The Effectiveness and Safety of Wu Tou Decoction on Rheumatoid Arthritis—A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Study Registration
2.3. Search Strategy
2.4. Inclusion and Exclusion Criteria
2.4.1. Inclusion Criteria
2.4.2. Exclusion Criteria
2.5. Study Selection and Data Extraction
2.5.1. The Characteristics of Study
2.5.2. Outcome Measures
2.6. Statistical Analysis
2.7. Quality Assessment
2.8. Publication Bias
3. Results
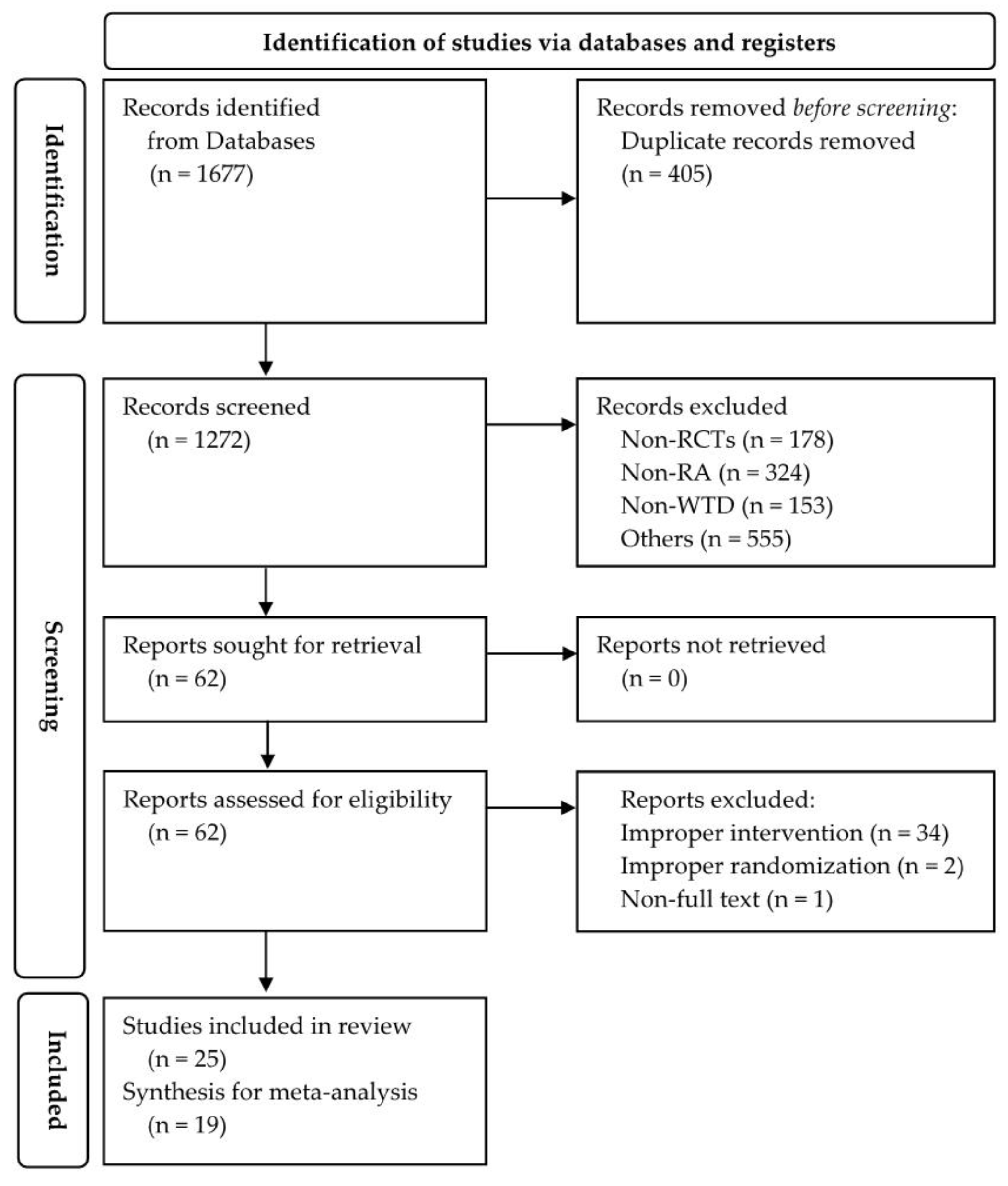
3.1. Study Selection
3.2. Characteristics of the Included Studies
3.3. Efficacy Assessment of WTD Monotherapy
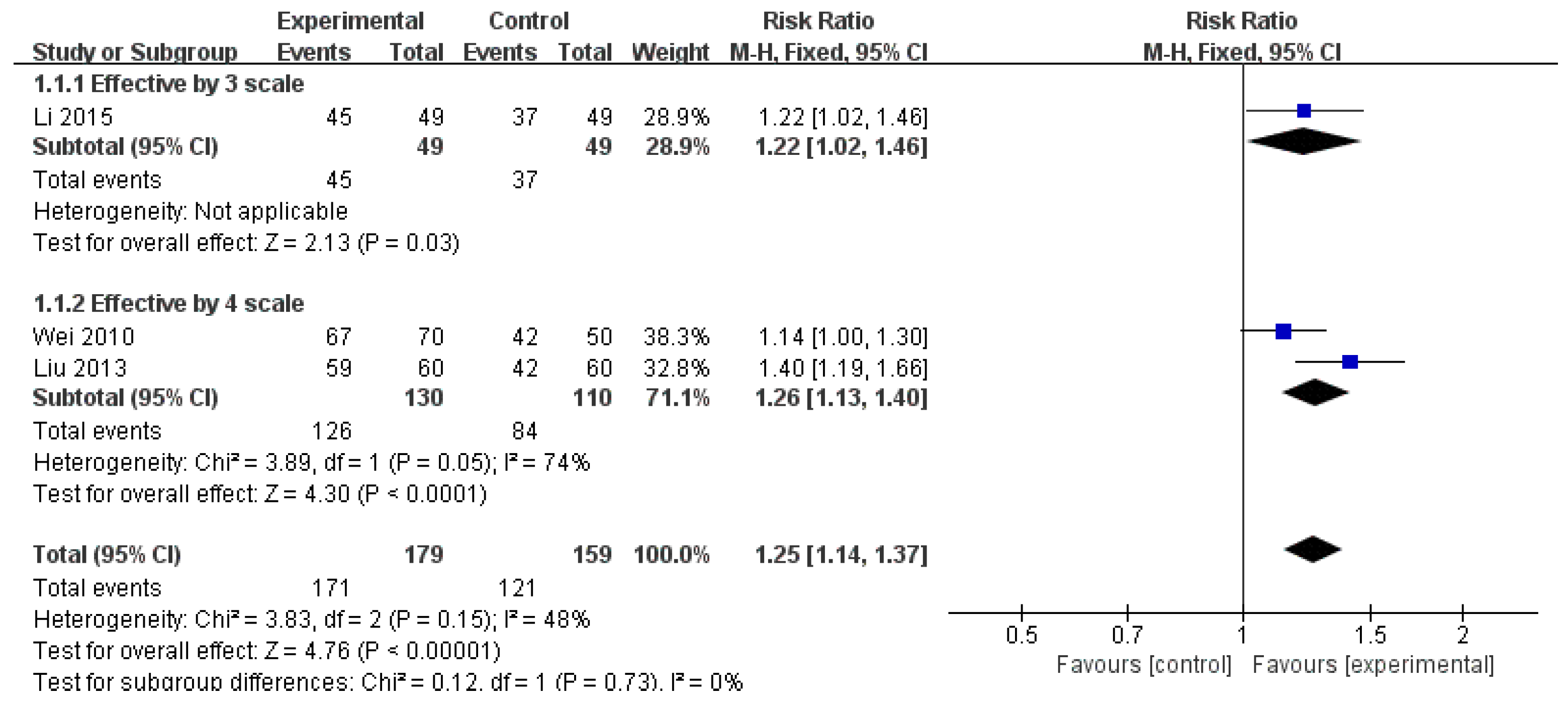
3.3.1. Effective Rate
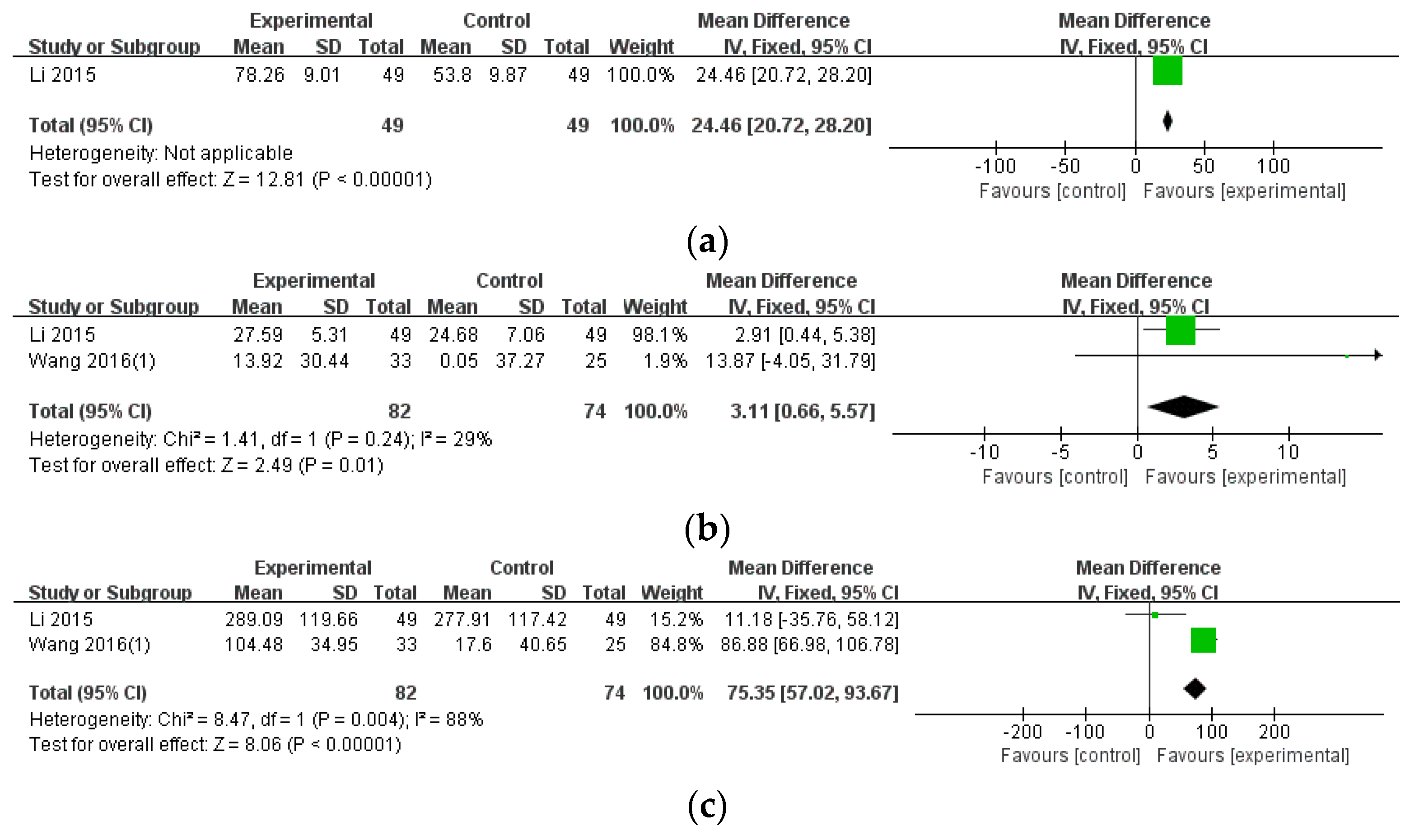
3.3.2. Blood Test Results
3.3.3. Other Outcome Measures of RA
3.4. Efficacy Assessment of WTD Combination Therapy
3.4.1. Effective Rate
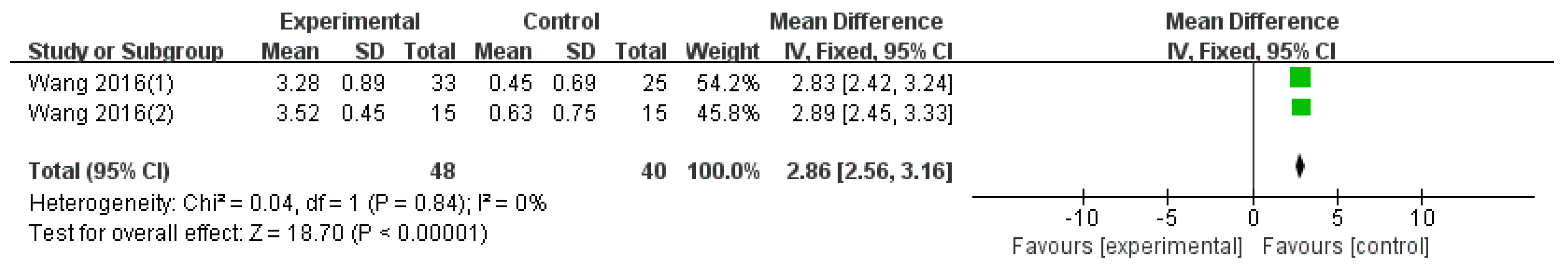
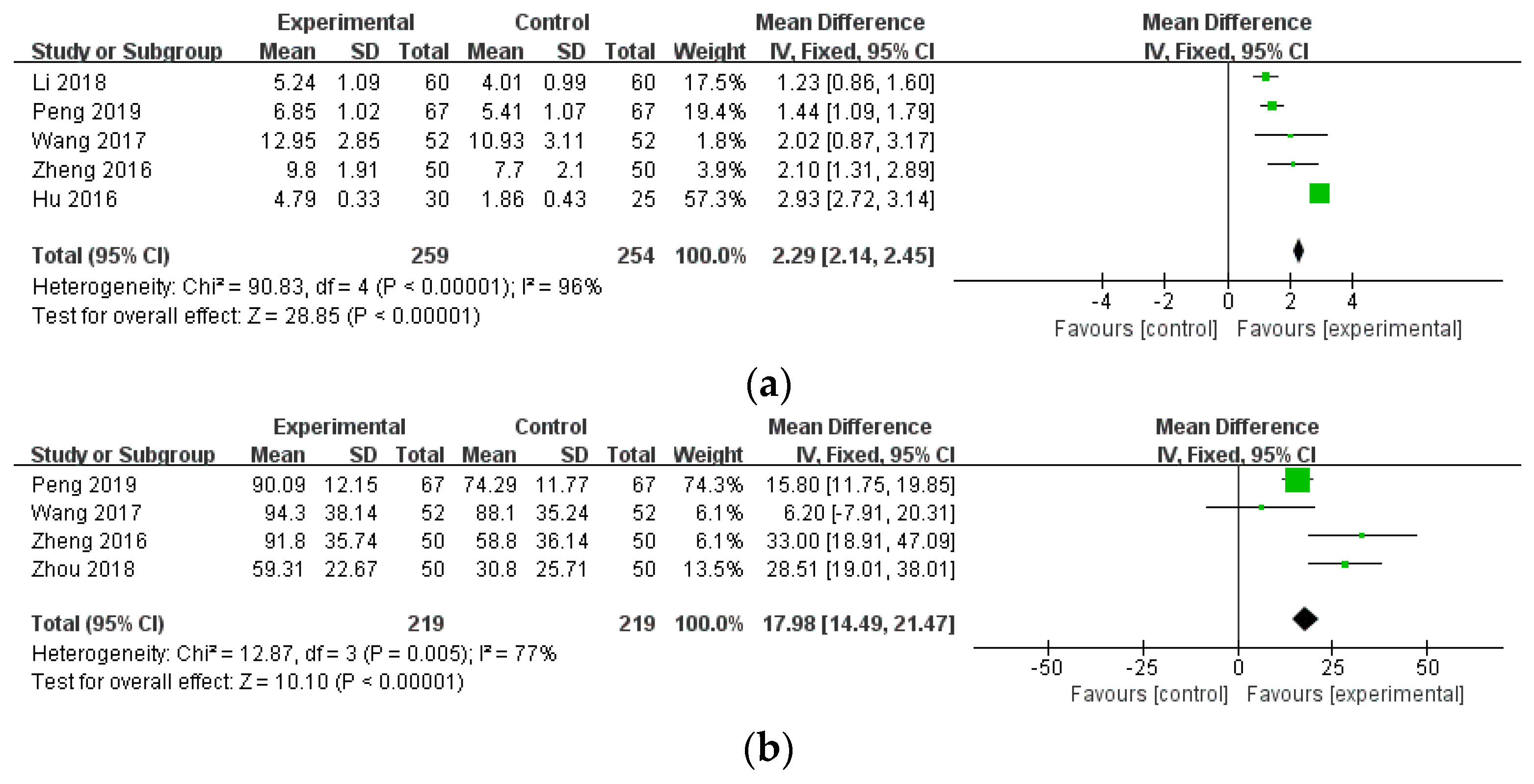
3.4.2. Disease Activity Outcomes
3.4.3. Blood Test Results
3.4.4. Other Outcome Measures of RA
3.5. Safety Assessment
3.6. Risk of Bias Assessment
3.7. Sensitivity Analysis
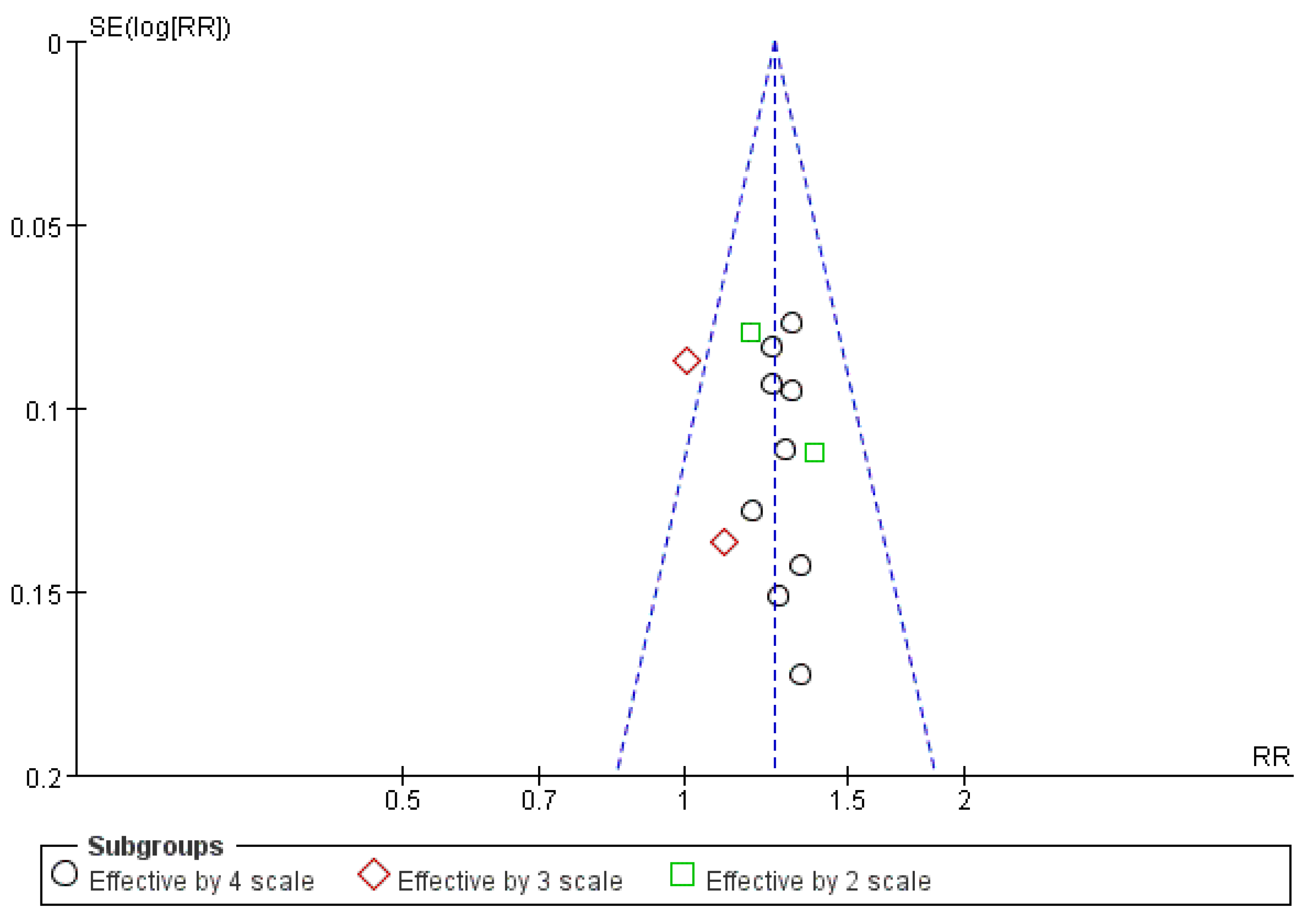
3.8. Publication Bias Assessment
3.9. Evidence Evaluation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Radu, A.F.; Bungau, S.G. Management of Rheumatoid Arthritis: An Overview. Cells 2021, 10, 2857. [Google Scholar] [CrossRef] [PubMed]
- Itoh, Y. Metalloproteinases: Potential therapeutic targets for rheumatoid arthritis. Endocr. Metab. Immune Disord. Drug Targets 2015, 15, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, S.E. The epidemiology of rheumatoid arthritis. Rheum. Dis. Clin. N. Am. 2001, 27, 269–281. [Google Scholar] [CrossRef]
- Sparks, J.A. Rheumatoid Arthritis. Ann. Intern. Med. 2019, 170, ITC1–ITC16. [Google Scholar] [CrossRef] [PubMed]
- Díaz-González, F.; Hernández-Hernández, M.V. Rheumatoid arthritis. Med. Clin. (Barc). 2023, 161, 533–542. [Google Scholar] [CrossRef]
- Komatsu, N.; Takayanagi, H. Mechanisms of joint destruction in rheumatoid arthritis—Immune cell-fibroblast-bone interactions. Nat. Rev. Rheumatol. 2022, 18, 415–429. [Google Scholar] [CrossRef]
- Wu, F.; Gao, J.; Kang, J.; Wang, X.; Niu, Q.; Liu, J.; Zhang, L. B Cells in Rheumatoid Arthritis: Pathogenic Mechanisms and Treatment Prospects. Front. Immunol. 2021, 12, 750753. [Google Scholar]
- Jang, S.; Kwon, E.J.; Lee, J.J. Rheumatoid Arthritis: Pathogenic Roles of Diverse Immune Cells. Int. J. Mol. Sci. 2022, 23, 905. [Google Scholar] [CrossRef]
- Wu, D.; Luo, Y.; Li, T.; Zhao, X.; Lv, T.; Fang, G.; Ou, P.; Li, H.; Luo, X.; Huang, A.; et al. Systemic complications of rheumatoid arthritis: Focus on pathogenesis and treatment. Front Immunol. 2022, 13, 1051082. [Google Scholar] [CrossRef]
- Conforti, A.; Cola, I.D.; Pavlych, V.; Ruscitti, P.; Berardicurti, O.; Ursini, F.; Giacomelli, R.; Cipriani, P. Beyond the joints, the extra-articular manifestations in rheumatoid arthritis. Autoimmun Rev. 2021, 20, 102735. [Google Scholar] [CrossRef]
- Kay, J.; Upchurch, K.S. ACR/EULAR 2010 rheumatoid arthritis classification criteria. Rheumatology 2012, 51, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Scherer, H.U.; Häupl, T.; Burmester, G.R. The etiology of rheumatoid arthritis. J Autoimmun. 2020, 110, 102400. [Google Scholar] [CrossRef]
