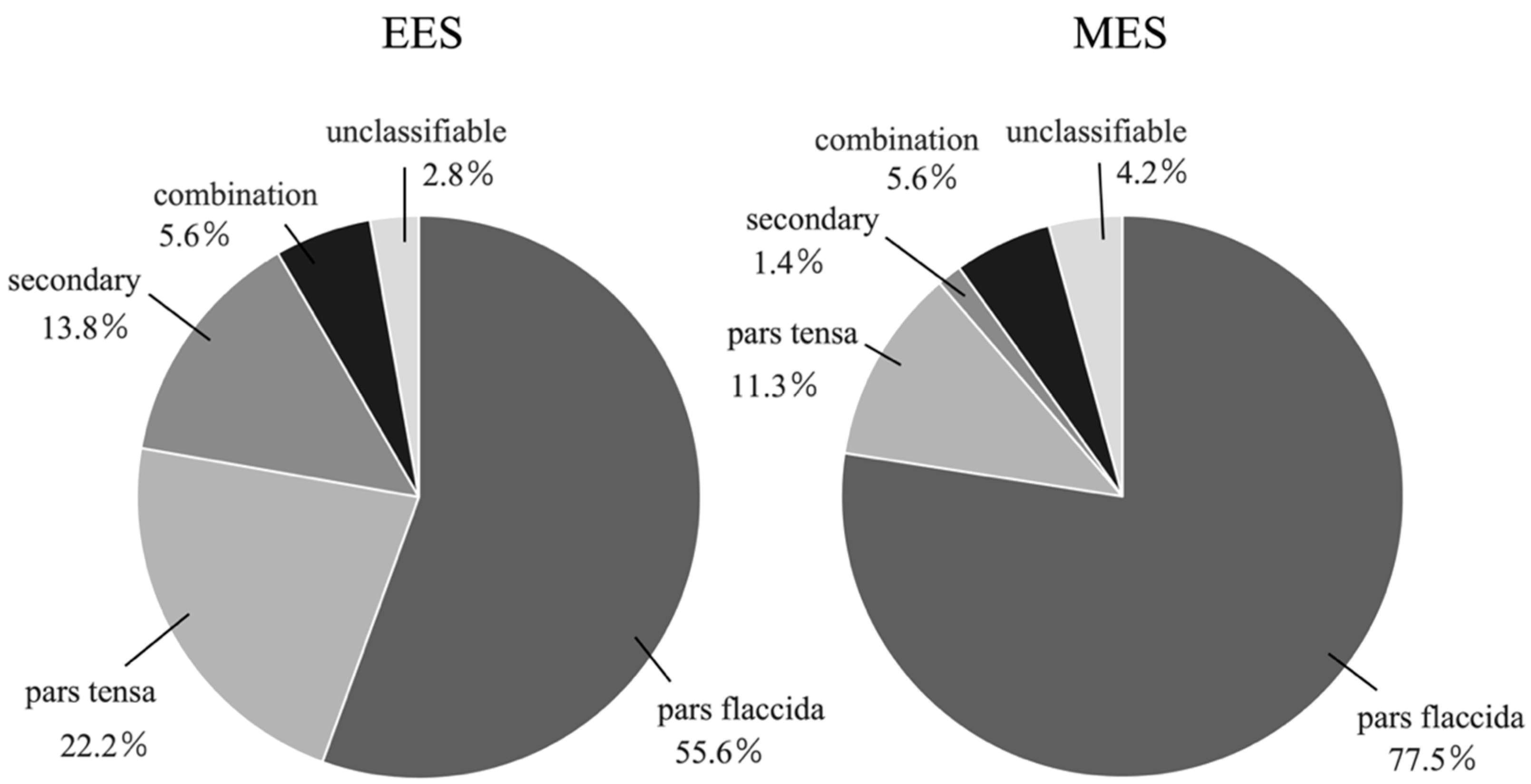
- Pars flaccida
- Pars tensa
- Secondary
- Combination
- Unclassifiable
- Stage I: Cholesteatoma localized in the primary site.
- Stage II: Cholesteatoma involving two or more sites.
- Stage III: Cholesteatoma with extracranial complications and/or intratemporal pathological conditions.
- Stage IV: Cholesteatoma with intracranial complications.
