Do Audible Sounds during a Lumbar Spine Thrust Manipulation Have an Impact on Brainwave Activity?
Abstract
1. Introduction
2. Material and Methods
Study Protocol
3. Results
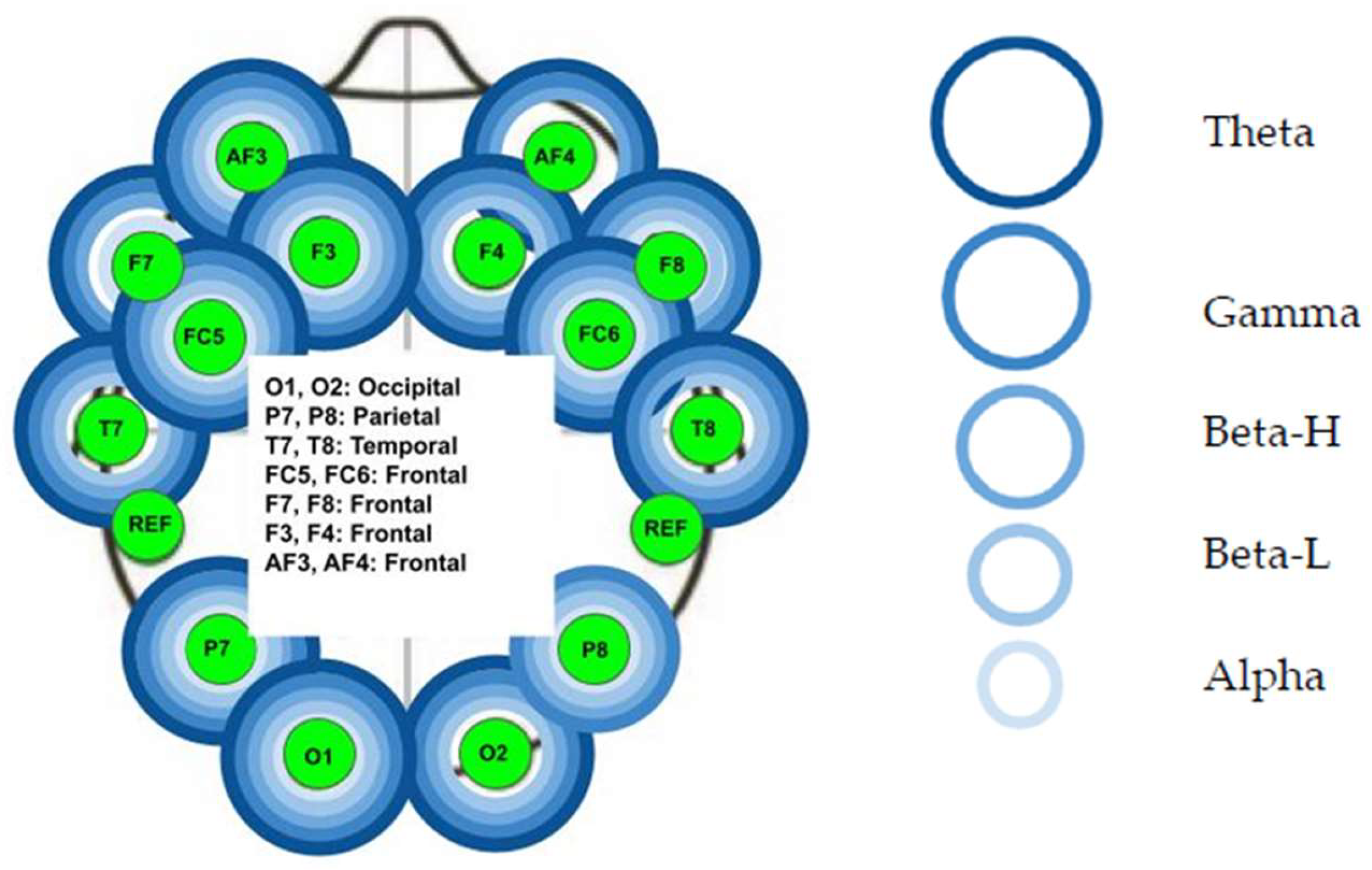
3.1. Audible Sound Group
- Left frontal lobe: the AF3 electrode for all five bands (p < 0.01), the F7 electrode for all bands (p < 0.01) except the Beta L band, the F3 for all five bands (p < 0.01), and the FC5 for all five bands (p < 0.01).