- Kerschbaumer, A.; Sepriano, A.; Smolen, J.S.; van der Heijde, D.; Dougados, M.; van Vollenhoven, R.; Mclnnes, I.B.; Bijlsma, J.W.; Burmester, G.R.; de Wit, M.; et al. Efficacy of pharmacological treatment in rheumatoid arthritis: A systematic literature research informing the 2019 update of the EULAR recommendations for management of rheumatoid arthritis. Ann. Rheum. Dis. 2020, 79, 744–759. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Wang, Y.; Xu, D.; Nossent, J.; Pavlos, N.J.; Xu, J. Rheumatoid arthritis: Pathological mechanisms and modern pharmacologic therapies. Bone Res. 2018, 6, 15. [Google Scholar] [CrossRef]
- Gaffo, A.; Saag, K.G.; Curtis, J.R. Treatment of rheumatoid arthritis. Am. J. Health-Syst. Pharm. 2006, 63, 2451–2465. [Google Scholar] [CrossRef]
- Smolen, J.S.; Landewé, R.B.M.; Bijlsma, J.W.J.; Burmester, G.R.; Dougados, M.; Kerschbaumer, A.; Mclnnes, I.B.; Sepriano, A.; van Vollenhoven, R.F.; de Wit, M.; et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann. Rheum. Dis. 2020, 79, 685–699. [Google Scholar] [CrossRef] [PubMed]
- Smolen, J.S.; Landewé, R.B.M.; Bergstra, S.A.; Kerschbaumer, A.; Sepriano, A.; Aletaha, D.; Caporali, R.; Edwards, C.J.; Hyrich, K.L.; Pope, J.E.; et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2022 update. Ann. Rheum. Dis. 2023, 82, 3–18. [Google Scholar] [CrossRef]
- Singh, J.A. Treatment Guidelines in Rheumatoid Arthritis. Rheum. Dis. Clin. N. Am. 2022, 48, 679–689. [Google Scholar] [CrossRef]
- Hyndman, I.J. Rheumatoid arthritis: Past, present, and future approaches to treating the disease. Int. J. Rheum. Dis. 2017, 20, 417–419. [Google Scholar] [CrossRef]
- Lin, W.; Shen, P.; Huang, Y.; Han, L.; Ba, X.; Huang, Y.; Yan, J.; Li, T.; Xu, L.; Qin, K.; et al. Wutou decoction attenuates the synovial inflammation of collagen-induced arthritis rats via regulating macrophage M1/M2 type polarization. J. Ethnopharmacol. 2023, 301, 115802. [Google Scholar] [CrossRef]
- Xu, T.; Li, S.; Sun, Y.; Pi, Z.; Liu, S.; Song, F.; Liu, Z. Systematically Characterize the Absorbed Effective Substances of Wutou Decoction and Their Metabolic Pathways in Rat Plasma Using UHPLC-Q-TOF-MS Combined with a Target Network Pharmacological Analysis. J. Pharm. Biomed. Anal. 2017, 141, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Li, S.; Pi, Z.; Song, F.; Lin, N.; Liu, S.; Liu, Z. Chemical profiling of Wu-tou decoction by UPLC–Q-TOF-MS. Talanta 2014, 118, 21–29. [Google Scholar] [CrossRef]
- Ba, X.; Huang, Y.; Shen, P.; Huang, Y.; Wang, H.; Han, L.; Lin, W.J.; Yan, H.J.; Xu, L.J.; Qin, K.; et al. WTD Attenuating Rheumatoid Arthritis via Suppressing Angiogenesis and Modulating the PI3K/AKT/mTOR/HIF-1α Pathway. Front. Pharmacol. 2021, 12, 696802. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Zheng, K.; Fan, D.; Zhao, Y.; Li, L.; Bian, Y.; Qiu, X.; Liu, X.; Zhang, G.; Ma, C.; et al. Wu-Tou Decoction in Rheumatoid Arthritis: Integrating Network Pharmacology and In Vivo Pharmacological Evaluation. Front. Pharmacol. 2017, 8, 230. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Mai, C.T.; Zheng, D.C.; He, Y.F.; Feng, S.L.; Li, Y.Z.; Liu, C.X.; Zhou, H.; Liu, L. Wutou decoction ameliorates experimental rheumatoid arthritis via regulating NF-kB and Nrf2: Integrating efficacy-oriented compatibility of traditional Chinese medicine. Phytomedicine 2021, 85, 1535. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Liu, C.; Sun, C.; Wang, J.; Zhi, K.; Sun, D.; Wang, H.; Wang, Q.; Lin, N. Wu-Tou Decoction Inhibits Angiogenesis in Experimental Arthritis by Targeting VEGFR2 Signaling Pathway. Rejuvenation Res. 2018, 21, 442–455. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.H.; Li, M.; Li, Y.B.; Lei, B.B.; Yuan, X.; Xing, X.K.; Xie, Y.F.; Wang, M.; Wang, L.; Yang, H.J.; et al. Benzoylaconitine Inhibits Production of IL-6 and IL-8 via MAPK, Akt, NF-κB Signaling in IL-1β-Induced Human Synovial Cells. Biol. Pharm. Bull. 2020, 43, 334–339. [Google Scholar] [CrossRef]
- Ahasm, H.; Irfam, H.M.; Alamgeer; Shahzad, M.; Asim, M.H.; Akram, M.; Zafar, M.S. Anti-rheumatic activity of pseudoephedrine (a substituted phenethylamine) in complete Freund’s adjuvant-induced arthritic rats by down regulating IL-1β, IL-6 and TNF-α as well as upregulating IL-4 and IL-10. Inflammopharmacology 2021, 29, 673–682. [Google Scholar]
- Jia, Z.; He, J. Paeoniflorin ameliorates rheumatoid arthritis in rat models through oxidative stress, inflammation and cyclooxygenase 2. Exp. Ther. Med. 2016, 11, 655–659. [Google Scholar] [CrossRef]
- Maruyama, M.; Shimizu, J.; Miyabe, C.; Yudo, K.; Miyabe, Y. Chemokines and chemokine receptors as promising targets in rheumatoid arthritis. Front. Immunol. 2023, 14, 1100869. [Google Scholar] [CrossRef]
- Ferrandiz, M.; Nacher-Juan, J.; Alcaraz, M. Nrf2 as a therapeutic target for rheumatic diseases. Biochem. Pharmacol. 2018, 152, 338–346. [Google Scholar] [CrossRef]
- Ilchovska, D.; Barrow, M. An Overview of the NF-kB mechanism of pathophysiology in rheumatoid arthritis, investigation of the NF-kB ligand RANKL and related nutritional interventions. Autoimmun. Rev. 2021, 20, 102741. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Pi, Z.; Zheng, Z.; Liu, S.; Song, F.; Liu, Z. Combined 16S rRNA gene sequencing and metabolomics to investigate the protective effects of Wu-tou decoction on rheumatoid arthritis in rats. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2022, 1199, 123249. [Google Scholar] [CrossRef] [PubMed]
- Yue, T.; Fan, X.; Zhang, Z.; Liu, Z.; Guo, M.; Wang, R.; Bai, F.; Qin, Y.; Wang, H. Wutou decoction, in combination with methotrexate, is more effective and equally tolerated, compared to methotrexate alone, in early active rheumatoid arthritis treatment. Int. J. Clin. Exp. Med. 2019, 12, 11930–11937. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.H.; Sung, W.S.; Choi, S.K.; Kim, J.H.; Suh, J.W.; Kim, J.H.; Seo, B.K.; Lee, S.D.; Kim, E.J. The effectiveness and safety of Wu tou decoction on rheumatoid arthritis: The effectiveness and safety of Wu tou decoction on rheumatoid arthritis. Medicine 2022, 101, e29105. [Google Scholar]
