Abstract
Objectives: Controlling the lifestyle associated with dementia risk can delay the process of cognitive decline. Subjective cognitive decline (SCD) and mild cognitive impairment (MCI) are early states in the development of dementia and are also the window period for early intervention in dementia. The purpose of this study was to explore the association between multi-domain lifestyle and objective cognitive impairment in elderly people with SCD and MCI in Chinese communities and to provide reference for effective implementation of precise health management measures to reduce the risk of dementia. Methods: A total of 265 middle-aged and elderly volunteers recruited from the community were divided into SCD group (107 cases), MCI group (80 cases), and healthy control (HC) group (78 cases). All participants received clinical interview, examination, and cognitive assessments. Results: The total Dementia Risk Reduction Lifestyle Scale (DRRLS) scores in the HC, SCD, and MCI groups [110.00 (11.25) vs. 101.00 (10.00) vs. 79.50 (20.75)] exhibited statistically significant differences among them. The total score of the DRRLS showed a significant negative correlation with the Trail-Making Test (TMT), and significant positive correlations with both the Verbal Fluency Test (VFT) and Auditory Verbal Learning Test (AVLT) scores (p < 0.05). After adjusting for confounding factors, such as age and years of education, multiple linear regression analysis revealed several points. In the SCD group, brain-strengthening exercise and interpersonal relationship scores were negatively correlated with TMT scores (β = −11.257, −15.077; all p < 0.05), while health responsibility, smoking control behavior, and interpersonal relationship scores were positively correlated with AVLT scores (β = 0.485, 0.344, and 0.406; all p < 0.05). In the MCI Group, brain-strengthening exercise, brain-healthy diet, and interpersonal relationship were negatively correlated with TMT (β = −22.011, −16.206, −11.696; all p < 0.01), whereas health responsibility, mental activity, smoking control behavior, interpersonal relationship, and stress management were positively correlated with AVLT (β = 0.450, 0.435, 0.308, 0.256, 0.607; all p < 0.05). Conclusions: In Chinese communities, the unhealthy lifestyle of elderly individuals with SCD and MCI is significantly associated with cognitive function impairment. The greater their unhealthy lifestyle habits, the more pronounced the scope and severity of cognitive function impairment becomes. Furthermore, different dimensions of lifestyle have varying impacts on cognitive domains.
1. Introduction
In China, as the population ages, the cognitive decline and cognitive disorders among the elderly have become a significant public health concern. Alzheimer’s disease (AD) is a major chronic disease that poses a serious threat to the health and lives of the elderly. China is a large country with a large elderly population, and the number of people with AD has exceeded 15 million, ranking first in the world, and is expected to exceed 40 million by 2050 [1]. The burden of family and society caused by AD will be a severe challenge to the whole society and healthy development of China. It is predicted that over the next decade, the Crude Mortality Rate (CMR) for AD and other dementias in China will increase to 9.66 per 100,000 people, while the Age-Standardized Mortality Rate (ASMR) is expected to decrease to 3.42 per 100,000 people. The upward trend in CMR and downward trend in ASMR suggest the further development of population aging and dementia mortality in the future decades in China [2]. Due to the complexity of the etiology and pathological mechanisms, there is no effective cure for the dementia stage of AD relative to the rapidly growing number of patients, but early non-pharmacological interventions for target populations at risk of progressing to AD can slow or delay the development of dementia. Subjective cognitive decline (SCD) and mild cognitive impairment (MCI) are precursors to AD and are common in the elderly population, where they are two to four times more likely to progress to AD than in normal elderly people. SCD is defined as a subjective perception of significant cognitive decline in a person with a normal objective cognitive examination, but there may be mild impairment in complex neurocognitive domains. A study indicates that 50% to 80% of elderly individuals assessed at memory clinics report a decline in some cognitive functions [3]. MCI indicates mild cognitive impairment, but the impact on daily activities and social functioning has not yet reached the level of AD. Globally, the prevalence of MCI is approximately 19.7%, and this proportion has been increasing over time, particularly after 2019 [4]. Both SCD and MCI patients have complaints of cognitive decline of their own origin and are able to actively seek medical help, which is the best window for early intervention in AD [5]. The aim of this study was to determine the cognitive status of the older population by using a localized Quick Cognitive Screening Scale for the Elderly (QCCS-E) questionnaire [6].
Dementia prevention, intervention, and care: 2024 report of the Lancet standing Commission recently identified 14 potentially modifiable risk factors for AD, most of which are associated with poor lifestyle, such as hypertension, obesity, smoking, depression, lack of physical activity, social isolation, excessive alcohol consumption, and so on [7]. It is estimated that interventions targeting modifiable risk factors in the preclinical stage of the disease, through the adoption of healthy lifestyle and behavior habits, may prevent or delay 40% of AD cases [8]. In recent years, the incidence of AD in developed countries has been on a downward trend by helping the population abandon bad lifestyles and controlling vascular risk factors [9].
In recent years, China has been taking action to promote healthy aging, building a community network for elderly cognitive impairment management, and the concept of preventing AD by changing unhealthy lifestyles is increasingly being recognized [10,11]. Since the changes in different cognitive domains at the clinically early stage of AD are not synchronized, and the degree of brain function damage caused by different categories of unhealthy lifestyles is different, it is necessary to study the correlation between lifestyle and different cognitive domains in SCD and MCI patients, providing reference data for healthcare professionals to develop targeted health management measures for high-risk AD populations [12].
In recent years, in order to achieve healthy aging, the Chinese government has established a community health management network for the elderly with cognitive impairment to promote the concept and practice of AD prevention and treatment to change the unhealthy lifestyle. In the early clinical stage of AD, the changes in different cognitive domains are not synchronized, and the characteristics of brain function impairment caused by different types of adverse lifestyle are also different. This study took elderly people in Chinese communities as samples to investigate the association between multi-domain lifestyle and objective cognitive impairment in patients with SCD and MCI, ho-ping to effectively reduce the risk of dementia in elderly people in communities through precise multi-domain healthy life interventions.