- Left temporal lobe: the T7 electrode for all bands (p < 0.01) except Alpha band.
- Left parietal lobe: the P7 electrode for all five bands (p < 0.01).
- Left occipital lobe: the O1 electrode for all five bands (p < 0.01).
- Right occipital lobe: the O2 electrode for all bands (p < 0.01) except Alpha band.
- Right parietal lobe: the P8 electrode for all five bands (p < 0.01).
- Right temporal lobe: not significant (p > 0.05).
- Right frontal lobe: the FC6 electrode for all five bands (p < 0.01), the F4 electrode for all bands (p < 0.01) except Alpha band, the F8 electrode for all five bands (p < 0.01), and the AF4 electrode for the Theta, Gamma, and Beta H bands (p < 0.01).
- Right occipital lobe: O2 Alpha and O2 Beta-L.
- Right parietal lobe: P8 Alpha.
- Right temporal lobe: T8 Alpha.
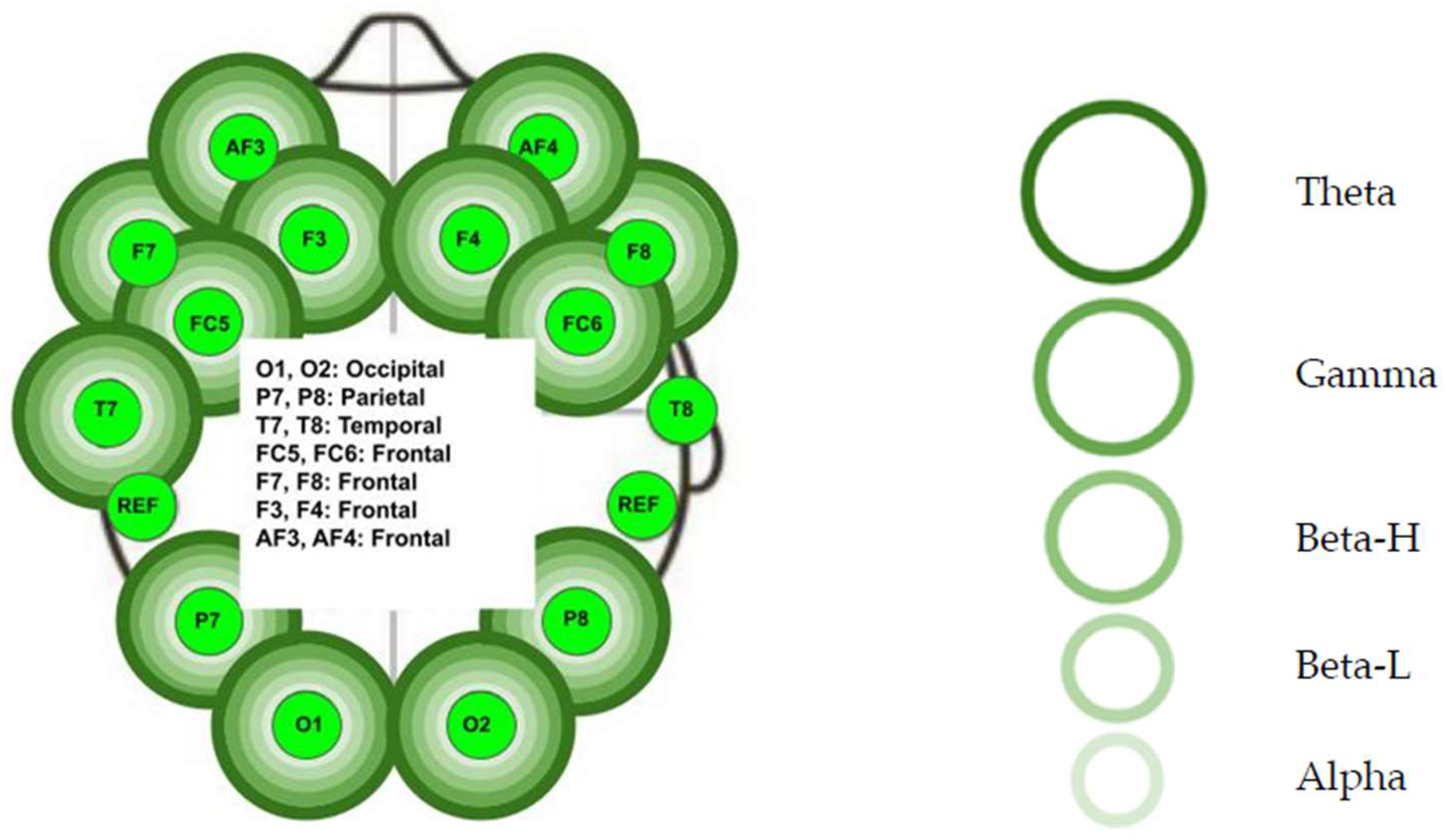
3.2. Non-Audible Sound Group
- Left frontal lobe: the AF3 electrode for all five bands (p < 0.01), the F7 electrode for all five bands (p < 0.01), the F3 for all five bands (p < 0.01), and the FC5 for all five bands (p < 0.01).
- Left temporal lobe: the T7 electrode for all five bands (p < 0.01).
- Left parietal lobe: the P7 electrode for all five bands (p < 0.01).
- Left occipital lobe: the O1 electrode for all five bands (p < 0.01).
- Right occipital lobe: the O2 electrode for all five bands (p < 0.01).
- Right parietal lobe: the P8 electrode for all bands (p < 0.01) except Beta-L (p > 0.05).