- Borenstein, M. How to understand and report heterogeneity in a meta-analysis: The difference between I-squared and prediction intervals. Integr. Med. Res. 2023, 12, 101014. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C. The Cochrane Collaboration’s tool for assessing risk of bias in randomized trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Neumann, I.; Pantoja, T.; Peñaloza, B.; Cifuentes, L.; Rada, G. The GRADE system: A change in the way of assessing the quality of evidence and the strength of recommendations. Rev. Medica De Chile 2014, 142, 630–635. [Google Scholar] [CrossRef]
- Li, W. Rheumatic Arthralgia Parallel Randomized Controlled Study Aconitum Treated. J. Pract. Tradit. Chin. Intern. Med. 2015, 29, 9–10. [Google Scholar]
- Liu, Y. Aconite Decoction in the Treatment of Rhematism of Random Parallel Control. J. Pract. Tradit. Chin. Intern. Med. 2013, 27, 38–39. [Google Scholar]
- Wei, G. Observation on the curative effect of Xuefengwutou Decoction on rheumatoid arthritis and observation of the curative effect of nursing Chinese medicine on 120 cases of rheumatoid arthritis. Med. Inf. Second. Ed. 2010, 23, 136–137. [Google Scholar]
- Wang, T.; Lin, J.; Zhou, S.; Kwang, H. Observation of clinical efficacy of Wutou decoction and its prescription on 116 cases of cold and wet rheumatoid arthritis. Chin. Med. 2016, 27, 145–146. [Google Scholar]
- Wang, T.; Lin, J.; Zhou, S.; Kwang, H. Clinical Observation on the Treatment of Cold and Wet Rheumatoid Arthritis with Orchid Decoction and Its Disassembly. New Chin. Med. 2016, 48, 130–132. [Google Scholar]
- Peng, Z.; Liu, L.; Liu, G.; Zhang, T. Clinical Study on Relationship Between Imaging Changes of Early Rheumatoid Arthritis and Modified Wutou Decoction. Liaoning J. Tradit. Chin. Med. 2019, 46, 1008–1012. [Google Scholar]
- Wang, Y.; Tu, S. Clinical Study on Modified Wutou Decoction in Treatment of Rheumatoid Arthritis. Acta Chin. Med. 2017, 32, 1706–1719. [Google Scholar]
- Li, Y.; Feng, C.; Liu, A.; Jia, J. Clinical Efficacy of Fufang Wutou Microemulsion on Rheumatoid Arthritis at Active Stage with Cold Dampness Syndrome. Chin. J. Exp. Tradit. Med. Formulae 2018, 24, 194–199. [Google Scholar]
- Chen, S. A Randomized Parallel Controlled Study of Wutou Decoction Combined with Western Medicine in Treatment of Rheumatoid Arthritis. J. Pract. Tradit. Chin. Intern. Med. 2015, 29, 31–33. [Google Scholar]
- Li, F. Effect of Wutou decoction on joint function and safety in patients of rheumatoid arthritis with cold-wet type. China’s Naturop. 2022, 30, 70–77. [Google Scholar]
- Zheng, W. Clinical curative effect observation of Wu Tou decoction combined with leflunomide in the treatment of rheumatoid arthritis. Chin. Community Dr. 2016, 32, 109–112. [Google Scholar]
- Li, S.; Xie, X. Clinical observation of methotrexate combined with aconitum decoction in the treatment of rheumatoid arthritis. J. Pract. Tradit. Chin. Med. 2016, 32, 794. [Google Scholar]
- Liu, X. Clinical observation of aconitum decoction combined with leflunomide in the treatment of rheumatoid arthritis with cold and wet paralysis. China Rural. Health 2015, 4, 20–21. [Google Scholar]
- Li, Y. Efficacy and nursing analysis of aconitum soup flavor in 46 patients with rheumatoid arthritis. China Pract. Med. 2013, 8, 226–227. [Google Scholar]
- Huang, Z.; Huang, C. The curative effect analysis of Aconite Decoction and 99Tc-MDP treated rheumatoid arthritis. China Mod. Dr. 2012, 50, 114–115. [Google Scholar]