2. Materials and Methods
2.1. Participants
A cross-sectional study was conducted in Wuxi, Jiangsu Province, China from October 2022 to June 2023. We recruited 300 volunteers aged 55 and above in the community through a questionnaire survey. The inclusion criteria were as follows: (1) age 55–80 years, primary school education and above; (2) having basic verbal expression and writing ability; (3) no serious physical illness affecting cognitive function; (4) voluntarily participated in this study and signed an informed consent form. The exclusion criteria were as follows: (1) significant cognitive impairment and severe mental illness; (2) any serious untreated systemic disease or unstable chronic somatic diseases. This study was approved by the Ethics Committee of Wuxi Mental Health Center (N0: WXMH-CIRB2021LLky001) and conformed to the Declaration of Helsinki. Stratified by sex and age, all participants were recruited directly by the researchers of this study. The purpose, risks, and benefits of the study and the principles of confidentiality and voluntary participation were explained to the 300 participants either orally or by text. Participants were allowed 1 week to consider whether to participate, and the final valid sample size was 265, all of whom signed the informed consent form.
2.2. Assessment and Grouping
2.2.1. General Information Questionnaire
Sociodemographic information that could potentially influence the participants cognition was collected using a simple questionnaire designed by the researcher. General information questionnaire includes gender, age, level of education, marital status, occupational classification, living situation, smoking history, alcohol consumption, hypertension, diabetes, and body mass index (BMI).
2.2.2. Subject Cognitive Assessment
Subjective cognitive symptoms were evaluated by the subjective cognitive decline questionnaire9 (SCD-Q9). It is a self-rating scale, including three dimensions, overall memory ability, daily activity ability, and time comparison, with nine entries and a total score of 0 to 9. Researchers conducted questionnaire assessments for the participants; the higher the score, the higher the likelihood of cognitive impairment. The questionnaire’s split-half reliability was 0.890, and the Cronbach’α coefficient was 0.880, indicating high reliability [13].
2.2.3. Global Cognitive Evaluation
Global cognitive function was evaluated by the QCSS-E. It contains 51 items that assess 12 cognitive domains: immediate memory, naming of objects, verbal fluency, visuospatial ability, digit span, auditory imitation, visual imitation, abstract ability, command, delayed memory, simple calculation, and temporal and spatial orientation. The total score of the QCSS-E distributes from 0 to 85, and the lower the score, the worse the impairment of cognitive function. The scale retest reliability was 0.972, and the Cronbach’α coefficient was 0.814. The QCSS-E showed good reliability and validity among older Chinese adults [14].
2.2.4. Dementia Risk Reduction Lifestyle Scale (DRRLS)
The status of healthy lifestyle was adopted using the DRRLS, developed by Zhang Jinying et al. It measures eight dimensions, health responsibility, brain-strengthening exercise, mental activity, brain-healthy diet, smoking control behavior, interpersonal relationship, stress management, and spiritual growth, with a total score of 32~128 points. Higher scores indicate that the lifestyle and behavioral habits of participants are more conducive to reducing the risk of dementia and promoting brain health. A scale with a split-half reliability of 0.909, re-test reliability of 0.864, and a Cronbach’α coefficient of 0.862 was used. Researchers assessed the quality of the dementia-risk lifestyles of participants based on their DRRLS scores [15].
2.2.5. Core Neurocognitive Assessment
Objective cognitive function was assessed using core neurocognitive tests (CNT), including Trail-Making Test (TMT), Verbal Fluency Text (VFT), and Auditory Verbal Learning Test (AVLT). The Trail-Making Test-A (TMT-A) requires participants to connect numbers from 1 to 25 in ascending order as quickly as possible; the Trail-Making Test-B (TMT-B) involves numbers embedded within squares and circles, requiring participants to alternate between the two shapes while connecting the numbers in sequence, thereby measuring information processing speed and flexibility, respectively. In this study, the time taken to complete TMT-B is used as the scoring criterion; the longer the time, the more severe the impairment of executive function. The VFT recorded the total number of animal names correctly uttered by participants within 1 min, with 1 point for each name correct; the lower the score, the more severe the impairment of verbal function. The AVLT recorded the number of long-delayed memories correctly recalled for 12 words in 3 different categories, with 1 point for each word correct; the lower the score, the more severe the impairment of verbal function. The AVLT recorded the number of correct long-delay recalls of 12 words in three different categories, with 1 point per correct word, and the lower the score, the more severe the delayed memory impairment; the sums of the sensitivity and specificity of the three tests in identifying mild cognitive impairment were 1.67, 1.80, and 1.94, respectively [16,17,18,19]. All instruments used in this study have been previously validated for use in the an elderly Chinese population, ensuring the reliability and comparability of our findings with other studies.
2.3. Data Collection and Clinical Grouping
All volunteers who met the recruitment criteria were notified to come to the hospital to complete the general information survey, scale assessment, core neurocognitive tests, psychiatric examination, necessary auxiliary examination. Finally, 265 participants completed all the tests. Based on the examination results, participants were divided into three groups: HC, SCD, and MCI. The SCD group was formulated with reference to the concepts of the 2014 International Working Group: (1) presence of subjective cognitive complaints, SCD-Q9 > 3; (2) normal overall cognition, QCSS-E ≥ 75 [5]. The MCI group was formulated with reference to Petersen’s diagnostic criteria [20]: (1) presence of subjective cognitive complaints, SCD-Q9 > 3; (2) mildly impaired overall cognition, QCSS-E: 65~75. Healthy control group (HC): (1) no subjective cognitive complaints, SCD-Q9 ≤ 3; (2) normal overall cognition, QCSS-E > 80. According to the criteria, there were 78 cases in the HC group, 107 cases in the SCD group, and 80 cases in the MCI group.
2.4. Statistical Method
All statistical analyses were performed using SPSS26.0 statistical software (IBM Corporation., Armonk, NY, USA), R Studio (version 4.3.2), and GraphPad Prism version 9.3.5. Quantitative information conforming to normal distribution was described as mean ± standard deviation ( ± s). Skewed-distribution quantitative information was expressed as median (interquartile spacing) [M(OR)]. Counting variables were expressed in terms of frequency and percentage of ingredients. Comparisons of measurement and count data among the three groups were performed using the Wilcoxon rank sum test and the Chi-square test, respectively. Spearman correlation coefficient was used to analyze the correlation between DRRLS scores and CNT scores in SCD and MCI groups. The correlation results have undergone multiple comparison adjustments. The effects of multi-domain lifestyle on objective cognitive function were analyzed via multiple linear regression. The level of significance was set at p < 0.05.
