Short-Term and Long-Term Risk of Diabetes Mellitus among Patients with Spinal Cord Injury: A Nationwide Retrospective Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source
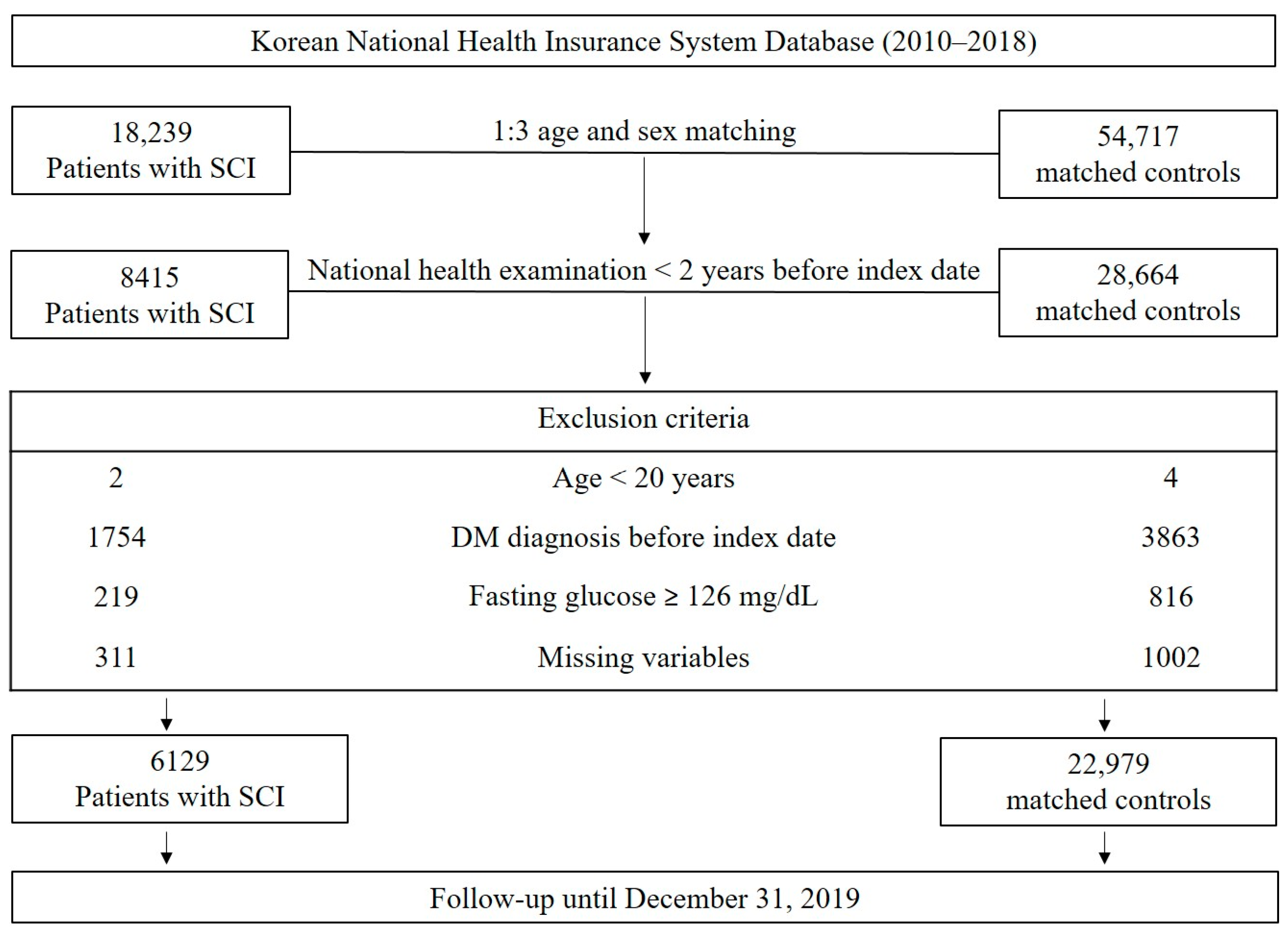
2.2. Study Design and Population
2.3. Primary Outcome
2.4. Status and Severity of Disability after SCI
2.5. Covariates
2.6. Statistical Analyses
3. Results
3.1. Baseline Characteristics
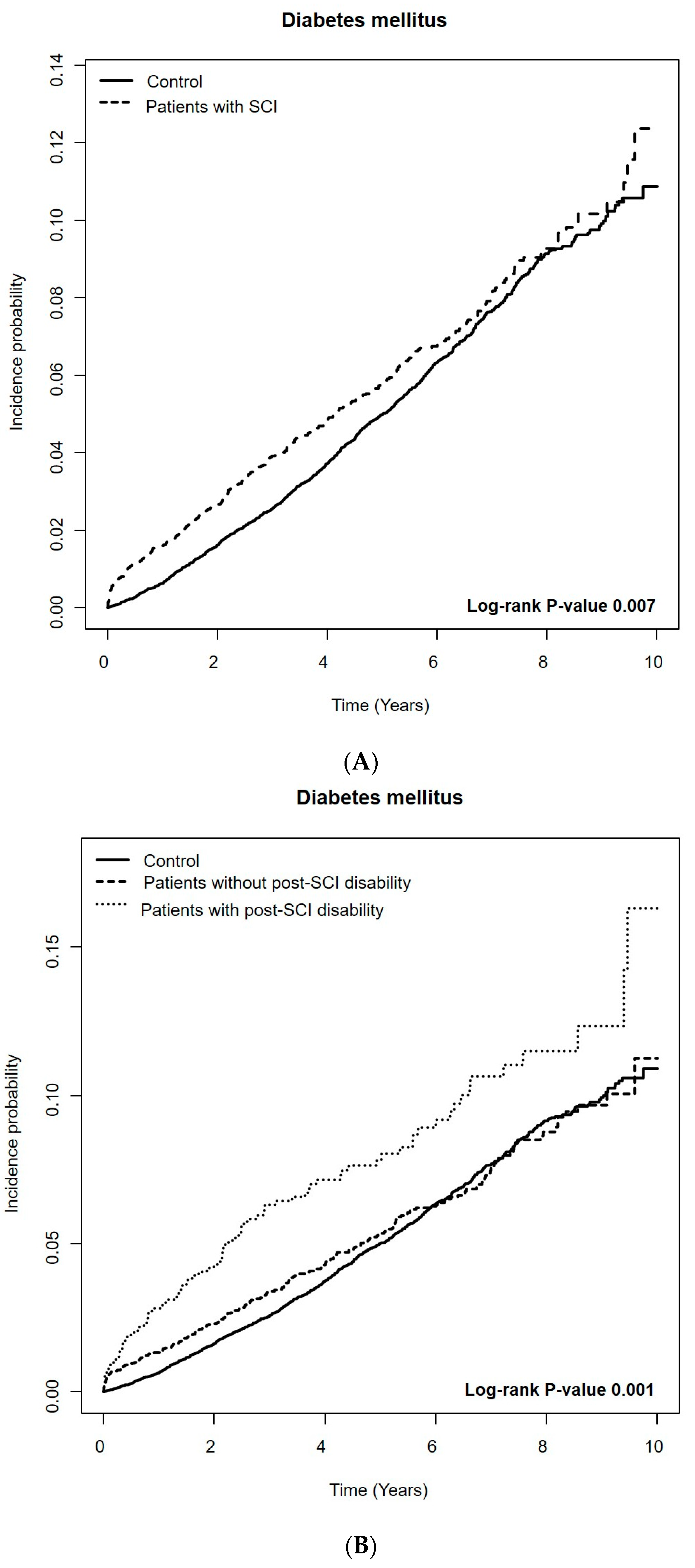
3.2. Short-Term Risk of DM among Patients with SCI Compared to the Controls
3.3. Long-Term Risk of DM among Patients with SCI Compared to Controls
4. Discussion
4.1. Investigation into Risk of DM among Patients with SCI
4.2. Strengths
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DM | diabetes mellitus |
| SCI | spinal cord injury |
| KNHIS | Korean National Health Insurance System |
| KNDRS | Korea National Disability Registration System |
| ICD-10 | International Classification of Diseases, 10th revision |
| eGFR | estimated glomerular filtration rate |
| IGF-1 | insulin-like growth factor |
| CCI | Charlson comorbidity index |
| OR | odds ratio |
| aHR | adjusted hazard ratio |
| CI | confidence interval |
References
- Ding, W.; Hu, S.; Wang, P.; Kang, H.; Peng, R.; Dong, Y.; Li, F. Spinal Cord Injury: The Global Incidence, Prevalence, and Disability from the Global Burden of Disease Study 2019. Spine 2022, 47, 1532–1540. [Google Scholar] [CrossRef] [PubMed]
- Nash, M.S.; Bilzon, J.L.J. Guideline Approaches for Cardioendocrine Disease Surveillance and Treatment Following Spinal Cord Injury. Curr. Phys. Med. Rehabil. Rep. 2018, 6, 264–276. [Google Scholar] [CrossRef]
- Ortega, M.A.; Fraile-Martinez, O.; García-Montero, C.; Haro, S.; Álvarez-Mon, M.; De Leon-Oliva, D.; Gomez-Lahoz, A.M.; Monserrat, J.; Atienza-Pérez, M.; Díaz, D.; et al. A comprehensive look at the psychoneuroimmunoendocrinology of spinal cord injury and its progression: Mechanisms and clinical opportunities. Mil. Med. Res. 2023, 10, 26. [Google Scholar] [CrossRef] [PubMed]
- Gater, D.R., Jr.; Farkas, G.J.; Tiozzo, E. Pathophysiology of Neurogenic Obesity after Spinal Cord Injury. Top. Spinal Cord. Inj. Rehabil. 2021, 27, 1–10. [Google Scholar] [CrossRef]
- Tune, J.D.; Goodwill, A.G.; Sassoon, D.J.; Mather, K.J. Cardiovascular consequences of metabolic syndrome. Transl. Res. 2017, 183, 57–70. [Google Scholar] [CrossRef]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef]
