Sleep Quality and Urinary Incontinence in Prostate Cancer Patients: A Data Analytics Approach with the ASCAPE Dataset
Abstract
:1. Introduction
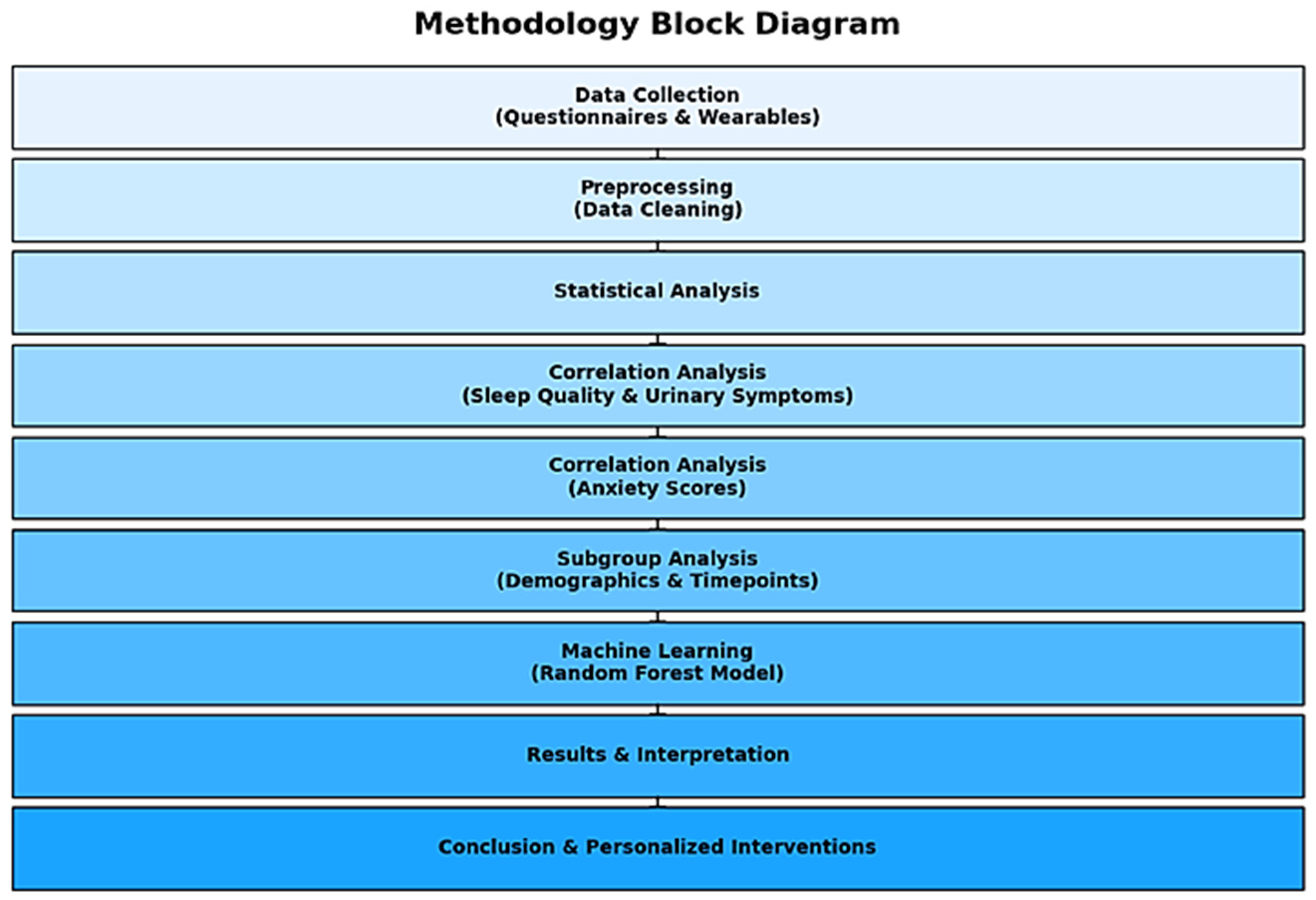
2. Methods
2.1. General Design
2.2. Participants
2.3. Data Collection
2.3.1. Standardized Questionnaires
- EORTC QLQ PR25 (European Organization for Research and Treatment of Cancer Quality of Life Questionnaire—Prostate Cancer Module): This questionnaire assesses specific QoL issues related to prostate cancer, including urinary symptoms, bowel symptoms, treatment-related side effects, and overall health status.
- HADS (Hospital Anxiety and Depression Scale): This scale measures the levels of anxiety and depression among patients, providing insights into their mental health status.
- IIEF (International Index of Erectile Function): This questionnaire evaluates erectile function, which is a critical aspect of QoL for prostate cancer patients.