- Right temporal lobe: not significant (p > 0.05).
- Right frontal lobe: the FC6 electrode for all five bands (p < 0.01), the F4 electrode for all five bands (p < 0.01), the F8 electrode for all five bands (p < 0.01), and the AF4 electrode for all five bands (p < 0.01).
- Right parietal lobe: P8 Beta-L.
- Right temporal lobe: T8 Alpha, T8 Beta-H, T8 Beta-L, T8 Gamma, and T8 Theta.
3.3. Group Comparison
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dieleman, J.L.; Cao, J.; Chapin, A.; Chen, C.; Li, Z.; Liu, A.; Horst, C.; Kaldjian, A.; Matyasz, T.; Scott, K.W.; et al. US Health Care Spending by Payer and Health Condition, 1996–2016. JAMA 2020, 323, 863–884. [Google Scholar] [CrossRef]
- Standaert, C.J.; Friedly, J.; Erwin, M.W.; Lee, M.J.; Rechtine, G.; Henrikson, N.B.; Norvell, D.C. Comparative effectiveness of exercise, acupuncture, and spinal manipulation for low back pain. Spine 2011, 36 (Suppl. S21), S120–S130. [Google Scholar] [CrossRef]
- Zhu, Y.; Haldeman, S.; Hsieh, C.Y.; Wu, P.; Starr, A. Do cerebral potentials to magnetic stimulation of paraspinal muscles reflect changes in palpable muscle spasm, low back pain, and activity scores? J. Manip. Physiol. Ther. 2000, 23, 458–464. [Google Scholar] [CrossRef][Green Version]
- Sillevis, R.; Cleland, J. Immediate effects of the audible pop from a thoracic spine thrust manipulation on the autonomic nervous system and pain: A secondary analysis of a randomized clinical trial. J. Manip. Physiol. Ther. 2011, 34, 37–45. [Google Scholar] [CrossRef]