2.2. Outcome Measures
2.3. Statistical Analysis
3. Results
3.1. Types of Cholesteatoma (Figure 3, Table 1)
| EES * | MES * | p-Value | |
|---|---|---|---|
| pars flaccida | 20 (55.6%) | 55 (77.5%) | 0.015 |
| pars tensa | 8 (22.2%) | 8 (11.3%) | 0.13 |
| secondary | 5 (13.8%) | 1 (1.4%) | 0.019 |
| combination | 2 (5.6%) | 4 (5.6%) | 1.0 |
| unclassifiable | 1 (2.8%) | 3 (4.2%) | 1.0 |
| total | 36 | 71 |
3.2. Staging of Cholesteatoma (Table 2)
| EES * | MES * | p-Value | |
|---|---|---|---|
| stage I | 15 (41.6%) | 12 (16.9%) | 0.0053 |
| stage II | 20 (55.6%) | 55 (77.5%) | 0.019 |
| stage III | 1 (2.8%) | 4 (5.6%) | 0.66 |
| total | 36 | 71 |
3.3. Location of Cholesteatoma (Supplementary Table S1)
3.4. Complications
3.5. Recurrence of Cholesteatoma
3.6. Hearing Outcomes (Table 3A–C)
| (A) | |||
| Preoperative (dB) | Postoperative (dB) | p-Value | |
| stage I (n = 13) | 47.12 ± 21.45 | 40.77 ± 19.62 | 0.04 |
| stage II (n =16) | 40.31 ± 14.31 | 41.09 ± 22.45 | 0.80 |
| all cases (n=30) | 42.67 ± 17.95 | 40.96 ± 20.50 | 0.44 |
| (B) | |||
| Preoperative (dB) | Postoperative (dB) | p-Value | |
| stage I (n = 4) | 57.19 ± 10.28 | 59.69 ± 29.87 | 0.82 |
| stage II (n =38) | 45.49 ± 18.08 | 41.90 ± 17.00 | 0.14 |
| all cases (n =45) | 46.36 ± 18.28 | 44.33 ± 19.42 | 0.37 |
| (C) | |||
| EES (dB) | MES (dB) | p-Value | |
| stage I (n =13 in EES, 4 in MES) | 6.35 ± 9.94 | −2.50 ± 21.04 | 0.25 |
| stage II (n =16 in EES, 38 in MES) | −0.78 ± 12.02 | 3.57 ± 14.50 | 0.30 |
| all cases (n =30 in EES, 45 in MES) | 1.71 ± 11.97 | 2.02 ± 14.98 | 0.92 |
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chiao, W.; Chieffe, D.; Fina, M. Endoscopic Management of Primary Acquired Cholesteatoma. Otolarayngol. Clin. N. Am. 2021, 54, 129–145. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.L.; Shiao, A.-S.; Yung, M.; Sakagami, M.; Sudhoff, H.; Wang, C.-H.; Hsu, C.-H.; Lien, C.-F. Updates and Knowledge Gaps in Cholesteatoma Research. Biomed. Res. Int. 2015, 2015, 854024. [Google Scholar] [CrossRef] [PubMed]
- Marchioni, D.; Alicandri-Ciufelli, M.; Piccinini, A.; Genovese, E.; Presutti, L. Inferior retrotympanum revisited: An endoscopic anatomic study. Laryngoscope 2010, 120, 1880–1886. [Google Scholar] [CrossRef] [PubMed]
- Tarabichi, M.; Nogueira, J.F.; Marchioni, D.; Presutti, L.; Pothier, D.D.; Ayache, S. Transcanal endoscopic management of cholesteatoma. Otolarayngol. Clin. N. Am. 2013, 46, 107–130. [Google Scholar] [CrossRef]
- Presutti, L.; Gioacchini, F.M.; Alicandri-Ciufelli, M.; Villari, D.; Marchioni, D. Results of endoscopic middle ear surgery for cholesteatoma treatment: A systematic review. Acta Otorhinolaryngol. Ital. 2014, 34, 153–157. [Google Scholar]
- Beckmann, S.; Mantokoudis, G.; Weder, S.; Borner, U.; Caversaccio, M.; Anschuetz, L. Endoscopic Cholesteatoma Surgery. J. Vis. Exp. 2022, 2022, e63315. [Google Scholar]
- Dalğıç, A.; Aksoy, Y.G.; Zorlu, M.E.; Delice, O.; Aysel, A. Total Transcanal Endoscopic Ear Surgery for Cholesteatoma. Turk. Arch. Otorhinolaryngol. 2023, 61, 1–7. [Google Scholar] [CrossRef]
- Hu, Y.; Teh, B.M.; Hurtado, G.; Yao, X.; Huang, J.; Shen, Y. Can endoscopic ear surgery replace microscopic surgery in the treatment of acquired cholesteatoma? A contemporary review. Int. J. Pediatr. Otorhinolaryngol. 2020, 131, 109872. [Google Scholar] [CrossRef]