2.3.2. Wearable Devices
- Fitbit Watches: These devices were provided to participants to continuously monitor their daily activity levels, sleep patterns, heart rate variability, and other vital health metrics. The wearable data complemented the subjective questionnaire responses, offering a comprehensive view of the patients’ health and QoL.
2.4. Data Management and Analysis
2.4.1. Data Analysis
2.4.2. Data Preparation
2.4.3. Correlation Analysis
2.4.4. Subgroup Analysis
2.4.5. Demographic and Clinical Features
2.4.6. Statistical Tests
3. Results
3.1. Demographic and Clinical Features
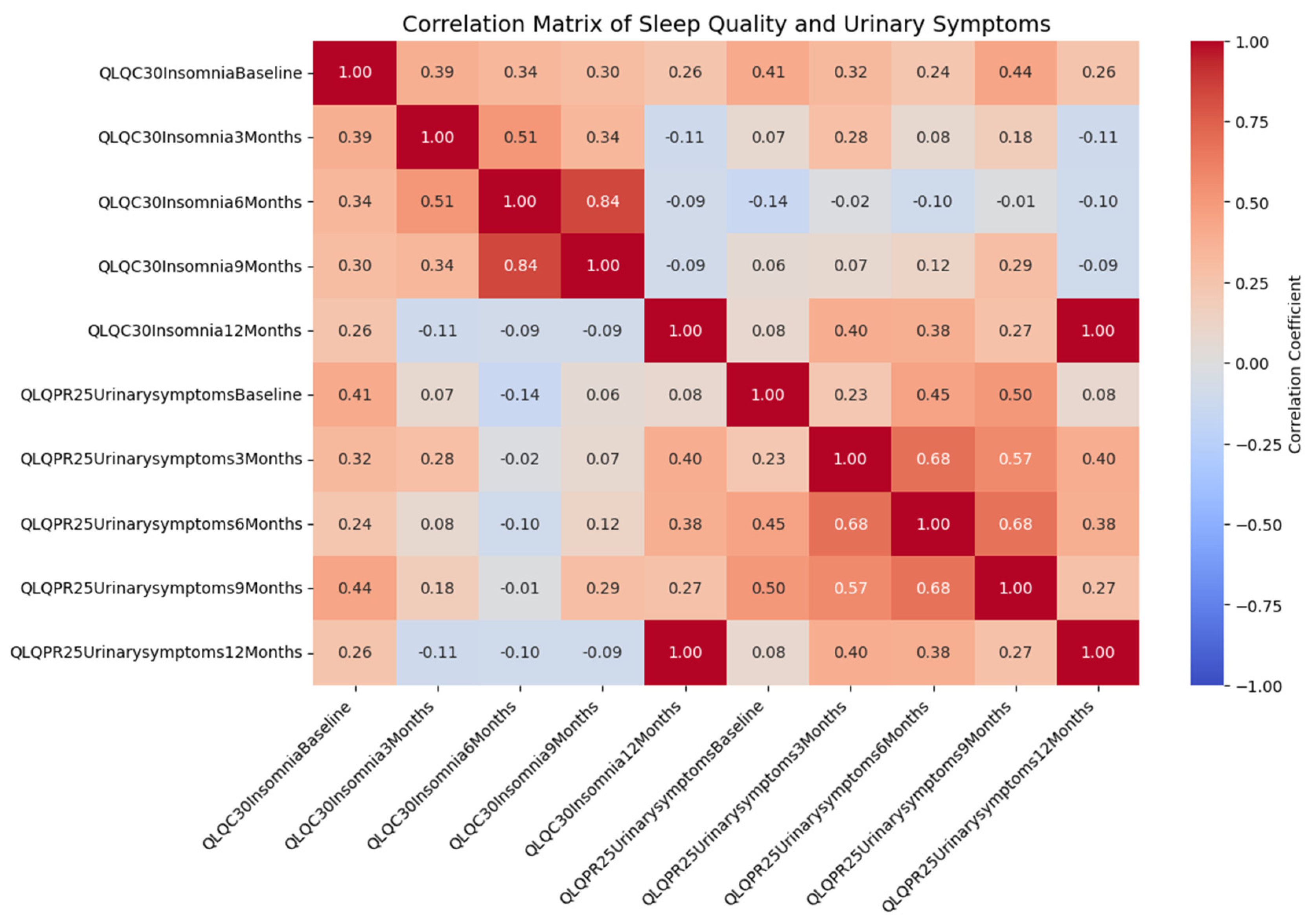
3.2. Correlation Analysis
- A moderate positive correlation between baseline insomnia and baseline urinary symptoms (r = 0.407, p = 0.011).