- Dunning, J.; Mourad, F.; Zingoni, A.; Iorio, R.; Perreault, T.; Zacharko, N.; de Las Penas, C.F.; Butts, R.; Cleland, J.A. Cavitation Sounds during Cervicothoracic Spinal Manipulation. Int. J. Sports Phys. Ther. 2017, 12, 642–654. [Google Scholar]
- Cleland, J.A.; Flynn, T.W.; Childs, J.D.; Eberhart, S. The audible pop from thoracic spine thrust manipulation and its relation to short-term outcomes in patients with neck pain. J. Man. Manip. Ther. 2007, 15, 143–154. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Flynn, T.W.; Fritz, J.M.; Wainner, R.S.; Whitman, J.M. The audible pop is not necessary for successful spinal high-velocity thrust manipulation in individuals with low back pain. Arch. Phys. Med. Rehabil. 2003, 84, 1057–1060. [Google Scholar] [CrossRef]
- Cramer, G.D.; Ross, J.K.; Raju, P.K.; Cambron, J.A.; Dexheimer, J.M.; Bora, P.; McKinnis, R.; Selby, S.; Habeck, A.R. Distribution of cavitations as identified with accelerometry during lumbar spinal manipulation. J. Manip. Physiol. Ther. 2011, 34, 572–583. [Google Scholar] [CrossRef] [PubMed]
- Brodeur, R. The audible release associated with joint manipulation. J. Manip. Physiol. Ther. 1995, 18, 155–164. [Google Scholar]