- Choi, S.H.; Sung, C.H.; Heo, D.R.; Jeong, S.Y.; Kang, C.N. Incidence of acute spinal cord injury and associated complications of methylprednisolone therapy: A national population-based study in South Korea. Spinal Cord. 2020, 58, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.J.; Jeong, J.H. Review: Steroid Use in Patients with Acute Spinal Cord Injury and Guideline Update. Korean J. Neurotrauma 2022, 18, 22–30. [Google Scholar] [CrossRef]
- Craven, B.C.; Cirnigliaro, C.M.; Carbone, L.D.; Tsang, P.; Morse, L.R. The Pathophysiology, Identification and Management of Fracture Risk, Sublesional Osteoporosis and Fracture among Adults with Spinal Cord Injury. J. Pers. Med. 2023, 13, 966. [Google Scholar] [CrossRef]
- Lagu, T.; Schroth, S.L.; Haywood, C.; Heinemann, A.; Kessler, A.; Morse, L.; Khan, S.S.; Kershaw, K.N.; Nash, M.S. Diagnosis and Management of Cardiovascular Risk in Individuals with Spinal Cord Injury: A Narrative Review. Circulation 2023, 148, 268–277. [Google Scholar] [CrossRef]
- Cao, Y.; DiPiro, N.; Krause, J.S. Association of Secondary Health Conditions with Future Chronic Health Conditions among Persons with Traumatic Spinal Cord Injury. Top. Spinal Cord. Inj. Rehabil. 2020, 26, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Cragg, J.J.; Noonan, V.K.; Dvorak, M.; Krassioukov, A.; Mancini, G.B.; Borisoff, J.F. Spinal cord injury and type 2 diabetes: Results from a population health survey. Neurology 2013, 81, 1864–1868. [Google Scholar] [CrossRef] [PubMed]
- DiPiro, N.D.; Murday, D.; Corley, E.H.; Krause, J.S. Prevalence of chronic health conditions and hospital utilization in adults with spinal cord injury: An analysis of self-report and South Carolina administrative billing data. Spinal Cord. 2019, 57, 33–40. [Google Scholar] [CrossRef]
- Jörgensen, S.; Hill, M.; Lexell, J. Cardiovascular Risk Factors among Older Adults with Long-Term Spinal Cord Injury. PM R 2019, 11, 8–16. [Google Scholar] [CrossRef] [PubMed]
- LaVela, S.L.; Evans, C.T.; Prohaska, T.R.; Miskevics, S.; Ganesh, S.P.; Weaver, F.M. Males aging with a spinal cord injury: Prevalence of cardiovascular and metabolic conditions. Arch. Phys. Med. Rehabil. 2012, 93, 90–95. [Google Scholar] [CrossRef]