3. Results
3.1. Comparison of Demographic Data and Clinical Features among the Three Groups
A total of 265 effective samples who met the inclusion criteria were finally obtained, including 126 males and 139 females, with an average age of 63.55 (±6.68) years. Among them, there were 78 healthy controls (HC group), 107 participants with SCD, and 80 participants with MCI. There were no significant differences in marital status, occupation classification, drinking, hypertension, or diabetes among the three groups, while there were significant differences in gender, age, level of education, living situation, smoking, BMI, QCSS-E, and SCD-Q9, as shown in Table 1.

Table 1.
Comparison of demographic characteristics and clinical data between the three groups (n = 265).
3.2. Comparison of the Results of the DRRLS and CNT Test Scores among the Three Groups
As shown in Table 2, there were no statistically significant differences in the stress management (p = 0.057) and spiritual growth (p = 0.866) scores of the DRRLS among the three groups. However, the total score of DRRLS and other dimensions were higher in the HC group than in the SCD group and higher in the SCD group than in the MCI group. Moreover, the MCI group exhibited the highest score in stress management and the lowest score in mental activity among the eight dimensions of DRRLS. There were statistically significant differences in the core neuropsychological scores among the three groups (p < 0.001); the HC group had the highest VFT and AVLT scores.

Table 2.
Comparison of DRRLS and CNT test results in three groups.
3.3. Correlation Results Correlation Analyses between DRRLS Score and Neurocognitive Test Score in SCD and MCI Groups
The Spearman correlation analysis of DRRLS scores and neurocognitive scores in SCD and MCI groups showed that the total score of DRRLS was negatively correlated with TMT (r = −0.259, −0.418, p < 0.05) and positively correlated with VFT (r = 0.235, 0.248, p < 0.05) and AVLT (r = 0.295, 0.398, p < 0.01). Among the DRRLS subfields, brain-strengthening exercise, brain-healthy diet, and interpersonal relationship were negatively correlated with TMT (r = −0.315–(−0.389), p < 0.01), while health responsibility, mental activity, smoking control behavior, interpersonal relationship, and stress management were positively correlated with AVLT (r = 0.209–0.377, p < 0.05), as shown in Table 3 and Figure 1. The correlation results have been adjusted with a correction for multiple comparisons.

Table 3.
Correlation analysis of DRRLS total and dimensional scores with core neurocognitive test in patients in the SCD and MCI groups (r—value).
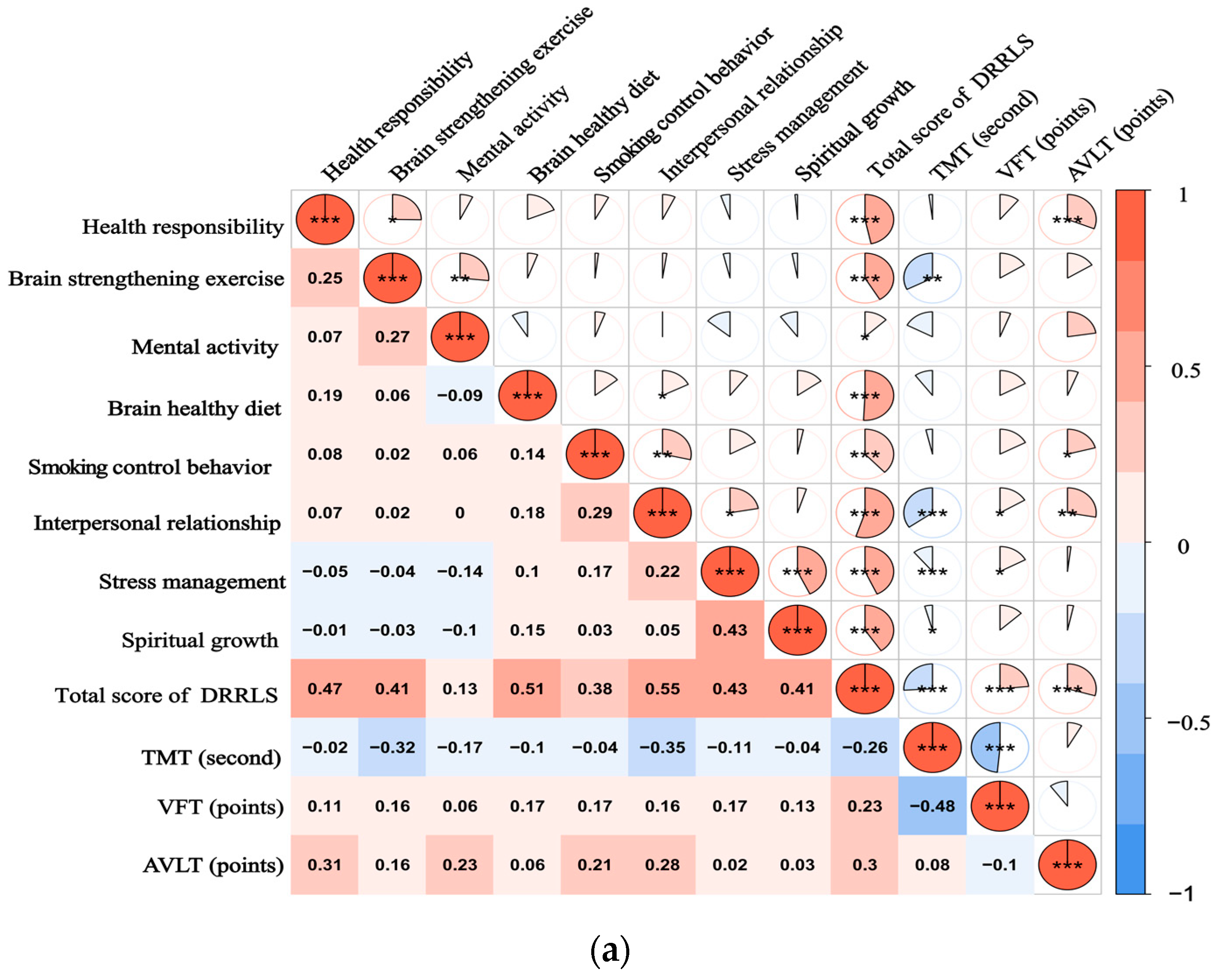
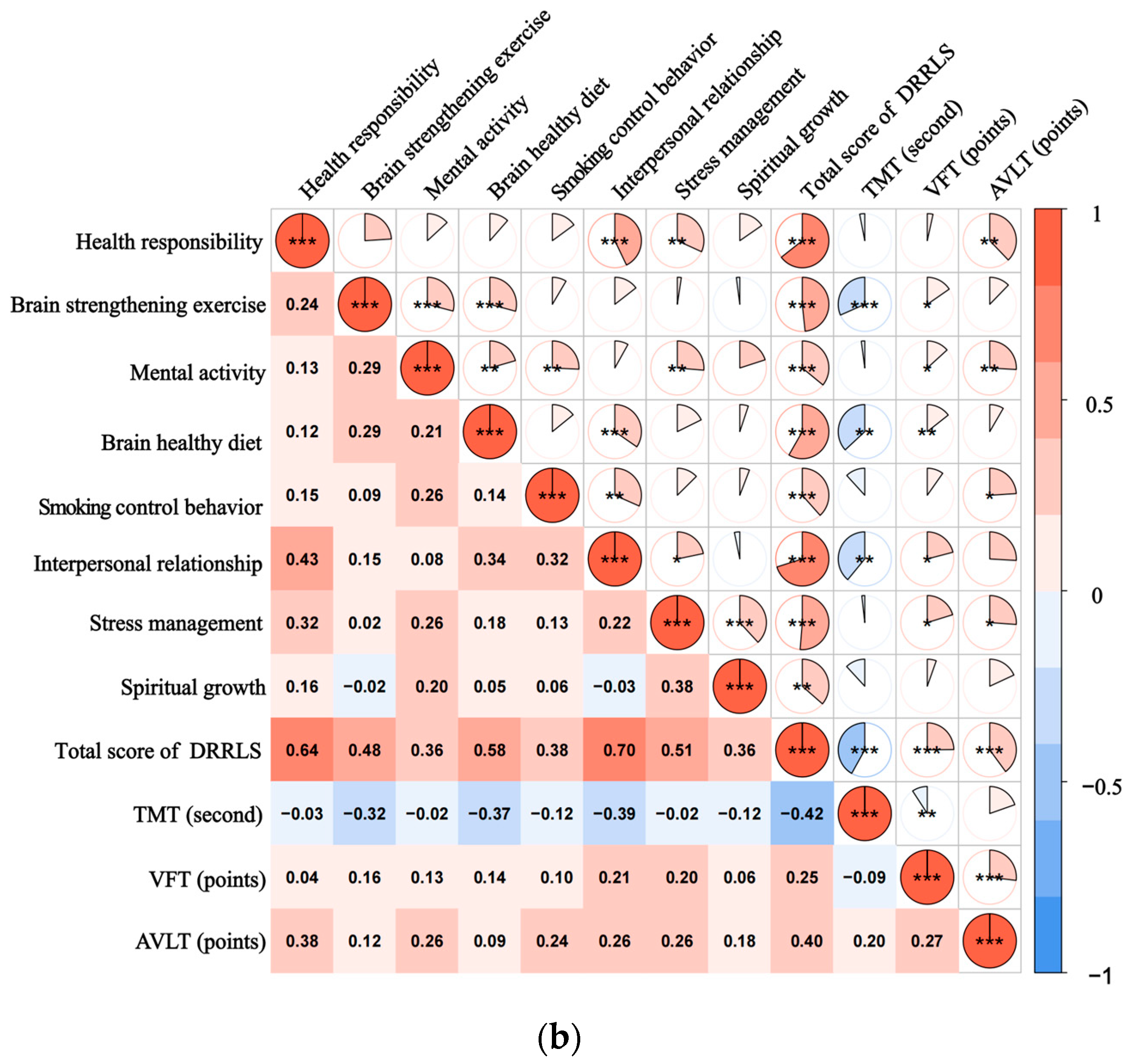
Figure 1.