- Hu, J.; Zhang, P. Effect of treatment of cold dampness blockage type rheumatoid arthritis with combination of TCM and Western Medicine. Chin. J. Clin. Ration. Drug Use 2016, 9, 1–3. [Google Scholar]
- Zhou, C.; Dong, Z. Treatment of 50 Cases of Rheumatoid Arthritis with Self-made Guizhi Wutou Decoction and Elamode. J. Sichuan Tradit. Chin. Med. 2018, 36, 160–163. [Google Scholar]
- Huang, L.; Zhu, Y. Observation and nursing of the curative effect of Xuehuangwutou Decoction on rheumatoid arthritis. Med. Inf. 2018, 21, 1424–1425. [Google Scholar]
- Bai, F.; Yue, T. Clinical observation of, Wutou Decoction and Methotrexate for early active rheumatoid arthritis patients. Int. J. Trad. Chin. Med. 2020, 42, 1074–1078. [Google Scholar]
- Luo, S. Observation of curative effect of Wutou decoction on 36 cases of rheumatoid arthritis. J. New Chin. Med. 2008, 40, 45–46. [Google Scholar]
- Luo, S. The curative effect Observation of Modified Aconiti Decoction on Reactiveness Rheumtoid Arthritis. J. Henan Univ. Chin. Med. 2009, 24, 71–72. [Google Scholar]
- Mao, L.; Huang, L.; Wu, D. Effect and Nursing of Erteng Aconite Decoction for Rheumatoid Arthitis in Activity. Hei Long Jiang Med. J. 2013, 37, 220–222. [Google Scholar]
- Zheng, H.; Huang, L.; Zhu, Y.; Yang, G. Effect of Xuefeng Wutou Decoction on Rheumatoid Arthritis Disease Activity and Platelets. Med. Inf. 2008, 21, 1388–1389. [Google Scholar]
- Zheng, X.; Mao, L.; Mai, T.; Huang, L.; Zhu, Y.; Liang, J. Clinical Study on the Treatment of Rheumatoid Arthritis with Ertu wutou Decoction. Shandong J. Tradit. Chin. Med. 2012, 31, 636–638. [Google Scholar]
- Worthington, J. Investigating the genetic basis of susceptibility to rheumatoid arthritis. J. Autoimmun. 2005, 25, 16–20. [Google Scholar] [CrossRef]
- Yu, R.; Zhang, J.; Zhuo, Y.; Hong, X.; Ye, J.; Tang, S.; Zhang, Y. Identification of Diagnostic Signatures and Immune Cell Infiltration Characteristics in Rheumatoid Arthritis by Integrating Bioinformatic Analysis and Machine-Learning Strategies. Front. Immunol. 2021, 12, 724934. [Google Scholar] [CrossRef]
- Aletaha, D.; Smolen, J.S. The rheumatoid arthritis patient in the clinic: Comparing more than 1300 consecutive DMARD courses. Rheumatology 2002, 41, 1367–1374. [Google Scholar] [CrossRef]
- Simon, L.S. DMARDs in the treatment of rheumatoid arthritis: Current agents and future developments. Int. J. Clin. Pract. 2000, 54, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Li, J.; Han, Y.; Yu, X.W.; Qin, L. Traditional Chinese medicine in the treatment of rheumatoid arthritis: A general review. Rheumatology International. 2010, 30, 713–718. [Google Scholar] [CrossRef]
- Lingyue, Z.; Zuoyuan, C.; Yeying, Y.; Tan, X.; Mao, J.; Su, L. Traditional Chinese medicine on treating active rheumatoid arthritis: A protocol for systematic review and meta-analysis. Medicine 2020, 99, e20642. [Google Scholar]
- Chae, S.Y.; Park, S.H.; Kim, J.H.; Kim, E.J.; Seo, B.K.; Park, S.S.; Sung, W.S. The efficacy and safety of Simiao Xiaobi decoction on rheumatoid arthritis: A systematic review and meta-analysis. Eur. J. Integr. Med. 2024, 65, 102322. [Google Scholar] [CrossRef]