- Peterson, M.D.; Berri, M.; Lin, P.; Kamdar, N.; Rodriguez, G.; Mahmoudi, E.; Tate, D. Cardiovascular and metabolic morbidity following spinal cord injury. Spine J. 2021, 21, 1520–1527. [Google Scholar] [CrossRef]
- Selassie, A.; Snipe, L.; Focht, K.L.; Welldaregay, W. Baseline prevalence of heart diseases, hypertension, diabetes, and obesity in persons with acute traumatic spinal cord injury: Potential threats in the recovery trajectory. Top. Spinal Cord. Inj. Rehabil. 2013, 19, 172–182. [Google Scholar] [CrossRef]
- Solinsky, R.; Betancourt, L.; Schmidt-Read, M.; Kupfer, M.; Owens, M.; Schwab, J.M.; Dusseau, N.B., 2nd; Szlachcic, Y.; Sutherland, L.; Taylor, J.A.; et al. Acute Spinal Cord Injury Is Associated with Prevalent Cardiometabolic Risk Factors. Arch. Phys. Med. Rehabil. 2022, 103, 696–701. [Google Scholar] [CrossRef] [PubMed]
- Raguindin, P.F.; Fränkl, G.; Itodo, O.A.; Bertolo, A.; Zeh, R.M.; Capossela, S.; Minder, B.; Stoyanov, J.; Stucki, G.; Franco, O.H.; et al. The neurological level of spinal cord injury and cardiovascular risk factors: A systematic review and meta-analysis. Spinal Cord. 2021, 59, 1135–1145. [Google Scholar] [CrossRef]
- Gordon, P.S.; Farkas, G.J.; Gater, D.R., Jr. Neurogenic Obesity-Induced Insulin Resistance and Type 2 Diabetes Mellitus in Chronic Spinal Cord Injury. Top. Spinal Cord. Inj. Rehabil. 2021, 27, 36–56. [Google Scholar] [CrossRef]
- Lai, Y.J.; Lin, C.L.; Chang, Y.J.; Lin, M.C.; Lee, S.T.; Sung, F.C.; Lee, W.Y.; Kao, C.H. Spinal cord injury increases the risk of type 2 diabetes: A population-based cohort study. Spine J. 2014, 14, 1957–1964. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.W.; Cho, J.; Park, J.H.; Cho, B. National General Health Screening Program in Korea: History, current status, and future direction. Precis. Future Med. 2022, 6, 9–31. [Google Scholar] [CrossRef]
- Kim, M.; Jung, W.; Kim, S.Y.; Park, J.H.; Shin, D.W. The Korea National Disability Registration System. Epidemiol. Health 2023, 45, e2023053. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.E.; Kim, M.; Kim, B.; Lee, H.; Chang, W.H.; Yoo, J.; Han, K.; Shin, D.W. Increased Risk of Myocardial Infarction, Heart Failure, and Atrial Fibrillation After Spinal Cord Injury. J. Am. Coll. Cardiol. 2024, 83, 741–751. [Google Scholar] [CrossRef]
- Jung, I.; Kwon, H.; Park, S.E.; Han, K.D.; Park, Y.G.; Rhee, E.J.; Lee, W.Y. The Prevalence and Risk of Type 2 Diabetes in Adults with Disabilities in Korea. Endocrinol. Metab. 2020, 35, 552–561. [Google Scholar] [CrossRef]