- Kawchuk, G.N.; Fryer, J.; Jaremko, J.L.; Zeng, H.; Rowe, L.; Thompson, R. Real-time visualization of joint cavitation. PLoS ONE 2015, 10, e0119470. [Google Scholar] [CrossRef]
- Bialosky, J.E.; Bishop, M.D.; Robinson, M.E.; George, S.Z. The relationship of the audible pop to hypoalgesia associated with high-velocity, low-amplitude thrust manipulation: A secondary analysis of an experimental study in pain-free participants. J. Manip. Physiol. Ther. 2010, 33, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Sillevis, R.; Unum, J.; Weiss, V.; Shamus, E.; Selva-Sarzo, F. The effect of a spinal thrust manipulation’s audible pop on brain wave activity: A quasi-experimental repeated measure design. PeerJ 2024, 12, e17622. [Google Scholar] [CrossRef] [PubMed]
- Michel, C.M.; Koenig, T. EEG microstates as a tool for studying the temporal dynamics of whole-brain neuronal networks: A review. Neuroimage 2018, 180 Pt B, 577–593. [Google Scholar] [CrossRef]
- Williams, N.S.; McArthur, G.M.; de Wit, B.; Ibrahim, G.; Badcock, N.A. A validation of Emotiv EPOC Flex saline for EEG and ERP research. PeerJ 2020, 8, e9713. [Google Scholar] [CrossRef]
- Kumar, J.S.; Bhuvaneswari, P. Analysis of Electroencephalography (EEG) signals and its categorization—A study. Procedia Eng. 2012, 38, 2525–2536. [Google Scholar] [CrossRef]
- Blanco, J.A.; Vanleer, A.C.; Calibo, T.K.; Firebaugh, S.L. Single-Trial Cognitive Stress Classification Using Portable Wireless Electroencephalography. Sensors 2019, 19, 499. [Google Scholar] [CrossRef]