- A positive correlation between baseline insomnia and urinary symptoms at 3 months (r = 0.321, p = 0.049).
- Significant correlations between insomnia at 12 months and urinary symptoms at 3 months (r = 0.396, p = 0.014) and at 6 months (r = 0.384, p = 0.017).
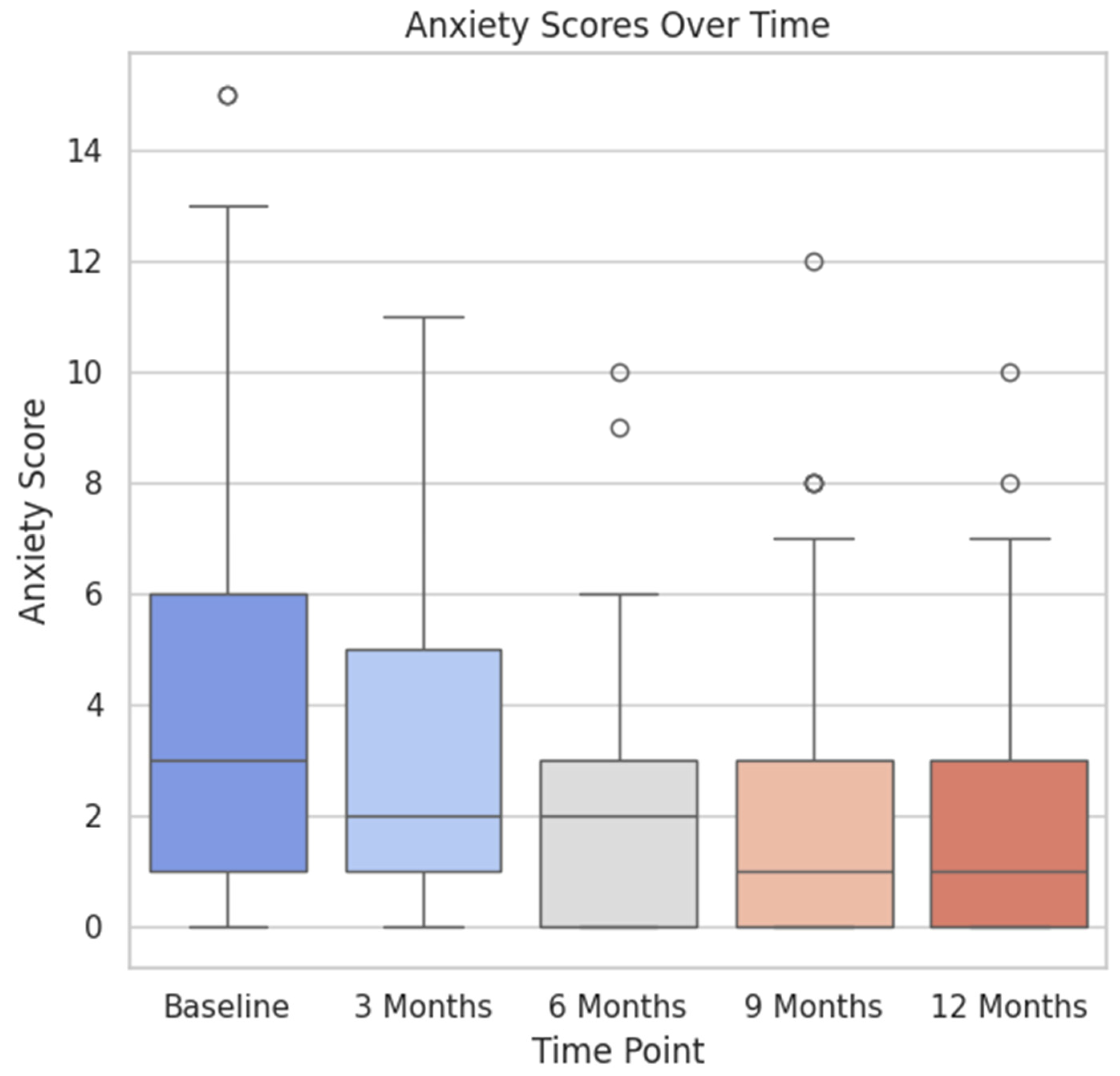
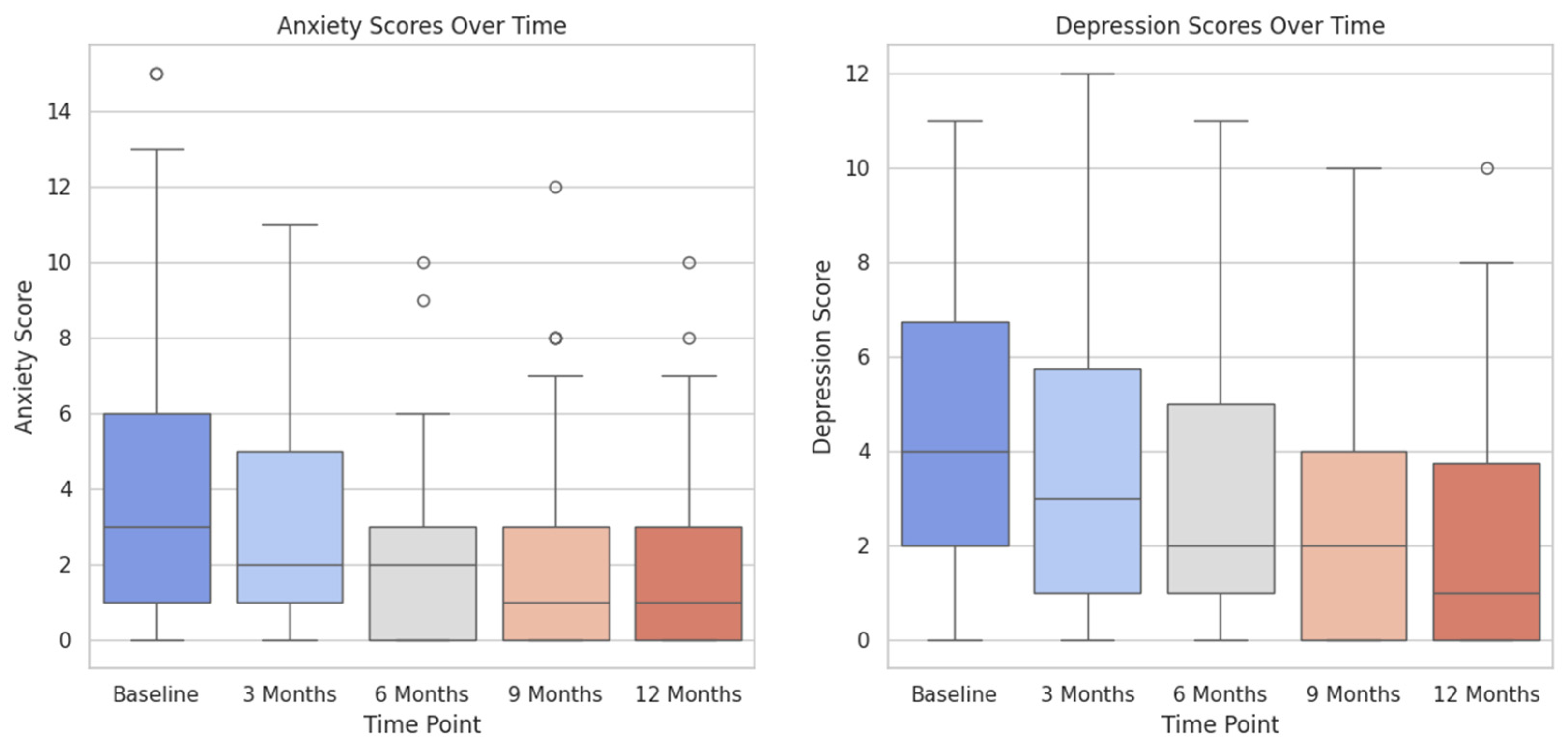
3.3. Subgroup Analysis
3.4. Statistical Tests
- Paired t-Test: Comparing baseline and 3-month insomnia scores yielded a t-statistic of 1.653 and a p-value of 0.107, indicating no significant change in sleep quality over this period.
- ANOVA: Analyzing insomnia scores across all time points (baseline, 3, 6, 9, and 12 months) resulted in an F-statistic of 0.987 and a p-value of 0.416, suggesting no significant differences in insomnia scores over time.
- Chi-Square Test: Examining the independence between smoking history and comorbidities produced a chi2 value of 16.648 and a p-value of 0.340, indicating no significant association between these variables.
- Linear Regression: Modeling the relationship between baseline insomnia and baseline urinary symptoms showed that baseline insomnia is significantly associated with baseline urinary symptoms (coef = 0.222, p = 0.036).
3.5. Visualization of Results
4. Discussion
4.1. Urinary Incontinence after Radical Prostatectomy
4.2. Correlation between Sleep Quality and Urinary Incontinence
4.3. Correlation between Anxiety and Urinary Incontinence
4.4. Implications for Patient Care
4.5. Need for Further Research
- Longitudinal Studies: Future studies should focus on monitoring these patients for an extended duration to evaluate the durability of the identified correlations and to determine any long-term effects of sleep quality on urinary health.
- Interventional Studies: Randomized controlled trials are necessary to assess the effectiveness of sleep enhancement therapies specifically designed for men with prostate cancer. Conducting such studies could provide insights into whether enhancing the quality of sleep has a direct impact on reducing urinary symptoms.