- Kim, K.H. Comparative study on three algorithms of the ICD-10 Charlson comorbidity index with myocardial infarction patients. J. Prev. Med. Public Health 2010, 43, 42–49. [Google Scholar] [CrossRef]
- Guest, J.; Datta, N.; Jimsheleishvili, G.; Gater, D.R., Jr. Pathophysiology, Classification and Comorbidities after Traumatic Spinal Cord Injury. J. Pers. Med. 2022, 12, 1126. [Google Scholar] [CrossRef]
- Sultan, I.; Lamba, N.; Liew, A.; Doung, P.; Tewarie, I.; Amamoo, J.J.; Gannu, L.; Chawla, S.; Doucette, J.; Cerecedo-Lopez, C.D.; et al. The safety and efficacy of steroid treatment for acute spinal cord injury: A Systematic Review and meta-analysis. Heliyon 2020, 6, e03414. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, Y.; He, L.; Pang, M.; Luo, C.; Liu, B.; Rong, L. High-dose methylprednisolone for acute traumatic spinal cord injury: A meta-analysis. Neurology 2019, 93, e841–e850. [Google Scholar] [CrossRef]
- Cheng, R.D.; Ren, W.; Sun, P.; Tian, L.; Zhang, L.; Zhang, J.; Li, J.B.; Ye, X.M. Spinal cord injury causes insulin resistance associated with PI3K signaling pathway in hypothalamus. Neurochem. Int. 2020, 140, 104839. [Google Scholar] [CrossRef]
- Doherty, J.G.; Burns, A.S.; O’Ferrall, D.M.; Ditunno, J.F., Jr. Prevalence of upper motor neuron vs lower motor neuron lesions in complete lower thoracic and lumbar spinal cord injuries. J. Spinal Cord. Med. 2002, 25, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Talifu, Z.; Zhang, C.J.; Gao, F.; Ke, H.; Pan, Y.Z.; Gong, H.; Du, H.Y.; Yu, Y.; Jing, Y.L.; et al. Mechanism of skeletal muscle atrophy after spinal cord injury: A narrative review. Front. Nutr. 2023, 10, 1099143. [Google Scholar] [CrossRef]
- Alazzam, A.M.; Goldsmith, J.A.; Khalil, R.E.; Khan, M.R.; Gorgey, A.S. Denervation impacts muscle quality and knee bone mineral density after spinal cord injury. Spinal Cord. 2023, 61, 276–284. [Google Scholar] [CrossRef]
- O’Brien, L.C.; Chen, Q.; Savas, J.; Lesnefsky, E.J.; Gorgey, A.S. Skeletal muscle mitochondrial mass is linked to lipid and metabolic profile in individuals with spinal cord injury. Eur. J. Appl. Physiol. 2017, 117, 2137–2147. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, L.C.; Wade, R.C.; Segal, L.; Chen, Q.; Savas, J.; Lesnefsky, E.J.; Gorgey, A.S. Mitochondrial mass and activity as a function of body composition in individuals with spinal cord injury. Physiol. Rep. 2017, 5, e13080. [Google Scholar] [CrossRef] [PubMed]