- Bae, M.R.; Kang, W.S.; Chung, J.W. Comparison of the clinical results of attic cholesteatoma treatment: Endoscopic versus microscopic ear suregery. Clin. Exp. Otorhinolaryngol. 2019, 12, 156–162. [Google Scholar] [CrossRef]
- Magliulo, G.; Lannella, G. Endoscopic versus microscopic approach in attic cholesteatoma surgery. Amerian J. Otolaryngol. 2018, 39, 25–30. [Google Scholar] [CrossRef]
- Hunter, J.B.; Zuniga, M.G.; Sweeney, A.D.; Bertrand, N.M.; Wanna, G.B.; Haynes, D.S.; Wootten, C.T.; Rivas, A. Peadiatric endoscopic cholesteatoma surgery. Otolaryngol. Head Neck Surg. 2016, 154, 1121–1127. [Google Scholar] [CrossRef] [PubMed]
- Yung, M.; Tono, T.; Olszewska, E.; Yamamoto, Y.; Sudhoff, H.; Sakagami, M.; Mulder, J.; Kojima, H.; İncesulu, A.; Trabalzini, F.; et al. EAONO/JOS Joint Consensus Statements on the Definitions, Classification and Staging of Middle Ear Cholesteatoma. J. Int. Adv. Otol. 2017, 13, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ardıç, F.N.; Mengi, E.; Tümkaya, F.; Kara, C.O.; Bir, F. Correlation between Surgical Outcome and Stage of Acquired Middle Ear Cholesteatoma: Revalidation of the EAONO/JOS Staging System. J. Int. Adv. Otol. 2020, 16, 34–39. [Google Scholar] [CrossRef]
- Fermi, M.; Bassano, E.; Villari, D.; Capriotti, V.; Calvaruso, F.; Bonali, M.; Alicandri-Ciufelli, M.; Marchioni, D.; Presutti, L. Prognostic role of EAONO/JOS, STAMCO, and ChOLE Staging for Exclusive Endoscopic and Endoscopic-Microscopic Tympanoplasty. Otolaryngol. Head Neck Surg. 2023, 168, 829–838. [Google Scholar] [CrossRef]
- Covelli, E.; Margani, V.; Filippi, C.; Elfarargy, H.H.; Volpini, L.; Romano, A.; Bozzao, A.; Barbara, M. Proposal of a magnetic resonance imaging follow-up protocol after cholesteatoma surgery: A prospective.study. Acta Oto-Laryngol. 2022, 142, 484–490. [Google Scholar] [CrossRef]
- Filipo, R.; Chiara D’Elia; Covelli, E.; Bertoli, G.A.; De Seta, E.; Manganaro, F.; Mancini, P. Haematoma after cochlear implantation: Management of a minor complication. Acta Oto-Laryngol. 2010, 130, 108–113. [Google Scholar] [CrossRef]
- Jenks, C.M.; Purcell, P.L.; Federici, G.; Villari, D.; Presutti, L.; James, A.L.; Hoff, S.R. Transcanal Endoscopic Ear Surgery for Congenital Cholesteatoma: A Multi-institutional Series. Otolaryngol. Head Neck Surg. 2022, 167, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Marchioni, D.; Soloperto, D.; Rubini, A.; Villari, D.; Genovese, E.; Artioli, F.; Presutti, L. Endoscopic exclusive transcanal approach to the tympanic cavity cholesteatoma in pediatric patients: Our experience. Int. J. Pediatr. Otorhinolaryngol. 2015, 79, 316–322. [Google Scholar] [CrossRef]
- Bennett, M.L.; Zhang, D.; Labadie, R.F.; Noble, J.H. Comparison of middle ear visualization with endoscopy and microscopy. Otol. Neurotol. 2016, 37, 362–366. [Google Scholar] [CrossRef]
- Esu, Y.; Tamii, S.; Kanazawa, H.; Iino, Y.; Yoshida, N. Surgical management of secondary acquired cholesteatoma depends on its characteristics. Auris Nasus Larynx 2024, 51, 465–471. [Google Scholar] [CrossRef]