(a) illustrates the spearman correlation heatmap between the DRRLS and CNT scores among individuals with SCD; (b) presents a similar correlation heatmap for participants diagnosed with MCI. The color intensity within the heatmap reflects the strength and direction of the correlation coefficients, with a color gradient ranging from blue, indicating negative correlations, to red, indicating positive correlations. * p-Value ˂ 0.05, ** p-Value ˂ 0.01, and *** p-Value ˂ 0.001. A scale bar is provided at the bottom of the heatmap to represent the range of correlation values from −1 to 1.
3.4. Multiple Linear Regression Analysis of Factors Influencing Patients with SCD and MCI
TMT, VFT, and AVLT scores were taken as dependent variables (assigned as measured values), and DRRLS factor scores and total scores with statistical significance in correlation analysis were taken as independent variables (assigned as measured values). After adjusting for confounding factors, such as age and years of education, multiple linear regression analysis was conducted. The results are shown in Table 4. In the SCD group, brain-strengthening exercise and interpersonal relationship were independently correlated with TMT (β = −11.257, −15.077, p < 0.05), and health responsibility, tobacco control behavior, and interpersonal relationship were independently correlated with AVLT (β = 0.485, 0.344, 0.406, p < 0.05). MCI Group: brain-strengthening exercise, brain-healthy diet, and interpersonal relationship were independently correlated with TMT (β = −22.011, −16.206, −11.696, p < 0.01), and health responsibility, mental activity, smoking control behavior, interpersonal relationship, and stress management were independently correlated with AVLT (β = 0.450, 0.435, 0.308, 0.256, 0.607, p < 0.05). The total DRRLS score was independently correlated with TMT, AVLT, and VFT in SCD and MCI patients (β = −1.253, 0.035, 0.070; −1.050, 0.058, 0.033, p < 0.01).

Table 4.
Results of Multiple Linear Regression Analysis of Factors Influencing Cognitive Function in Patients with SCD and MCI.
4. Discussion
AD has a covert onset, and the cognitive decline in the elderly is often regarded as a normal part of aging. By the time most patients seek medical attention, they are already in the middle to late stages of the disease, where irreversible brain damage has occurred, and the optimal window for treatment has been missed. SCD and MCI represent different stages in the pre-AD phase. Longitudinal observational studies have found that SCD occurs on average about 10 years before a dementia diagnosis, while MCI may progress to dementia within 5 to 7 years [21]. During these two stages, implementing diverse and effective interventions targeting modifiable risk factors can delay or slow down the onset of dementia.