- DiSabato, D.J.; Marion, C.M.; Mifflin, K.A.; Alfredo, A.N.; Rodgers, K.A.; Kigerl, K.A.; Popovich, P.G.; McTigue, D.M. System failure: Systemic inflammation following spinal cord injury. Eur. J. Immunol. 2024, 54, e2250274. [Google Scholar] [CrossRef]
- Gorgey, A.S.; Dudley, G.A. Skeletal muscle atrophy and increased intramuscular fat after incomplete spinal cord injury. Spinal Cord. 2007, 45, 304–309. [Google Scholar] [CrossRef]
- Boehl, G.; Raguindin, P.F.; Valido, E.; Bertolo, A.; Itodo, O.A.; Minder, B.; Lampart, P.; Scheel-Sailer, A.; Leichtle, A.; Glisic, M.; et al. Endocrinological and inflammatory markers in individuals with spinal cord injury: A systematic review and meta-analysis. Rev. Endocr. Metab. Disord. 2022, 23, 1035–1050. [Google Scholar] [CrossRef]
- Byers, J.S.; Huguenard, A.L.; Kuruppu, D.; Liu, N.K.; Xu, X.M.; Sengelaub, D.R. Neuroprotective effects of testosterone on motoneuron and muscle morphology following spinal cord injury. J. Comp. Neurol. 2012, 520, 2683–2696. [Google Scholar] [CrossRef]
- Bluvshtein, V.; Korczyn, A.D.; Pinhas, I.; Vered, Y.; Gelernter, I.; Catz, A. Insulin resistance in tetraplegia but not in mid-thoracic paraplegia: Is. the mid-thoracic spinal cord involved in glucose regulation? Spinal Cord. 2011, 49, 648–652. [Google Scholar] [CrossRef]
- Goodus, M.T.; McTigue, D.M. Hepatic dysfunction after spinal cord injury: A vicious cycle of central and peripheral pathology? Exp. Neurol. 2020, 325, 113160. [Google Scholar] [CrossRef] [PubMed]
- Wulf, M.J.; Tom, V.J. Consequences of spinal cord injury on the sympathetic nervous system. Front. Cell. Neurosci. 2023, 17, 999253. [Google Scholar] [CrossRef]
- Kimball, A.L.; Petrie, M.A.; McCue, P.M.; Johnson, K.A.; Shields, R.K. Impaired Glucose Tolerance and Visceral Adipose Tissue Thickness among Lean and Non-Lean People with and without Spinal Cord Injury. J. Funct. Morphol. Kinesiol. 2023, 8, 123. [Google Scholar] [CrossRef]
- Farkas, G.J.; Gater, D.R. Neurogenic obesity and systemic inflammation following spinal cord injury: A review. J. Spinal Cord. Med. 2018, 41, 378–387. [Google Scholar] [CrossRef]