- Kotowski, K.; Stapor, K.; Leski, J.; Kotas, M. Validation of Emotiv EPOC+ for extracting ERP correlates of emotional face processing. Biocybern. Biomed. Eng. 2018, 38, 773–781. [Google Scholar] [CrossRef]
- Anderson, E.W.; Potter, K.C.; Matzen, L.E.; Shepherd, J.F.; Preston, G.A.; Silva, C.T. A user study of visualization effectiveness using EEG and cognitive load. In Computer Graphics Forum; Wiley Online Library: Hoboken, NJ, USA, 2011; Volume 30, pp. 791–800. [Google Scholar]
- Morita, I.; Sakuma, S.; Shimomura, J.; Hayashi, N.; Toda, S. Brain activity in response to the touch of a hand on the center of the back. PLoS ONE 2018, 13, e0206451. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Bauer, M.; Chowanski, W.; Sui, Y.; Atkinson, D.; Baurley, S.; Fry, M.; Evans, J.; Bianchi-Berthouze, N. The brain’s response to pleasant touch: An EEG investigation of tactile caressing. Front. Hum. Neurosci. 2014, 8, 893. [Google Scholar] [CrossRef]
- Hartman, L. Handbook of Osteopathic Techniques; Stanley Thornes (Publishers) Ltd.: Kingston upon Thames, UK, 1997. [Google Scholar]
- Kliuchko, M.; Heinonen-Guzejev, M.; Vuust, P.; Tervaniemi, M.; Brattico, E. A window into the brain mechanisms associated with noise sensitivity. Sci. Rep. 2016, 6, 39236. [Google Scholar] [CrossRef]
- Bakker, M.; Miller, J. Does an audible release improve the outcome of a chiropractic adjustment? J. Can. Chiropr. Assoc. 2004, 48, 237–239. [Google Scholar]