The latest epidemiological survey data indicate that the prevalence rates of SCD and MCI in the elderly are 25.0~50.0% and 5.0~36.7%, respectively, representing a significant proportion of the population in the early stages of AD [22,23,24]. This study’s sample was derived from a community-dwelling elderly population, with the proportions of SCD and MCI being 40.4% and 30.2%, respectively, which are at a medium level. Although SCD and MCI share similar neuropathological patterns with AD, there are differences in the specific domains and severity of cognitive impairment [5]. Therefore, exploring the levels of lifestyle modifications that reduce the risk of dementia in SCD and MCI, as well as their association with cognitive deficits, can assist clinical practitioners in delivering personalized and precise health management strategies for different target populations.
This study demonstrates that the total score of DRRLS, as well as the scores of brain-strengthening exercise, brain-healthy diet, mental activity, health responsibility, interpersonal relationship, and smoking control behavior, were all significantly higher in the normal control group compared to both the SCD and MCI groups. It indicates that the health level of lifestyle and behavior habits of patients with pre-AD has been reduced in multiple dimensions, and primary healthcare personnel can carry out multi-field intervention in brain health from sports, nutrition, cognition, and other aspects service. The research findings both domestically and internationally also underscore the significance of cognitive engagement and cerebral stimulation in community health management, deeming it a primary preventive measure against dementia within the targeted population [25,26].
Although SCD is defined as the absence of objective cognitive impairment, most studies have concluded that there is mild cognitive impairment in SCD, and due to the compensatory effect of cognitive reserve, abnormalities are difficult to be detected by conventional cognitive tests at the individual level, and need to be captured by sensitive and complex neuropsychological tests above the group level [27].
In this study, the score of the core neuropsychological test of SCD was between the normal control and MCI patients, and the difference was statistically significant, indicating that executive function, language function, and delayed memory were changed in SCD stage. Compared with executive function and delayed memory, the impairment of language function was relatively slow with the progression of the disease. These results indicate that the changes in different cognitive domains in the early stage of AD are non-synchronous, which suggests that to accurately reduce the risk of dementia and delay the occurrence of dementia, health management measures with appropriate content and intensity should be formulated for different cognitive domains at different stages. The findings of the prevention of cognitive decline team in high-income countries suggest that undifferentiated prevention of cognitive decline is not sufficient, and that the cognitive benefits in the intervention mode mainly depend on the correct identification of the target population, the appropriate form and intensity of the intervention [28,29]. A healthy lifestyle and behavioral habits could lower the risk of dementia, and evidence-based interventions have become an important strategy for the primary prevention of AD [30]. This study showed that brain-strengthening exercise was independently associated with TMT in patients with SCD and MCI, indicating that insufficient physical activity has a negative impact on executive function. Several studies have shown that the prefrontal cortex is closely related to executive function, and moderate aerobic exercise can keep the hippocampus and prefrontal cortex regions of older adults flexible and responsive [31,32].
There is widespread lack of physical activity among the elderly in China, which may be related to traditional misconceptions. Indeed, most adverse events related to physical activities in the elderly stem from inappropriate exercise [33]. The China Geriatric Nursing Alliance advocates for brain-strengthening exercises as the primary lifestyle intervention for addressing the prevalence of AD. Furthermore, it is recommended that elderly individuals with SCD and MCI receive a tailored exercise prescription, including moderate aerobic exercise and comprehensive sports activities based on their individual physical condition [34,35]. The reserve hypothesis posits that maintaining positive interpersonal relationships in older adults serves as a beneficial stress stimulus, which can safeguard or enhance neuron growth [36]. Our findings demonstrate an independent association between interpersonal relationship and executive function, as well as delayed memory, in both individuals with SCD and MCI patients. Hirabayashi et al. reported that social isolation is linked to brain atrophy and cognitive decline, while exposing older adults to socially stimulating groups can potentially prevent or even reverse the decline in brain volume and improve cognitive function [37,38]. These collective findings underscore the significance of interventions aimed at ameliorating social isolation for dementia prevention and treatment [39]. It is recommended that communities actively engage in group interventions tailored for older adults, such as chess and card activities or virtual social interaction training utilizing internet technology.
Balanced dietary nutrition may affect the occurrence and development of AD through the “gut–brain” axis and epigenetic pathways. Studies have found that the microflora with anti-inflammatory activity in the gut of patients with MCI and AD decreased, inducing neuroinflammatory changes and affecting brain function [40,41]. We found that a brain-healthy diet was an influential factor in executive function in patients with MCI. Horie et al. reported that dietary intervention can significantly improve the executive function of patients with MCI [42]. In community services, healthcare workers should promote the importance of healthy eating habits for brain health and actively recommend the Mediterranean diet pattern [43]. Health responsibility and smoking control behavior were independently correlated with delayed memory in patients with SCD and MCI [44]. Health responsibility includes self-care awareness, ability to obtain and apply health information and services, etc. The stronger the sense of health responsibility, the better the individual’s ability to prevent dementia. Serper et al. reported that the health responsibility of the elderly is closely related to attention, computing ability, and memory, which is consistent with the results of this study [45]. Although the role of smoking in AD is still a controversial topic, long-term smoking can damage the endothelium of blood vessels, lead to arteriosclerosis, and trigger chronic cerebral ischemia. In addition, the high levels of carbon monoxide in cigarette smoke cause oxygen deprivation to the brain, both of which can lead to memory loss. The present study demonstrated a positive association between mental activity, stress management, and AVLT performance in patients with SCD and MCI. Additionally, these factors were independently associated with delayed memory in patients with MCI. Some studies have reported that cognitive training and decompression are beneficial to the overall cognitive function of the elderly in different life cycles, among which delayed memory and memory retention rate are the most significant improvements. The mechanism may be related to the activation of neuroplasticity in the dorsolateral prefrontal cortex and bilateral parietal cortex by cognitive training and the increase in gray matter volume in the hippocampus and cingulate gyrus by self-relaxation and decompression [46,47,48].
Due to the cross-sectional nature of this comparative study, certain limitations should be noted. Firstly, causal inference cannot be definitively drawn from the study results. Secondly, potential confounding factors, such as AD risk genes or other somatic diseases, may impact our findings. Additionally, our sample, although reasonably sized, is derived from a specific geographical area and may not be fully representative of the broader population, potentially affecting the accuracy of our data. Future research should aim to address these limitations by employing longitudinal study designs to better understand the causal relationships between lifestyle factors and cognitive health. Additionally, studies with larger and more diverse samples, incorporating both objective measures of lifestyle behaviors and a broader range of cognitive assessments, would enhance the validity and generalizability of the findings.