- Shojaei, M.H.; Alavinia, S.M.; Craven, B.C. Management of obesity after spinal cord injury: A systematic review. J. Spinal Cord. Med. 2017, 40, 783–794. [Google Scholar] [CrossRef] [PubMed]
- McDonald, J.W.; Sadowsky, C. Spinal-cord injury. Lancet 2002, 359, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Majdan, M.; Plancikova, D.; Nemcovska, E.; Krajcovicova, L.; Brazinova, A.; Rusnak, M. Mortality due to traumatic spinal cord injuries in Europe: A cross-sectional and pooled analysis of population-wide data from 22 countries. Scand. J. Trauma Resusc. Emerg. Med. 2017, 25, 64. [Google Scholar] [CrossRef]
- Maruyama, Y.; Mizuguchi, M.; Yaginuma, T.; Kusaka, M.; Yoshida, H.; Yokoyama, K.; Kasahara, Y.; Hosoya, T. Serum leptin, abdominal obesity and the metabolic syndrome in individuals with chronic spinal cord injury. Spinal Cord. 2008, 46, 494–499. [Google Scholar] [CrossRef]
| SCI | Control (n = 22,979) | p-Value a | p-Value b | |||
|---|---|---|---|---|---|---|
| Total (n = 6129) | Without Disability (n = 5026) | With Disability (n = 1103) | ||||
| Age, years | 56.8 ± 13.2 | 56.3 ± 13.3 | 59.0 ± 12.5 | 56.6 ± 13.3 | 0.271 | <0.001 |
| Sex, male, n (%) | 4172 (68.1) | 3379 (67.2) | 793 (71.9) | 15,928 (69.3) | 0.061 | 0.002 |
| Income, lowest 25%, n (%) | 1279 (20.9) | 1014 (20.2) | 265 (24.0) | 4042 (17.6) | <0.001 | <0.001 |
| Residence, urban, n (%) | 2288 (37.3) | 1873 (37.3) | 415 (37.6) | 10,404 (45.3) | <0.001 | <0.001 |
| BMI, kg/m2 | 24.0 ± 3.2 | 24.0 ± 3.1 | 24.0 ± 3.4 | 24.0 ± 3.1 | 0.747 | 0.909 |
| Hypertension, n (%) | 2498 (40.8) | 1969 (39.2) | 529 (48.0) | 8128 (35.4) | <0.001 | <0.001 |
| Dyslipidemia, n (%) | 1734 (28.3) | 1402 (27.9) | 332 (30.1) | 6071 (26.4) | 0.003 | 0.004 |
| Charlson Comorbidity Index | 2.7 ± 2.3 | 2.5 ± 2.2 | 3.5 ± 2.3 | 1.2 ± 1.5 | <0.001 | <0.001 |
| Systolic blood pressure (mmHg) | 124.7 ± 15.3 | 124.6 ± 15.1 | 125.2 ± 15.9 | 124.3 ± 14.6 | 0.080 | 0.103 |
| Diastolic blood pressure (mmHg) | 77.3 ± 10.0 | 77.2 ± 10.0 | 77.6 ± 10.2 | 77.0 ± 9.76 | 0.103 | 0.142 |
| Fasting serum glucose (mg/dL) | 95.5 ± 11.5 | 95.4 ± 11.3 | 96.0 ± 12.0 | 95.3 ± 11.3 | 0.207 | 0.135 |
| Total cholesterol (mg/dL) | 194.8 ± 37.6 | 195.6 ± 37.4 | 191.3 ± 38.0 | 197.2 ± 36.5 | <0.001 | <0.001 |
| eGFR (mL/min/1.73 m2) | 92.1 ± 49.3 | 91.8 ± 50.1 | 93.7 ± 45.3 | 89.0 ± 44.3 | <0.001 | <0.001 |
| Regular exercise, n (%) | 1290 (21.1) | 1056 (21.0) | 234 (21.2) | 5057 (22.0) | 0.106 | 0.268 |