- Bishop, M.D.; Mintken, P.E.; Bialosky, J.E.; Cleland, J.A. Patient expectations of benefit from interventions for neck pain and resulting influence on outcomes. J. Orthop. Sports Phys. Ther. 2013, 43, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Bishop, M.D.; Bialosky, J.E.; Cleland, J.A. Patient expectations of benefit from common interventions for low back pain and effects on outcome: Secondary analysis of a clinical trial of manual therapy interventions. J. Man. Manip. Ther. 2011, 19, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Sparks, C.L.; Liu, W.C.; Cleland, J.A.; Kelly, J.P.; Dyer, S.J.; Szetela, K.M.; Elliott, J.M. Functional Magnetic Resonance Imaging of Cerebral Hemodynamic Responses to Pain Following Thoracic Thrust Manipulation in Individuals with Neck Pain: A Randomized Trial. J. Manip. Physiol. Ther. 2017, 40, 625–634. [Google Scholar] [CrossRef] [PubMed]
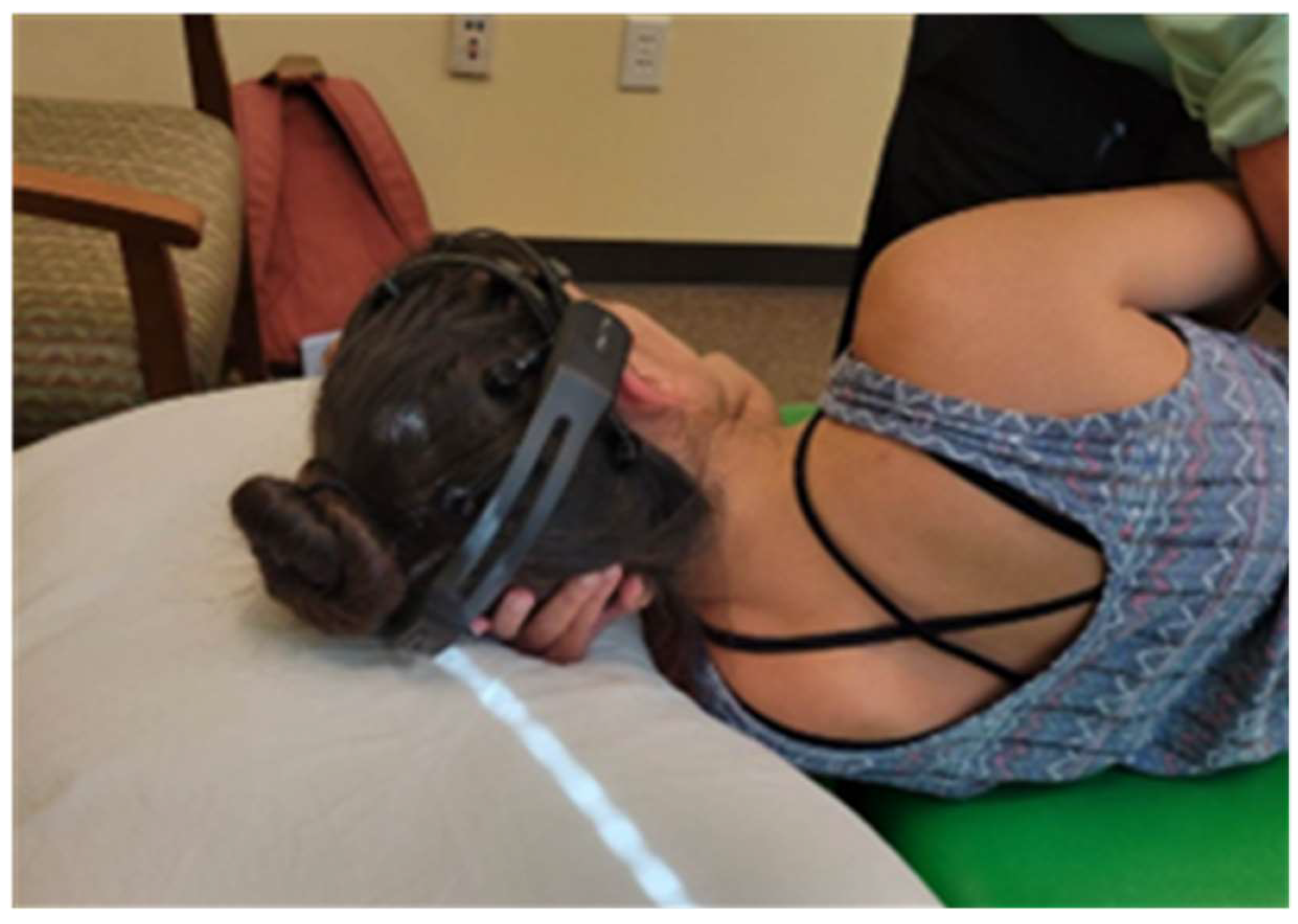

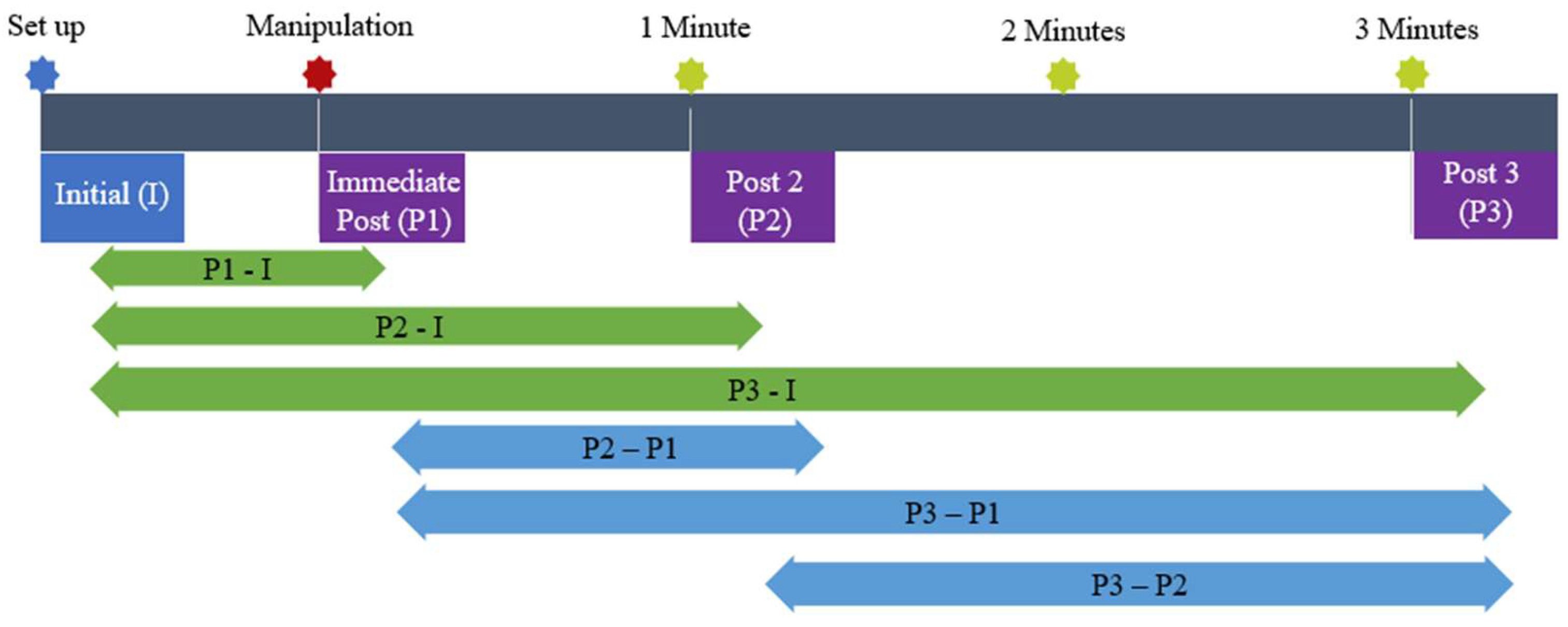
| Name | Time | Position | Tactile Touch | |
|---|---|---|---|---|
| I | Initial | Immediately before manipulation | Manipulation position | Present |
| P1 | Post 1 | Immediately after manipulation executed | Manipulation position | Present |
| P2 | Post 2 | 1 min after manipulation | Relaxed side-lying | Absent |
| P3 | Post 3 | 3 min after manipulation | Relaxed side-lying | Absent |