5. Conclusions
The unhealthy lifestyle of elderly individuals with SCD and MCI in Chinese communities is closely associated with objective cognitive impairment, and various types of lifestyles exert distinct effects on the domain and extent of cognitive function. We recommend that personalized and targeted interventions be developed based on assessment results when implementing lifestyle modifications for the prevention and treatment of AD.
Given the heterogeneity of lifestyle factors and their distinct influences on cognitive domains, we advocate for the development of personalized interventions that are informed by comprehensive assessments. These interventions should be multimodal, encompassing physical exercise interventions, nutritional therapy, psychosocial interventions, raising health awareness, etc. Emphasis is placed on the customization of healthcare based on individual differences in genetics, environment, and lifestyle. In terms of future research, longitudinal studies are warranted to assess the long-term impact of such interventions on the cognitive trajectories of individuals with SCD and MCI [49]. In the future, we can rely on digital means, such as digital detection and prevention methods, and various digital technologies were independently used by people aged 50 years or older for health promotion and disease prevention [50]. Healthcare professionals can use internet platforms to enhance the information support for cognitive decline, provide accurate and effective disease knowledge, and pay attention to the cognitive follow-up for individuals with multiple risk factors [51].
Author Contributions
Conceptualization, Y.S. and Y.W.; methodology, Y.S.; software, Y.S.; validation, J.Y.; formal analysis, Y.S. and Y.W.; investigation, Y.S.; resources, R.Z., Z.M. and Y.Z.; data curation, Y.S. and J.Y.; writing—original draft preparation, Y.S.; writing—review and editing, Y.W.; visualization, Y.W.; supervision, Y.W.; project administration, Y.W. and R.Z.; funding acquisition, Y.W. and R.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by Scientific and technological achievements of Wuxi Municipal Health Commission and appropriate technology extension projects (T202023); Wuxi Taihu Talent Project (No. WXTTP2020008 and WXTTP2021); Wuxi Nursing Association Surface Project (M202107).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of Wuxi Mental Health Center (N0: WXMH-CIRB2021LLky001, dates of approval: 19 April 2021).
Informed Consent Statement
Informed consent was obtained from all participants involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Jia, L.; Du, Y.; Chu, L.; Zhang, Z.; Li, F.; Lyu, D.; Li, Y.; Li, Y.; Zhu, M.; Jiao, H.; et al. Prevalence, risk factors, and management of dementia and mild cognitive impairment in adults aged 60 years or older in China: A cross-sectional study. Lancet Public Health 2020, 5, e661–e671. [Google Scholar] [CrossRef] [PubMed]
- Lv, B.; Liang, L.; Chen, A.; Yang, H.; Zhang, X.; Guo, F.; Qian, H. Mortality of Alzheimer’s Disease and Other Dementias in China: Past and Future Decades. Int. J. Public Health 2023, 68, 1605129. [Google Scholar] [CrossRef] [PubMed]
- Zetterberg, H.; Schott, J.M. Objectifying Subjective Cognitive Decline: The Prognostic Role of Alzheimer Biomarkers. Neurology 2022, 99, 735–736. [Google Scholar] [CrossRef] [PubMed]
- Song, W.X.; Wu, W.W.; Zhao, Y.Y.; Xu, H.L.; Chen, G.C.; Jin, S.Y.; Chen, J.; Xian, S.X.; Liang, J.H. Evidence from a meta-analysis and systematic review reveals the global prevalence of mild cognitive impairment. Front. Aging Neurosci. 2023, 15, 1227112. [Google Scholar] [CrossRef]
- Jessen, F.; Amariglio, R.E.; van Boxtel, M.; Breteler, M.; Ceccaldi, M.; Chételat, G.; Dubois, B.; Dufouil, C.; Ellis, K.A.; van der Flier, W.M.; et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimer’s Dement. 2014, 10, 844–852. [Google Scholar] [CrossRef]
- WU, Y.; XU, W.; Cheng, Z.; Wu, B.; Gu, J.; Zhou, X. Quick cognitive screening scale for the elder: Development, reliability and validity. Chin. J. Behav. Med. Brain Sci. 2013, 22, 1129–1132. [Google Scholar] [CrossRef]
- Livingston, G.; Huntley, J.; Liu, K.Y.; Costafreda, S.G.; Selbæk, G.; Alladi, S.; Ames, D.; Banerjee, S.; Burns, A.; Brayne, C.; et al. Dementia prevention, intervention, and care: 2024 report of the Lancet standing Commission. Lancet 2024, 404, 572–628. [Google Scholar] [CrossRef]
- Montero-Odasso, M.; Ismail, Z.; Livingston, G. One third of dementia cases can be prevented within the next 25 years by tackling risk factors. The case “for” and “against”. Alzheimers Res. Ther. 2020, 12, 81. [Google Scholar] [CrossRef]
- Grande, G.; Qiu, C.; Fratiglioni, L. Prevention of dementia in an ageing world: Evidence and biological rationale. Ageing Res. Rev. 2020, 64, 101045. [Google Scholar] [CrossRef] [PubMed]
- Borgeest, G.S.; Henson, R.N.; Shafto, M.; Samu, D.; Kievit, R.A. Greater lifestyle engagement is associated with better age-adjusted cognitive abilities. PLoS ONE 2020, 15, e0230077. [Google Scholar] [CrossRef]