| Smoking, n (%) | <0.001 | <0.001 | ||||
| Never-smoker | 3069 (50.1) | 2522 (50.2) | 547 (49.6) | 11,906 (51.8) | ||
| Ex-smoker | 1182 (19.3) | 942 (18.7) | 240 (21.8) | 5388 (23.5) | ||
| Current smoker | 1878 (30.6) | 1562 (31.1) | 316 (28.7) | 5685 (24.7) | ||
| Alcohol Consumption, n (%) | <0.001 | <0.001 | ||||
| None | 2947 (48.1) | 2359 (46.9) | 588 (53.3) | 11,681 (50.8) | ||
| Mild (<30g/d) | 2404 (39.2) | 2007 (39.9) | 397 (36.0) | 9363 (40.8) | ||
| Heavy (≥30g/d) | 778 (12.7) | 660 (13.1) | 118 (10.7) | 1935 (8.4) | ||
| Follow-up duration, years | 4.6 ± 2.6 | 4.6 ± 2.6 | 4.7 ± 2.6 | 5.0 ± 2.5 | <0.001 | <0.001 |
| Events | IR | OR (95% Confidence Interval) | |
|---|---|---|---|
| Comparison between patients with SCI and the controls | |||
| Control | 145 | 0.006 | 1.00 (ref.) |
| SCI | 96 | 0.016 | 2.51 (1.91, 3.27) |
| Comparison between patients with SCI and the controls by disability status | |||
| Control | 145 | 0.006 | 1.00 (ref.) |
| SCI without disability | 65 | 0.013 | 2.06 (1.51, 2.79) |
| SCI with disability | 31 | 0.028 | 4.55 (2.97, 6.79) |
| Comparison between patients with SCI and the controls by the degree of disability | |||
| Control | 145 | 0.006 | 1.00 (ref.) |
| SCI without disability | 65 | 0.013 | 2.06 (1.51, 2.79) |
| SCI with mild disability (Grade 4–6) | 16 | 0.025 | 3.98 (2.20, 6.73) |
| SCI with severe disability (Grade 1–3) | 15 | 0.033 | 5.38 (2.91, 9.27) |
| Comparison between patients with SCI and the controls by the level of SCI | |||
| Control | 145 | 0.006 | 1.00 (ref.) |
| SCI without disability | |||
| Cervical level | 58 | 0.015 | 2.42 (1.75, 3.31) |
| Thoracic level | 3 | 0.012 | 1.98 (0.40, 5.99) |
| Lumbar level | 4 | 0.004 | 0.67 (0.18, 1.75) |
| SCI with disability | |||
| Cervical level | 25 | 0.030 | 4.93 (3.07, 7.63) |
| Thoracic level | 3 | 0.023 | 3.78 (0.76, 11.52) |
| Lumbar level | 3 | 0.020 | 3.17 (0.64, 9.63) |
| Total Follow-Up Period | After a 1-Year Lag Period | |||||
|---|---|---|---|---|---|---|
| Events | IR | aHR (95% CI) | Events | IR | aHR (95% CI) | |
| Comparison between patients with SCI and the controls | ||||||
| Control | 1227 | 10.7 | 1.00 (ref.) | 1082 | 11.8 | 1.00 (ref.) |
| SCI | 354 | 12.5 | 1.13 (1.00, 1.27) | 258 | 11.5 | 0.93 (0.81, 1.06) |
| Comparison between patients with SCI and the controls by disability status | ||||||