| Audible Sound Group | |
|---|---|
| Intervals | % of Significance |
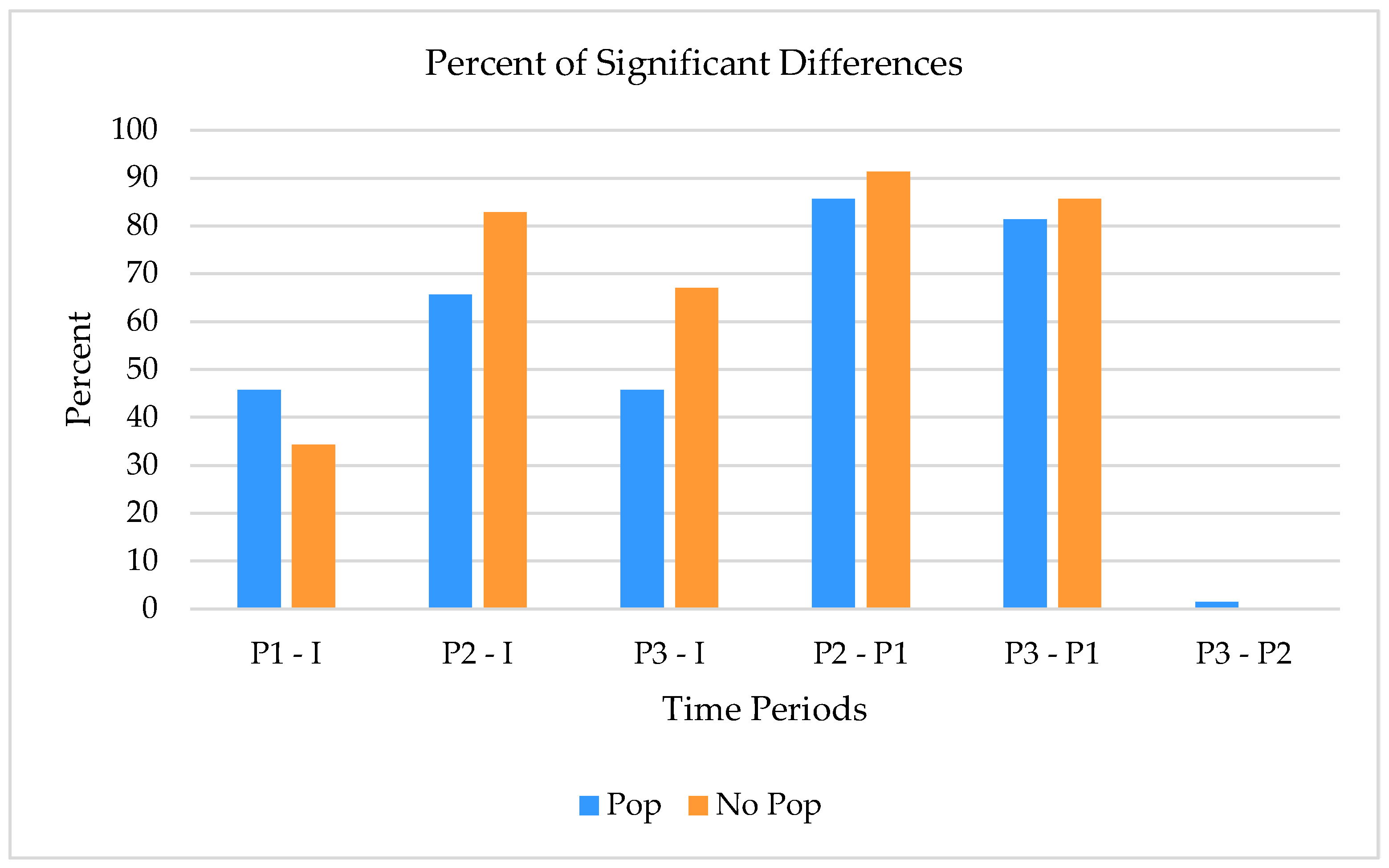
| P1-I | 45.7% |
| P2-I | 65.7% |
| P3-I | 45.7% |
| P2–P1 | 85.7% |
| P3–P1 | 81.4% |
| P3–P2 | 1.4% |
| Non-Audible Sound Group | |
|---|---|
| Intervals | % of Significance |
| P1–I | 34.3% |
| P2–I | 82.9% |
| P3–I | 67.1% |
| P2–P1 | 91.4% |
| P3–P1 | 85.7% |
| P3–P2 | 0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sillevis, R.; de Zayas, T.; Hansen, A.W.; Krisinski, H. Do Audible Sounds during a Lumbar Spine Thrust Manipulation Have an Impact on Brainwave Activity? Healthcare 2024, 12, 1783. https://doi.org/10.3390/healthcare12171783
Sillevis R, de Zayas T, Hansen AW, Krisinski H. Do Audible Sounds during a Lumbar Spine Thrust Manipulation Have an Impact on Brainwave Activity? Healthcare. 2024; 12(17):1783. https://doi.org/10.3390/healthcare12171783
Chicago/Turabian StyleSillevis, Rob, Tiffanny de Zayas, Anne Weller Hansen, and Halle Krisinski. 2024. "Do Audible Sounds during a Lumbar Spine Thrust Manipulation Have an Impact on Brainwave Activity?" Healthcare 12, no. 17: 1783. https://doi.org/10.3390/healthcare12171783
APA StyleSillevis, R., de Zayas, T., Hansen, A. W., & Krisinski, H. (2024). Do Audible Sounds during a Lumbar Spine Thrust Manipulation Have an Impact on Brainwave Activity? Healthcare, 12(17), 1783. https://doi.org/10.3390/healthcare12171783