- Zhang, J.; Eggink, E.; Zhang, X.; Li, X.; Jiang, B.; Liu, H.; Ge, S.; Zhang, W.; Lyu, J.; Niu, Y.; et al. Needs and views on healthy lifestyles for the prevention of dementia and the potential role for mobile health (mHealth) interventions in China: A qualitative study. BMJ Open 2022, 12, e061111. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Jiang, H.; Han, Y.; Huang, R.; Gao, X.; Feng, W.; Guo, Z. Lifestyle Influence on Mild Cognitive Impairment Progression: A Decision Tree Prediction Model Study. Neuropsychiatr. Dis. Treat. 2024, 20, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Jia, J.; Xing, Y.; Han, Y. An application study-subjective cognitive decline Questionnaire9 in detecting mild cognitive impairment (MCI). Aging Ment. Health 2022, 26, 2014–2021. [Google Scholar] [CrossRef]
- Wu, Y.; Mao, Z.; Cui, F.; Fan, J.; Yuan, Z.; Tang, L. Neurocognitive Characteristics of Subjective Cognitive Decline and Its Association with Objective Cognition, Negative Emotion, and Sleep Quality in Chinese Elderly. Neuropsychiatr. Dis. Treat. 2023, 19, 2261–2270. [Google Scholar] [CrossRef]
- Zhang, J.; Li, H.; Liu, X.; Wang, L.; Peng, Y.; Yang, Y. Development, Reliability and Validity of the Dementia Risk Reduction Lifestyle Scale. Chin. Gen. Pract. 2022, 25, 1595–1602. [Google Scholar] [CrossRef]
- Zhao, Q.; Guo, Q.; Li, F.; Zhou, Y.; Wang, B.; Hong, Z. The Shape Trail Test: Application of a new variant of the Trail making test. PLoS ONE 2013, 8, e57333. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Zhao, Q.; Teramukai, S.; Ding, D.; Guo, Q.; Fukushima, M.; Hong, Z. Executive function predicts survival in Alzheimer disease: A study in Shanghai. J. Alzheimer’s Dis. 2010, 22, 673–682. [Google Scholar] [CrossRef]
- Zhao, Q.; Lv, Y.; Zhou, Y.; Hong, Z.; Guo, Q. Short-term delayed recall of auditory verbal learning test is equivalent to long-term delayed recall for identifying amnestic mild cognitive impairment. PLoS ONE 2012, 7, e51157. [Google Scholar] [CrossRef]
- Huang, L.; Li, Q.; Lu, Y.; Pan, F.; Cui, L.; Wang, Y.; Miao, Y.; Chen, T.; Li, Y.; Wu, J.; et al. Consensus on rapid screening for prodromal Alzheimer’s disease in China. Gen. Psychiatry 2024, 37, e101310. [Google Scholar] [CrossRef]
- Petersen, R.C.; Caracciolo, B.; Brayne, C.; Gauthier, S.; Jelic, V.; Fratiglioni, L. Mild cognitive impairment: A concept in evolution. J. Intern. Med. 2014, 275, 214–228. [Google Scholar] [CrossRef]
- Michel, B.; Sambuchi, N.; Geda, Y.; Muraccioli, I.; Petersen, R. From Subjective Cognitive Impairment (PRE-MCI) to Alzheimer’s Disease: A Two Year Follow UP Study of 143 Subjects (P2.229). Neurology 2016, 86, P2.229. [Google Scholar] [CrossRef]
- Jonker, C.; Geerlings, M.I.; Schmand, B. Are memory complaints predictive for dementia? A review of clinical and population-based studies. Int. J. Geriatr. Psychiatry 2000, 15, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Parnetti, L.; Chipi, E.; Salvadori, N.; D’Andrea, K.; Eusebi, P. Prevalence and risk of progression of preclinical Alzheimer’s disease stages: A systematic review and meta-analysis. Alzheimers Res. Ther. 2019, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Albert, M.S.; DeKosky, S.T.; Dickson, D.; Dubois, B.; Feldman, H.H.; Fox, N.C.; Gamst, A.; Holtzman, D.M.; Jagust, W.J.; Petersen, R.C.; et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011, 7, 270–279. [Google Scholar] [CrossRef]
- Ito, E.; Nouchi, R.; Dinet, J.; Cheng, C.H.; Husebø, B.S. The Effect of Music-Based Intervention on General Cognitive and Executive Functions, and Episodic Memory in People with Mild Cognitive Impairment and Dementia: A Systematic Review and Meta-Analysis of Recent Randomized Controlled Trials. Healthcare 2022, 10, 1462. [Google Scholar] [CrossRef] [PubMed]
- James, C.E.; Altenmüller, E.; Kliegel, M.; Krüger, T.H.C.; Van De Ville, D.; Worschech, F.; Abdili, L.; Scholz, D.S.; Jünemann, K.; Hering, A.; et al. Train the brain with music (TBM): Brain plasticity and cognitive benefits induced by musical training in elderly people in Germany and Switzerland, a study protocol for an RCT comparing musical instrumental practice to sensitization to music. BMC Geriatr. 2020, 20, 418. [Google Scholar] [CrossRef]
- Hao, L.X.; Sun, Y.; Li, Y.; Wang, J.Y.; Wang, Z.Z.; Zhang, Z.Y.; Wei, Z.Y.; Gao, G.; Jia, J.G.; Xing, Y.; et al. Demographic characteristics and neuropsychological assessments of subjective cognitive decline (SCD) (plus). Ann. Clin. Transl. Neur. 2020, 7, 1002–1012. [Google Scholar] [CrossRef]
- Rao, R.V.; Subramaniam, K.G.; Gregory, J.; Bredesen, A.L.; Coward, C.; Okada, S.; Kelly, L.; Bredesen, D.E. Rationale for a Multi-Factorial Approach for the Reversal of Cognitive Decline in Alzheimer’s Disease and MCI: A Review. Int. J. Mol. Sci. 2023, 24, 1659. [Google Scholar] [CrossRef]
- Isaacson, R.S.; Hristov, H.; Saif, N.; Hackett, K.; Hendrix, S.; Melendez, J.; Safdieh, J.; Fink, M.; Thambisetty, M.; Sadek, G.; et al. Individualized clinical management of patients at risk for Alzheimer’s dementia. Alzheimer’s Dement. 2019, 15, 1588–1602. [Google Scholar] [CrossRef]
- WHO Guidelines Approved by the Guidelines Review Committee. Risk Reduction of Cognitive Decline and Dementia: WHO Guidelines; World Health Organization: Geneva, Switzerland, 2019.