- Miller, A.; Mahtani, K.R.; Waterfield, M.A.; Timms, A.; Misbah, S.A.; Luqmani, R.A. Is rheumatoid factor useful in primary care? A retrospective cross-sectional study. Clin. Rheumatol. 2013, 32, 1089–1093. [Google Scholar] [CrossRef] [PubMed]
- Ingegnoli, F.; Castelli, R.; Gualtierotti, R. Rheumatoid factors: Clinical applications. Dis. Markers 2013, 35, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Sansonno, D.; Lauletta, G.; Nisi, L.; Gatti, P.; Pesola, F.; Pansini, N.; Dammacco, F. Non-enveloped HCV core protein as constitutive antigen of cold-precipitable immune complexes in type II mixed cryoglobulinemia. Clin. Exp. Immunol. 2003, 133, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Michael, J.A. Management of Rheumatoid Arthritis: Update From ACR. Am. Fam. Physician 2022, 106, 340–342. [Google Scholar]
- Wang, W.; Zhou, H.; Liu, L. Side effects of methotrexate therapy for rheumatoid arthritis: A systematic review. Eur. J. Med. Chem. 2018, 156, 502–516. [Google Scholar] [CrossRef]
- Duong, S.Q.; Crowson, C.S.; Athreya, A.; Athreya, A.; Atkinson, E.J.; Davis, J.M., 3rd; Warrington, K.J.; Matteson, E.L.; Weinshilboum, R.; Wang, L.; et al. Clinical predictors of response to methotrexate in patients with rheumatoid arthritis: A machine learning approach using clinical trial data. Arthritis Res. Ther. 2022, 24, 162. [Google Scholar] [CrossRef]
- van de Putte, L.B.A.; Atkins, C.; Malaise, M.; Sany, J.; Russell, A.S.; van Riel, P.L.C.M.; Settas, L.; Bijlsma, J.W.; Todesco, S.; Dougados, M.; et al. Efficacy and safety of adalimumab as monotherapy in patients with rheumatoid arthritis for whom previous disease modifying antirheumatic drug treatment has failed. Ann. Rheum. Dis. 2004, 63, 508–516. [Google Scholar] [CrossRef]




| Study (y) | Number of RA Patients (Male/Female) | Duration of RA | Treatment Details of WTD Group | Treatment Details of CTR Group | Duration of Treatment | Outcome Measures (Primary/Secondary/ Other Measures Used in Meta-Analysis) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| WTD | CTR | WTD | CTR | Name of Treatment | Frequency Sessions of Treatment | Name of Treatment Type of Administration (Oral or Injection) | Frequency Sessions of Treatment | |||
| Li 2015 [40] | 49 (26/23) | 49 (24/25) | 2.23 ±1.34 (m) | 2.34 ±1.26 (m) | Modified WTD | BID | LEF (50 mg for first 3 d + 10~20 mg) by oral | QD | 30 d | ER/ESR, CRP, RF, AEs |
| Liu 2013 [41] | 60 (35/25) | 60 (40/20) | 1 ± 2.1 (y) | 1 ±2.2 (y) | Modified WTD | QD | LEF (50 mg for first 3 d + 10 mg) by oral | QD | 30 d | ER/AEs |
| Wei 2010 [42] | 70 (30/40) | 50 (24/26) | 2–8 (y) | 1–7(y) | Modified WTD | BID | MTX (15 mg), Prednisone (10 mg), Nimesulide (0.1 g), Glucosides capsule (2 cap) by oral | BID, TID, BID, TID | 4 w | ER/AEs |
| Wang 2016(1) [43] | 33 (16/17) | 25 (12/13) | 11.94 ±8.02 (y) | 8.04 ±6.98 (y) | WTD | BID | MTX (15 mg) by oral | QW | 8 w | CRP, RF/DAS28/ Cyclic citrullinated peptide antibody |
| Wang 2016(2) [44] | 15 (8/7) | 15 (7/8) | 7.67 ±3.12 (y) | 7.27 ±3.69 (y) | WTD | BID | MTX (15 mg) by oral | QW | 8 w | DAS28/TNF-a, IL-6, Vascular endothelial growth factor, IL-17, HGB, PLT |
| Peng 2019 [45] | 67 (20/47) | 67 (19/48) | 7.28 ±3.65 (m) | 7.33 ±3.54 (m) | Modified WTD +CTR | BID | LEF (20 mg), MTX (10 mg) by oral | QD, QW | 3 m | ER, TJC, MS/ESR, CRP, RF, AEs/ DAS28, SJP |