- Mechanistic Studies: Gaining a comprehensive understanding of the fundamental mechanisms that link sleep issues with urinary symptoms could offer more profound insights. This involves investigating the impact of hormone fluctuations, stress, and other physiological factors that may influence this association [29].
4.6. Broader Context and Future Directions
4.7. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miller, K.D.; Nogueira, L.; Mariotto, A.B.; Rowland, J.H.; Yabroff, K.R.; Alfano, C.M.; Jemal, A.; Kramer, J.L.; Siegel, R.L. Cancer treatment and survivorship statistics, 2019. CA Cancer J. Clin. 2019, 69, 363–385. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Nardin, S.; Mora, E.; Varughese, F.M.; D’Avanzo, F.; Vachanaram, A.R.; Rossi, V.; Saggia, C.; Rubinelli, S.; Gennari, A. Breast Cancer Survivorship, Quality of Life, and Late Toxicities. Front. Oncol. 2020, 10, 864. [Google Scholar] [CrossRef] [PubMed]
- Shakeel, S.; Tung, J.; Rahal, R.; Finley, C. Evaluation of Factors Associated with Unmet Needs in Adult Cancer Survivors in Canada. JAMA Netw. Open 2020, 3, e200506. [Google Scholar] [CrossRef]
- Paterson, C.; Robertson, A.; Smith, A.; Nabi, G. Identifying the unmet supportive care needs of men living with and beyond prostate cancer: A systematic review. Eur. J. Oncol. Nurs. 2015, 19, 405–418. [Google Scholar] [CrossRef]
- Watson, E.; Shinkins, B.; Frith, E.; Neal, D.; Hamdy, F.; Walter, F.; Weller, D.; Wilkinson, C.; Faithfull, S.; Wolstenholme, J.; et al. Symptoms, unmet needs, psychological well-being, and health status in survivors of prostate cancer: Implications for redesigning follow-up. BJU Int. 2016, 117, E10–E19. [Google Scholar] [CrossRef]
- Hazarika, I. Artificial intelligence: Opportunities and implications for the health workforce. Int. Health 2020, 12, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Tzelves, L.; Manolitsis, I.; Varkarakis, I.; Ivanovic, M.; Kokkonidis, M.; Useros, C.S.; Kosmidis, T.; Muñoz, M.; Grau, I.; Athanatos, M.; et al. Artificialintelligence supporting cancer patients across Europe-The ASCAPE project. PLoS ONE 2022, 17, e0265127. [Google Scholar] [CrossRef]
- Manolitsis, I.; Tzelves, L.; Feretzakis, G.; Kalles, D.; Verykios, V.S.; Katsimperis, S.; Anastasiou, A.; Koutsouris, D.; Kosmidis, T.; Deliveliotis, C.; et al. Acceptance of Artificial Intelligence in Supporting Cancer Patients. Stud. Health Technol. Inform. 2023, 305, 572–575. [Google Scholar] [CrossRef]
- van Andel, G.; Bottomley, A.; Fosså, S.D.; Efficace, F.; Coens, C.; Guerif, S.; Kynaston, H.; Gontero, P.; Thalmann, G.; Akdas, A.; et al. An international field study of the EORTC QLQ-PR25: A questionnaire for assessing the health-related quality of life of patients with prostate cancer. Eur. J. Cancer 2008, 44, 2418–2424. [Google Scholar] [CrossRef]
- Zigmond, A.S.; Snaith, R.P. The Hospital Anxiety and Depression Scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Rosen, R.C.; Riley, A.; Wagner, G.; Osterloh, I.H.; Kirkpatrick, J.; Mishra, A. The international index of erectile function (IIEF): A multidimensional scale for assessment of erectile dysfunction. Urology 1997, 49, 822–830. [Google Scholar] [CrossRef]
- Haghayegh, S.; Khoshnevis, S.; Smolensky, M.H.; Diller, K.R.; Castriotta, R.J. Accuracy of Wristband Fitbit Models in Assessing Sleep: Systematic Review and Meta-Analysis. J. Med. Internet Res. 2019, 21, e16273. [Google Scholar] [CrossRef]
- Mondschein, C.F.; Monda, C. The EU’s General Data Protection Regulation (GDPR) in a Research Context. In Fundamentals of Clinical Data Science; Kubben, P., Dumontier, M., Dekker, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 55–71. [Google Scholar] [CrossRef]
- Manolitsis, I.; Feretzakis, G.; Tzelves, L.; Kalles, D.; Katsimperis, S.; Angelopoulos, P.; Anastasiou, A.; Koutsouris, D.; Kosmidis, T.; Verykios, V.S.; et al. Training ChatGPT Models in Assisting Urologists in Daily Practice. Stud. Health Technol. Inform. 2023, 305, 576–579. [Google Scholar] [CrossRef] [PubMed]
- Raschka, S.; Patterson, J.; Nolet, C. Machine learning in python: Main developments and technology trends in data science, machine learning, and artificial intelligence. Information 2020, 11, 193. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.r-project.org/ (accessed on 5 August 2024).