| Control | 1227 | 10.7 | 1.00 (ref.) | 1082 | 11.8 | 1.00 (ref.) |
| SCI without disability | 264 | 11.4 | 1.05 (0.92, 1.20) | 199 | 10.9 | 0.93 (0.81, 1.06) |
| SCI with disability | 90 | 17.4 | 1.41 (1.14, 1.74) | 59 | 14.4 | 1.05 (0.81, 1.37) |
| Comparison between patients with SCI and the controls by the degree of disability | ||||||
| Control | 1227 | 10.7 | 1.00 (ref.) | 1082 | 11.8 | 1.00 (ref.) |
| SCI without disability | 264 | 11.4 | 1.05 (0.92, 1.20) | 199 | 10.9 | 0.93 (0.81, 1.06) |
| SCI with mild disability (Grade 4–6) | 50 | 16.2 | 1.19 (0.89, 1.58) | 34 | 13.9 | 0.91 (0.65, 1.29) |
| SCI with severe disability (Grade 1–3) | 40 | 19.0 | 1.83 (1.34, 2.51) | 25 | 15.0 | 1.32 (0.89, 1.96) |
| Comparison between patients with SCI and the controls by the level of SCI | ||||||
| Control | 1227 | 10.7 | 1.00 (ref.) | 1082 | 11.8 | 1.00 (ref.) |
| SCI without disability | ||||||
| Cervical level | 201 | 11.7 | 1.06 (0.91, 1.23) | 143 | 10.6 | 0.86 (0.72, 1.02) |
| Thoracic level | 15 | 15.0 | 1.17 (0.70, 1.95) | 12 | 15.5 | 1.10 (0.62, 1.94) |
| Lumbar level | 48 | 9.60 | 0.98 (0.74, 1.32) | 44 | 10.8 | 0.98 (0.73, 1.33) |
| SCI with disability | ||||||
| Cervical level | 68 | 18.0 | 1.42 (1.11, 1.82) | 43 | 14.5 | 1.03 (0.76, 1.39) |
| Thoracic level | 11 | 18.8 | 1.92 (1.06, 3.47) | 8 | 17.4 | 1.65 (0.82, 3.31) |
| Lumbar level | 11 | 13.3 | 1.05 (0.58, 1.91) | 8 | 11.9 | 0.85 (0.42, 1.70) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Han, K.-D.; Kim, B.; Min, J.-H.; Chang, W.H.; Cho, I.Y.; Shin, D.W. Short-Term and Long-Term Risk of Diabetes Mellitus among Patients with Spinal Cord Injury: A Nationwide Retrospective Cohort Study. Healthcare 2024, 12, 1859. https://doi.org/10.3390/healthcare12181859
Kim S, Han K-D, Kim B, Min J-H, Chang WH, Cho IY, Shin DW. Short-Term and Long-Term Risk of Diabetes Mellitus among Patients with Spinal Cord Injury: A Nationwide Retrospective Cohort Study. Healthcare. 2024; 12(18):1859. https://doi.org/10.3390/healthcare12181859
Chicago/Turabian StyleKim, Seonghye, Kyung-Do Han, Bongseong Kim, Ju-Hong Min, Won Hyuk Chang, In Young Cho, and Dong Wook Shin. 2024. "Short-Term and Long-Term Risk of Diabetes Mellitus among Patients with Spinal Cord Injury: A Nationwide Retrospective Cohort Study" Healthcare 12, no. 18: 1859. https://doi.org/10.3390/healthcare12181859