| Wang 2017 [46] | 52 (26/26) | 52 (28/24) | 4.4 ±2.1 (NR) | 4.7 ±2.2 (NR) | Modified WTD +CTR | QD | LEF (20 mg), MTX (10 mg) by oral | QD, QW | 3 m | ER, TJC, MS/ESR, CRP, RF/ DAS28, ACR20, ACR50, ACR70, HAQ, SJP |
| Li 2018 [47] | 60 (24/36) | 60 (26/34) | 4.49 ±3.75 (y) | 4.37 ±3.42 (y) | Modified WTD + CTR | TID | Meloxicam Tab (7.5 mg), DCF (NR), Baishao GLS Cap (2 Cap) by oral | QD, TID, TID | 4 w | TJC/ESR, CRP, RF, AEs/ DAS28, ACR20, ACR50, ACR70, HAQ, SJP |
| Chen 2015(2) [48] | 26 (19/7) | 26 (21/5) | 6.87 ±2.33 (y) | 7.57 ±1.98 (y) | Modified WTD +CTR | QD | MTX (10 mg), Tripterygium GLS (20 mg) by oral | QW | 6 m | ER/AEs |
| Li 2022 [49] | 78 (33/45) | 78 (32/46) | 2.23 ±1.34 (m) | 2.34 ±1.26 (m) | Modified WTD +CTR | BID | DCF (25 mg), LEF (10 mg) by oral | TID, QD | 30 d | ER/ESR, CRP, AEs |
| Zheng 2016 [50] | 50 (23/27) | 50 (22/28) | 6.5 ±5.4 (y) | 5.8 ±6.3 (y) | WTD +CTR | BID | LEF (20 mg) by oral | BID | 6 m | ER, TJC, MS/ESR, CRP |
| Li 2016 [51] | 50 (29/21) | 50 (NR/NR) | 6.88 ±2.34 (y) | 7.56 ±1.97 (y) | Modified WTD +CTR | BID | MTX (10 mg), Tripterygium GLS (20 mg) by oral | QW, TID | 3 m | ER |
| Liu 2015 [52] | 26 (NR/NR) | 26 (NR/NR) | NR | NR | Modified WTD +CTR | TID | LEF (20 mg) by oral | QD | 3 m | ER |
| Li 2013 [53] | 23 (NR/NR) | 23 (NR/NR) | NR | NR | Modified WTD +CTR | BID | DCF (0.1 g), SSZ (0.75 g), Tripteryzgium GLS (0.02 g) by oral | QD, TID, TID | 1 m | ER/AEs |
| Huang 2012 [54] | 28 (NR/NR) | 28 (NR/NR) | NR | NR | Modified WTD +CTR | BID | 99mTc-Methyl diphosphonate (5 mg) by injection | NR | 2 m | ER/ESR, RF, AEs |
| Hu 2016 [55] | 30 (NR/NR) | 25 (NR/NR) | NR | NR | WTD +CTR | NR | DCF (50 mg), MTX (10 mg), LEF (20 mg) by oral | BID, QW, QD | 15 d | ER, TJC/ESR, CRP, AEs |
| Zhou 2018 [56] | 50 (32/18) | 50 (28/22) | 39.20 ±11.38 (m) | 39.12 ±11.04 (m) | Modified WTD +CTR | BID | Igurtimod (25 mg) by oral | BID | 3 m | ER, MS/ESR, CRP, RF, AEs/ ACR 20, ACR 50, ACR70/ADL, QOL, SJP, Joint pain, Joint swelling, Joint heatness, Joint hardness/Lansbury Score |
| Huang 2008 [57] | 35 (NR/NR) | 34 (NR/NR) | NR | NR | Modified WTD +CTR | BID | Ibuprofen (0.3 mg), MTX (15 mg) by oral | BID, QW | 6 w | ER |
| Bai 2020 [58] | 131 (29/102) | 93 (17/76) | 1.0 ±0.5 (y) | 0.9 ±0.4 (y) | Modified WTD +CTR | QD | MTX (7.5–15 mg) by oral | QW | 12 w | ER/ESR, CRP/DAS28, HAQ/LDA |
| Luo 2008 [59] | 36 (NR/NR) | 34 (NR/NR) | NR | NR | Modified WTD +CTR | BID | Celecoxib (0.2 g), MTX (15 mg) | BID, QW | 6 w | TJC, MS/ESR, CRP, RF, AEs/ GS, WT20, BPC |
| Luo 2009 [60] | 32 (NR/NR) | 30 (NR/NR) | NR | NR | Modified WTD +CTR | BID | Celecoxib (0.2 g), MTX (15 mg) | BID, QW | 6 w | TJC, MS/ESR, CRP, RF, AEs/ GS, WT20 |
| Mao 2013 [61] | 32 (NR/NR) | 30 (NR/NR) | NR | NR | Modified WTD +CTR | BID | Ibuprofen (0.3 g), MTX (15 mg) | BID, QW | 6 w | TJC, MS/ESR, CRP, RF, AEs/ SJP, GS, WT20, RBC, HGB |
| Zheng 2008 [62] | 32 (NR/NR) | 30 (NR/NR) | NR | NR | Modified WTD +CTR | BID | Celecoxib (0.2 g), MTX (15 mg) | BID, QW | 6 w | TJC, MS/ESR, CRP, RF, AEs/ GS, WT20, PLT |
| Zheng 2012 [63] | 32 (NR/NR) | 30 (NR/NR) | NR | NR | Modified WTD +CTR | BID | Ibuprofen (0.3 g), MTX (15 mg) | BID, QW | 6 w | TJC, MS/ESR, CRP, RF, AEs/Grip strength, 20-min walking time, TNF-a, IL-6, PAF |