- Chatzi, A.; Doody, O. The one-way ANOVA test explained. Nurse Res. 2023, 31, 8–14. [Google Scholar] [CrossRef]
- Kleinbaum, D.G.; Kupper, L.L.; Muller, K.E.; Nizam, A. Applied Regression Analysis and Other Multivariable Methods; Duxbury Press: Pacific Grove, CA, USA, 1998; pp. 639–655. [Google Scholar]
- Felde, G.; Engeland, A.; Hunskaar, S. Urinary incontinence associated with anxiety and depression: The impact of psychotropic drugs in a cross-sectional study from the Norwegian HUNT study. BMC Psychiatry 2020, 20, 521. [Google Scholar] [CrossRef]
- Arcila-Ruiz, M.; Brucker, B.M. The Role of Urodynamics in Post-Prostatectomy Incontinence. Curr. Urol. Rep. 2018, 19, 21. [Google Scholar] [CrossRef]
- Yu, K.; Bu, F.; Jian, T.; Liu, Z.; Hu, R.; Chen, S.; Lu, J. Urinary incontinence rehabilitation of after radical prostatectomy: A systematic review and network meta-analysis. Front. Oncol. 2024, 13, 1307434. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Braun, A.E., 3rd; Washington, S.L.; Cowan, J.E.; Hampson, L.A.; Carroll, P.R. Impact of Stress Urinary Incontinence After Radical Prostatectomy on Time to Intervention, Quality of Life and Work Status. Urology 2023, 180, 242–248. [Google Scholar] [CrossRef]
- Azevedo, C.; da Mata, L.R.F.; Izidoro, L.C.d.R.; Moura, C.d.C.; Araújo, B.B.A.; Pereira, M.G.; Chianca, T.C.M. Effectiveness of auricular acupuncture and pelvic floor muscle training in the management of urinary incontinence following surgical treatment for prostate cancer: A randomized clinical trial. Eur. J. Oncol. Nurs. 2024, 68, 102490. [Google Scholar] [CrossRef] [PubMed]
- de Zambotti, M.; Cellini, N.; Goldstone, A.; Colrain, I.M.; Baker, F.C. Wearable Sleep Technology in Clinical and Research Settings. Med. Sci. Sports Exerc. 2019, 51, 1538–1557. [Google Scholar] [CrossRef] [PubMed]
- Mirchandaney, R.; Barete, R.; Asarnow, L.D. Moderators of Cognitive Behavioral Treatment for Insomnia on Depression and Anxiety Outcomes. Curr. Psychiatry Rep. 2022, 24, 121–128. [Google Scholar] [CrossRef]
- Hertenstein, E.; Trinca, E.; Wunderlin, M.; Schneider, C.L.; Züst, M.A.; Fehér, K.D.; Su, T.; Straten, A.V.; Berger, T.; Baglioni, C.; et al. Cognitive behavioral therapy for insomnia in patients with mental disorders and comorbid insomnia: A systematic review and meta-analysis. Sleep Med. Rev. 2022, 62, 101597. [Google Scholar] [CrossRef]