- Yamamoto, K.; Yaguchi, Y.; Kojima, H. Clinical analysis of secondary acquired cholesteatoma. Am. J. Otolaryngol. 2014, 35, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Uranaka, T.; Matsumoto, Y.; Hoshi, Y.; Iwasaki, S.; Kakigi, A.; Yamasoba, T. Classification of the Chorda Tympani: An Endoscopic Study. Otol. Neurotol. 2021, 42, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Liu, Q.; Gao, B.; Huang, S.; Yang, N. Comparison of endoscopic and microscopic management of attic cholesteatoma: A randomized controlled trial. Am. J. Otolaryngol. 2022, 43, 103378. [Google Scholar] [CrossRef]
- Glikson, E.; Yousovich, R.; Mansour, J.; Wolf, M.; Migirov, L.; Shapira, Y. Transcanal Endoscopic Ear Surgery for Middle Ear Cholesteatoma. Otol. Neurotol. 2017, 38, e41–e45. [Google Scholar] [CrossRef]
- Migirov, L.; Shapira, Y.; Horowitz, Z.; Wolf, M. Exclusive endoscopic ear surgery for acquired cholesteatoma: Preliminary results. Otol. Neurotol. 2011, 32, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Killeen, D.E.; Tolisano, A.M.; Kou, Y.F.; Kutz, J.W.; Isaacson, B. Recidivism After Endoscopic Treatment of Cholesteatoma. Otol. Neurotol. 2019, 40, 1313–1321. [Google Scholar] [CrossRef]
- Li, B.; Zhou, L.; Wang, M.; Wang, Y.; Zou, J. Endoscopic versus microscopic surgery for treatment of middle ear cholesteatoma: A systematic review and meta-analysis. Am. J. Otolaryngol. 2021, 42, 102451. [Google Scholar] [CrossRef]
- Yamauchi, D.; Honkura, Y.; Kawamura, Y.; Shimizu, Y.; Sunose, T.; Hara, Y.; Ohta, J.; Suzuki, J.; Kawase, T.; Katori, Y. Underwater Endoscopic Ear Surgery for Closure of Cholesteatomatous Labyrinthine Fistula With Preservation of Auditory Function. Otol. Neurotol. 2021, 42, E1669–E1676. [Google Scholar] [CrossRef]
- Guo, Y.-N.; Qian, M.; Li, J.; Xu, J.; Chen, H.; Zhang, H. Clinical retrospective study on the efficacy and safety of endoscopic ear surgery for adhesive otitis media. Ann. Transl. Med. 2022, 10, 1211. [Google Scholar] [CrossRef]
- Barbara, M.; Covelli, E.; Monini, S.; Bandiera, G.; Filippi, C.; Margani, V.; Volpini, L.; Salerno, G.; Romano, A.; Bozzao, A. Early non-EPI DW-MRI after cholesteatoma surgery. Ear Nose Throat J. Open Access 2024, 103, 435–441. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otsuka, A.; Koyama, H.; Kashio, A.; Matsumoto, Y.; Yamasoba, T. Comparison of Endoscopic and Microscopic Surgery for the Treatment of Acquired Cholesteatoma by EAONO/JOS Staging. Healthcare 2024, 12, 1737. https://doi.org/10.3390/healthcare12171737
Otsuka A, Koyama H, Kashio A, Matsumoto Y, Yamasoba T. Comparison of Endoscopic and Microscopic Surgery for the Treatment of Acquired Cholesteatoma by EAONO/JOS Staging. Healthcare. 2024; 12(17):1737. https://doi.org/10.3390/healthcare12171737
Chicago/Turabian StyleOtsuka, Ayaka, Hajime Koyama, Akinori Kashio, Yu Matsumoto, and Tatsuya Yamasoba. 2024. "Comparison of Endoscopic and Microscopic Surgery for the Treatment of Acquired Cholesteatoma by EAONO/JOS Staging" Healthcare 12, no. 17: 1737. https://doi.org/10.3390/healthcare12171737
APA StyleOtsuka, A., Koyama, H., Kashio, A., Matsumoto, Y., & Yamasoba, T. (2024). Comparison of Endoscopic and Microscopic Surgery for the Treatment of Acquired Cholesteatoma by EAONO/JOS Staging. Healthcare, 12(17), 1737. https://doi.org/10.3390/healthcare12171737