| Yue 2019 [34] | 40 (NR/NR) | 37 (NR/NR) | NR | NR | WTD +CTR | NR | MTX (10–15 mg) | QW | 12 w | CRP, ESR, AEs/DAS28, HAQ/LDA, Remission rate, Overall response rate |
| Adverse Events | Total Number of Adverse Events | |
|---|---|---|
| Treatment Group | Control Group | |
| Skin irritation | 6 [42,45,47,49,56] | 13 [42,45,47,49,56] |
| Gastrointestinal problem (with nausea and vomiting) | 9 [42,48,49,53,55,56] | 18 [42,48,49,53,55,56] |
| Diarrhea with vomiting | 8 [40,45] | 11 [40,45] |
| Liver failure | 2 [45] | 3 [45] |
| Decreased WBC | 0 [42] | 1 [42] |
| Upper abdominal pain and reflux | 0 [54] | 2 [54] |
| Outcome | Included RCTs (Participants) | Effect Estimate (95% CI) | I2 | Quality of Evidence | Reasons |
|---|---|---|---|---|---|
| ER (monotherapy) | 3 (338) | RR 1.25 (1.14, 1.37) | 48% | ⨁⨁◯◯ Low | Risk of bias, inconsistency |
| ESR (monotherapy) | 1 (98) | MD 24.46 (20.72, 28.20) | Not applicable | ⨁⨁◯◯ Low | Serious imprecision |
| CRP (monotherapy) | 2 (156) | MD 3.11 (0.66, 5.57) | 29% | ⨁⨁◯◯ Low | Serious imprecision |
| RF (monotherapy) | 2 (156) | MD 75.35 (57.02, 93.67) | 88% | ⨁◯◯◯ Very Low | Serious imprecision, inconsistency |
| ER (combination therapy | 13 (1248) | RR 1.25 (1.18, 1.33) | 0% | ⨁⨁⨁◯ Moderate | Risk of bias |
| TJC (combination therapy) | 5 (513) | MD 2.29 (2.14, 2.45) | 96% | ⨁⨁◯◯ Low | Risk of bias, inconsistency |
| MS (combination therapy) | 4 (438) | MD 17.98 (14.49, 21.47) | 77% | ⨁⨁◯◯ Low | Risk of bias, inconsistency |
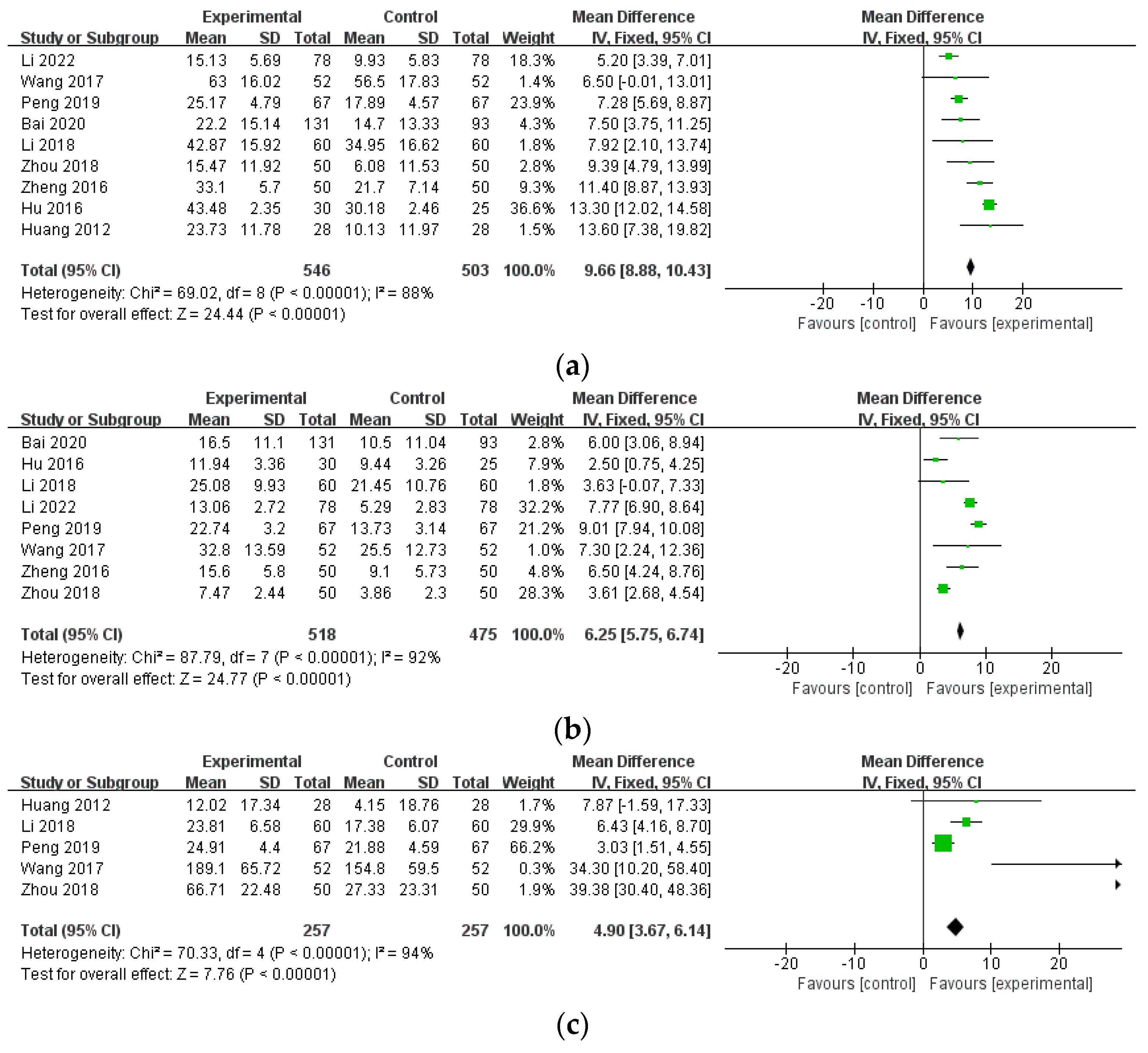
| ESR (combination therapy) | 9 (1049) | MD 9.66 (8.88, 10.43) | 88% | ⨁⨁⨁◯ Moderate | inconsistency |
| CRP (combination therapy) | 8 (993) | MD 6.25 (5.75, 6.74) | 92% | ⨁⨁⨁◯ Moderate | inconsistency |
| RF (combination therapy) | 5 (514) | MD 4.90 (3.67, 6.14) | 94% | ⨁⨁⨁◯ Moderate | inconsistency |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, J.-H.; Park, G.; Kwon, C.-Y.; Kim, J.-H.; Kim, E.-J.; Seo, B.-K.; Lee, S.-D.; Hong, S.-U.; Sung, W.-S. The Effectiveness and Safety of Wu Tou Decoction on Rheumatoid Arthritis—A Systematic Review and Meta-Analysis. Healthcare 2024, 12, 1739. https://doi.org/10.3390/healthcare12171739
Moon J-H, Park G, Kwon C-Y, Kim J-H, Kim E-J, Seo B-K, Lee S-D, Hong S-U, Sung W-S. The Effectiveness and Safety of Wu Tou Decoction on Rheumatoid Arthritis—A Systematic Review and Meta-Analysis. Healthcare. 2024; 12(17):1739. https://doi.org/10.3390/healthcare12171739
Chicago/Turabian StyleMoon, Jeong-Hyun, Gyoungeun Park, Chan-Young Kwon, Joo-Hee Kim, Eun-Jung Kim, Byung-Kwan Seo, Seung-Deok Lee, Seung-Ug Hong, and Won-Suk Sung. 2024. "The Effectiveness and Safety of Wu Tou Decoction on Rheumatoid Arthritis—A Systematic Review and Meta-Analysis" Healthcare 12, no. 17: 1739. https://doi.org/10.3390/healthcare12171739
APA StyleMoon, J.-H., Park, G., Kwon, C.-Y., Kim, J.-H., Kim, E.-J., Seo, B.-K., Lee, S.-D., Hong, S.-U., & Sung, W.-S. (2024). The Effectiveness and Safety of Wu Tou Decoction on Rheumatoid Arthritis—A Systematic Review and Meta-Analysis. Healthcare, 12(17), 1739. https://doi.org/10.3390/healthcare12171739