- Islam, U.; Mehmood, G.; Al-Atawi, A.A.; Khan, F.; Alwageed, H.S.; Cascone, L. NeuroHealth guardian: A novel hybrid approach for precision brain stroke prediction and healthcare analytics. J. Neurosci. Methods 2024, 409, 110210. [Google Scholar] [CrossRef]
- Kohn, T.P.; Kohn, J.R.; Haney, N.M.; Pastuszak, A.W.; Lipshultz, L.I. The effect of sleep on men’s health. Transl. Androl. Urol. 2020, 9 (Suppl. S2), S178–S185. [Google Scholar] [CrossRef]
- Constand, M.K.; MacDermid, J.C.; Dal Bello-Haas, V.; Law, M. Scoping review of patient-centered care approaches in healthcare. BMC Health Serv. Res. 2014, 14, 271. [Google Scholar] [CrossRef] [PubMed]
- Shevach, J.; Weiner, A.; Morgans, A.K. Quality of Life-Focused Decision-Making for Prostate Cancer. Curr. Urol. Rep. 2019, 20, 57. [Google Scholar] [CrossRef]
- Bellos, T.; Manolitsis, I.; Katsimperis, S.; Juliebø-Jones, P.; Feretzakis, G.; Mitsogiannis, I.; Varkarakis, I.; Somani, B.K.; Tzelves, L. Artificial Intelligence in Urologic Robotic Oncologic Surgery: A Narrative Review. Cancers 2024, 16, 1775. [Google Scholar] [CrossRef]
- Talyshinskii, A.; Hameed, B.M.Z.; Ravinder, P.P.; Naik, N.; Randhawa, P.; Shah, M.; Rai, B.P.; Tokas, T.; Somani, B.K. Catalyzing Precision Medicine: Artificial Intelligence Advancements in Prostate Cancer Diagnosis and Management. Cancers 2024, 16, 1809. [Google Scholar] [CrossRef]
- Baydoun, A.; Jia, A.Y.; Zaorsky, N.G.; Kashani, R.; Rao, S.; Shoag, J.E.; Vince, R.A.; Bittencourt, L.K.; Zuhour, R.; Price, A.T.; et al. Artificial intelligence applications in prostate cancer. Prostate Cancer Prostatic Dis. 2024, 27, 37–45. [Google Scholar] [CrossRef] [PubMed]
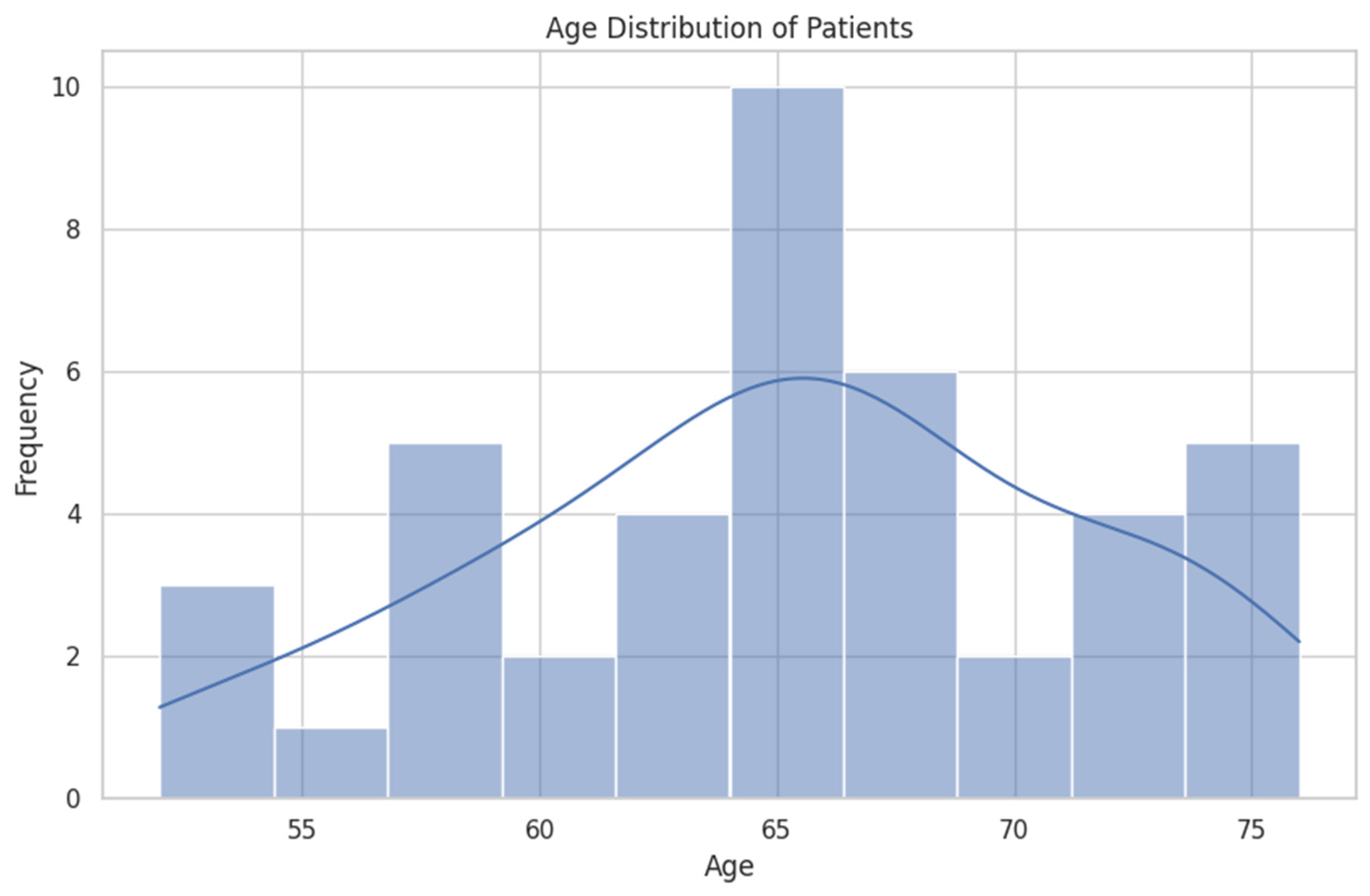
| Feature | Mean (SD) | Median (IQR) |
|---|---|---|
| Age | 65.1 (6.3) | 66.0 (61.3–68.8) |
| Presenting Symptoms (LUTS) | 0.3 (0.5) | 0.0 (0.0–1.0) |
| Comorbidities | 1.9 (1.3) | 2.0 (1.0–3.0) |
| Drug Regimen | 2.7 (2.3) | 2.5 (1.0–4.0) |
| PSA Pre-op (ng/mL) | 8.9 (7.2) | 7.7 (5.7–9.0) |
| Gleason Score (Biopsy) | 6.9 (0.8) | 7.0 (6.0–7.0) |
| Blood Loss in OR (mL) | 530.5 (149.5) | 550.0 (450.0–650.0) |
| Length of Stay (days) | 5.9 (1.9) | 5.0 (5.0–6.0) |
| Duration of Surgery (min) | 153.3 (32.4) | 155.0 (130.0–170.0) |
| BCR | 0.2 (0.4) | 0.0 (0.0–0.0) |
| Static Variables (Only at Baseline) | |||||