- Steventon, J.J.; Foster, C.; Furby, H.; Helme, D.; Wise, R.G.; Murphy, K. Hippocampal Blood Flow Is Increased after 20 min of Moderate-Intensity Exercise. Cereb. Cortex 2020, 30, 525–533. [Google Scholar] [CrossRef]
- Heath, M.; Weiler, J.; Gregory, M.A.; Gill, D.P.; Petrella, R.J. A Six-Month Cognitive-Motor and Aerobic Exercise Program Improves Executive Function in Persons with an Objective Cognitive Impairment: A Pilot Investigation Using the Antisaccade Task. J. Alzheimer’s Dis. 2016, 54, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Tomioka, K.; Kurumatani, N.; Hosoi, H. Positive and negative influences of social participation on physical and mental health among community-dwelling elderly aged 65–70 years: A cross-sectional study in Japan. BMC Geriatr. 2017, 17, 111. [Google Scholar] [CrossRef] [PubMed]
- López-Ortiz, S.; Lista, S.; Valenzuela, P.L.; Pinto-Fraga, J.; Carmona, R.; Caraci, F.; Caruso, G.; Toschi, N.; Emanuele, E.; Gabelle, A.; et al. Effects of physical activity and exercise interventions on Alzheimer’s disease: An umbrella review of existing meta-analyses. J. Neurol. 2023, 270, 711–725. [Google Scholar] [CrossRef] [PubMed]
- Müllers, P.; Taubert, M.; Müller, N.G. Physical Exercise as Personalized Medicine for Dementia Prevention? Front. Physiol. 2019, 10, 672. [Google Scholar] [CrossRef] [PubMed]
- Ihle, A.; Oris, M.; Baeriswyl, M.; Zuber, S.; Cullati, S.; Maurer, J.; Kliegel, M. The longitudinal relation between social reserve and smaller subsequent decline in executive functioning in old age is mediated via cognitive reserve. Int. Psychogeriatr. 2021, 33, 461–467. [Google Scholar] [CrossRef]
- Walter, A.E.; Sandsmark, D.K. The Importance of Social Contact on Brain Atrophy among Older Individuals. Neurology 2023, 101, 459–460. [Google Scholar] [CrossRef]
- Hirabayashi, N.; Honda, T.; Hata, J.; Furuta, Y.; Shibata, M.; Ohara, T.; Tatewaki, Y.; Taki, Y.; Nakaji, S.; Maeda, T.; et al. Association between Frequency of Social Contact and Brain Atrophy in Community-Dwelling Older People without Dementia: The JPSC-AD Study. Neurology 2023, 101, e1108–e1117. [Google Scholar] [CrossRef]
- Dodge, H.H.; Hooker, K.; Antonucci, T.C. I-Conect Project: Can Social Interaction Improve Cognitive Functions among Socially Isolated Older Adults? Innov. Aging 2019, 3, S224. [Google Scholar] [CrossRef]
- Kesika, P.; Suganthy, N.; Sivamaruthi, B.S.; Chaiyasut, C. Role of gut-brain axis, gut microbial composition, and probiotic intervention in Alzheimer’s disease. Life Sci. 2021, 264, 118627. [Google Scholar] [CrossRef]
- Wu, S.; Liu, X.; Jiang, R.; Yan, X.; Ling, Z. Roles and Mechanisms of Gut Microbiota in Patients with Alzheimer’s Disease. Front. Aging Neurosci. 2021, 13, 650047. [Google Scholar] [CrossRef]
- Erickson, K.I.; Leckie, R.L.; Weinstein, A.M. Physical activity, fitness, and gray matter volume. Neurobiol. Aging 2014, 35 (Suppl. S2), S20–S28. [Google Scholar] [CrossRef] [PubMed]
- Shannon, O.M.; Stephan, B.C.M.; Granic, A.; Lentjes, M.; Hayat, S.; Mulligan, A.; Brayne, C.; Khaw, K.T.; Bundy, R.; Aldred, S.; et al. Mediterranean diet adherence and cognitive function in older UK adults: The European Prospective Investigation into Cancer and Nutrition-Norfolk (EPIC-Norfolk) Study. Am. J. Clin. Nutr. 2019, 110, 938–948. [Google Scholar] [CrossRef]
- Rajczyk, J.I.; Ferketich, A.; Wing, J.J. Relation between Smoking Status and Subjective Cognitive Decline in Middle Age and Older Adults: A Cross-Sectional Analysis of 2019 Behavioral Risk Factor Surveillance System Data. J. Alzheimer’s Dis. 2023, 91, 215–223. [Google Scholar] [CrossRef]
- Serper, M.; Patzer, R.E.; Curtis, L.M.; Smith, S.G.; O’Conor, R.; Baker, D.W.; Wolf, M.S. Health Literacy, Cognitive Ability, and Functional Health Status among Older Adults. Health Serv. Res. 2014, 49, 1249–1267. [Google Scholar] [CrossRef] [PubMed]
- Behfar, Q.; Richter, N.; Kural, M.; Clemens, A.; Behfar, S.K.; Folkerts, A.K.; Fassbender, R.; Kalbe, E.; Fink, G.R.; Onur, O.A. Improved connectivity and cognition due to cognitive stimulation in Alzheimer’s disease. Front. Aging Neurosci. 2023, 15, 1140975. [Google Scholar] [CrossRef] [PubMed]
- Donix, M.; Ercoli, L.M.; Siddarth, P.; Brown, J.A.; Martin-Harris, L.; Burggren, A.C.; Miller, K.J.; Small, G.W.; Bookheimer, S.Y. Influence of Alzheimer disease family history and genetic risk on cognitive performance in healthy middle-aged and older people. Am. J. Geriatr. Psychiatry 2012, 20, 565–573. [Google Scholar] [CrossRef]
- Peter, J.; Schumacher, L.V.; Landerer, V.; Abdulkadir, A.; Kaller, C.P.; Lahr, J.; Klöppel, S. Biological Factors Contributing to the Response to Cognitive Training in Mild Cognitive Impairment. J. Alzheimer’s Dis. 2018, 61, 333–345. [Google Scholar] [CrossRef]
- Pérez-Blanco, L.; Felpete, A.; Mallo, S.C.; Campos-Magdaleno, M.; Nieto-Vieites, A.; Lojo-Seoane, C.; Facal, D.; Pereiro, A.X. Longitudinal study on subjective cognitive complains from patients and informants: Differences between stable and worsening SCD and MCI participants and prediction of cognitive decline. Alzheimer’s Dement. 2021, 17, e052335. [Google Scholar] [CrossRef]
- De Santis, K.K.; Mergenthal, L.; Christianson, L.; Busskamp, A.; Vonstein, C.; Zeeb, H. Digital Technologies for Health Promotion and Disease Prevention in Older People: Scoping Review. J. Med. Internet Res. 2023, 25, e43542. [Google Scholar] [CrossRef]
- Giansanti, D. Ten Years of TeleHealth and Digital Healthcare: Where Are We? Healthcare 2023, 11, 875. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).