| Variable | Baseline (Mean ± SD) | ||||
| Age (years) | 61.54 ± 6.56 | ||||
| Weight (Kg) | 86.86 ± 11.41 | ||||
| PSA (ng/mL) | 9.11 ± 7.53 | ||||
| Gleason Score (Biopsy) | 6.92 ± 0.80 | ||||
| Time-Dependent Variables | |||||
| Variable | Baseline (Mean ± SD) | 3 Months (Mean ± SD) | 6 Months (Mean ± SD) | 9 Months (Mean ± SD) | 12 Months (Mean ± SD) |
| Urinary Symptoms (QLQ-PR25) | 21.4 ± 17.49 | 20.65 ± 18.61 | 15.31 ± 13.50 | 15.99 ± 16.51 | 11.40 ± 6.85 |
| Erectile Function (IIEF) | 39.11 ± 23.67 | 16.89 ± 10.91 | 18.41 ± 15.34 | 15.38 ± 13.27 | 15.59 ± 12.23 |
| Anxiety Time Point | UI Time Point | Correlation (r) | p-Value |
|---|---|---|---|
| HADSAnxietyBaseline | QLQPR25Urinarysymptoms12Months | 0.46 | 0.0037 |
| HADSAnxiety3Months | QLQPR25Urinarysymptoms6Months | 0.34 | 0.0373 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manolitsis, I.; Feretzakis, G.; Tzelves, L.; Anastasiou, A.; Koumpouros, Y.; Verykios, V.S.; Katsimperis, S.; Bellos, T.; Lazarou, L.; Varkarakis, I. Sleep Quality and Urinary Incontinence in Prostate Cancer Patients: A Data Analytics Approach with the ASCAPE Dataset. Healthcare 2024, 12, 1817. https://doi.org/10.3390/healthcare12181817
Manolitsis I, Feretzakis G, Tzelves L, Anastasiou A, Koumpouros Y, Verykios VS, Katsimperis S, Bellos T, Lazarou L, Varkarakis I. Sleep Quality and Urinary Incontinence in Prostate Cancer Patients: A Data Analytics Approach with the ASCAPE Dataset. Healthcare. 2024; 12(18):1817. https://doi.org/10.3390/healthcare12181817
Chicago/Turabian StyleManolitsis, Ioannis, Georgios Feretzakis, Lazaros Tzelves, Athanasios Anastasiou, Yiannis Koumpouros, Vassilios S. Verykios, Stamatios Katsimperis, Themistoklis Bellos, Lazaros Lazarou, and Ioannis Varkarakis. 2024. "Sleep Quality and Urinary Incontinence in Prostate Cancer Patients: A Data Analytics Approach with the ASCAPE Dataset" Healthcare 12, no. 18: 1817. https://doi.org/10.3390/healthcare12181817
APA StyleManolitsis, I., Feretzakis, G., Tzelves, L., Anastasiou, A., Koumpouros, Y., Verykios, V. S., Katsimperis, S., Bellos, T., Lazarou, L., & Varkarakis, I. (2024). Sleep Quality and Urinary Incontinence in Prostate Cancer Patients: A Data Analytics Approach with the ASCAPE Dataset. Healthcare, 12(18), 1817. https://doi.org/10.3390/healthcare12181817







