Abstract
Background: Identifying mediators between obesity-related traits and lower respiratory tract infections (LRTIs) would inform preventive and therapeutic strategies to reduce the burden of LRITs. We aimed to recognize whether lung function and inflammatory factors mediate their associations. Methods: We conducted a two-step, two-sample Mendelian randomization (MR) analysis. Two-sample MR was performed on (1) obesity-related traits (i.e., body mass index [BMI], waist circumference [WC], and waist-to-hip ratio [WHR]) and LRTIs (i.e., acute bronchitis, acute bronchiolitis, bronchiectasis, influenza, and pneumonia), (2) obesity-related traits and potential mediators, and (3) potential mediators and LRTIs. Next, two-step MR was applied to infer whether the mediation effects exist. Results: We found that C-reactive protein (CRP), interleukin-6 (IL-6), and forced expiratory volume in the first second (FEV1) mediated 32.59% (95% CI: 17.90%, 47.27%), 7.96% (95% CI: 1.79%, 14.14%), and 4.04% (95% CI: 0.34%, 7.74%) of the effect of BMI on pneumonia, and they mediated 26.90% (95% CI: 13.98%, 39.83%), 10.23% (95% CI: 2.72%, 17.73%), and 4.67% (95% CI: 0.25%, 9.09%) of the effect of WC on pneumonia, respectively. Additionally, CRP, forced vital capacity (FVC), and FEV1 mediated 18.66% (95% CI: 8.70%, 28.62%), 8.72% (95% CI: 1.86%, 15.58%), and 8.41% (95% CI: 2.77%, 14.06%) of the effect of BMI on acute bronchitis, and they mediated 19.96% (95% CI: 7.44%, 32.48%), 12.19% (95% CI: 2.00%, 22.39%), and 12.61% (95% CI: 2.94%, 22.29%) of the effect of WC on acute bronchitis, respectively. Conclusions: Health interventions linked to reducing inflammation and maintaining normal lung function could help mitigate the risk of obesity-related LRTIs.
1. Introduction
Lower respiratory tract infections (LRTIs) are a significant public health threat [1]. In 2019, there were 488.9 million cases of LRTIs, and LRTIs resulted in 2.4 million deaths globally [2], being the fourth cause of death [3]. The risk of LRTIs is affected by environmental factors as well as individual characteristics, such as age and health status (e.g., obesity) [4,5,6]. Over the past five years, the global incidence of obesity has continued to rise, becoming a public health issue of widespread concern [7]. According to the World Obesity Atlas, the number of obese individuals (body mass index [BMI] ≥ 30 kg/m2) aged over five years reached 988 million in 2020, accounting for 14% of the global population. It is estimated that, by 2035, this number will rise to 1.2 billion, representing 17% of the global population [8].
Previous studies have suggested associations between specific obesity-related traits and LRTIs. A systematic review indicated that obesity (BMI ≥ 30 kg/m2) was associated with an increased risk and severity of influenza and COVID-19 [9]. A prospective cohort study in the United States suggested that excessive weight gain was associated with an increased risk of community-acquired pneumonia [10]. A population-based prospective cohort study showed that both overweight (BMI > the 85th percentile) and obesity (BMI > the 95th percentile) were associated with an increased risk of bronchitis in adolescents [11]. Previous Mendelian randomization (MR) studies have revealed the effect of obesity (measured by BMI and waist circumference [WC]) on the risk of respiratory diseases [12,13,14]. Waist-to-hip ratio (WHR) is an important indicator of obesity, reflecting fat distribution [15]; however, prior studies merely reported the association of WHR with COVID-19 [16,17]. The associations with other types of LRTIs warrant further investigations. Clarifying the mediating pathways between obesity and LRTIs would inform preventive and therapeutic strategies to reduce the burden of LRTIs. Some lung function indicators and inflammatory factors have been linked to specific obesity-related traits and certain LRTIs, such as COVID-19 [18,19]. It is hypothesized that some lung function traits and inflammatory indicators play mediating roles between obesity and LRTIs. Further efforts are needed to identity the mediators of the associations between different obesity-related traits and various types of LRTIs and to what extent the mediators influence the associations.
The MR design utilizes genetic variations, typically single-nucleotide polymorphisms (SNPs), as instrumental variables to deduce the causal effect of exposure on the outcome. It effectively mitigates confounding bias and prevents reverse causality, provided that the core assumptions are satisfied [20]. Previous studies have applied the MR approach to examine the influential factors of infectious disease [21,22]. Herein, we conducted a two-step, two-sample MR to determine the roles of lung function indicators and inflammatory factors in the association between obesity and LRTIs. In this study, BMI, WC, and WHR were employed as the obesity-related traits [23]. We considered five common types of LRTIs, acute bronchitis, acute bronchiolitis, bronchiectasis, influenza, and pneumonia, as outcomes. Additionally, two lung function indicators and 13 inflammatory factors were considered as potential mediators.
2. Methods
2.1. Data Source
Summary statistics of the genome-wide association studies (GWAS) on BMI and WHR were obtained from a GWAS meta-analysis of 694,649 individuals of European ancestry [24]. Genetic associations with WC were extracted from a GWAS study of 462,166 UK Biobank participants of European ancestry. To avoid sample overlap for GWAS between genetically predicted obesity-related traits and genetically predicted potential mediators, we included an additional set of GWAS summary statistics for BMI, WC, and WHR from Genetic Investigation of Anthropometric Traits (GIANT), which did not include UK Biobank participants (Figure 1 and Table 1) [25,26].
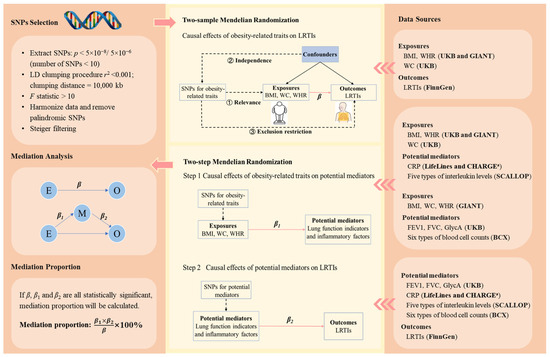
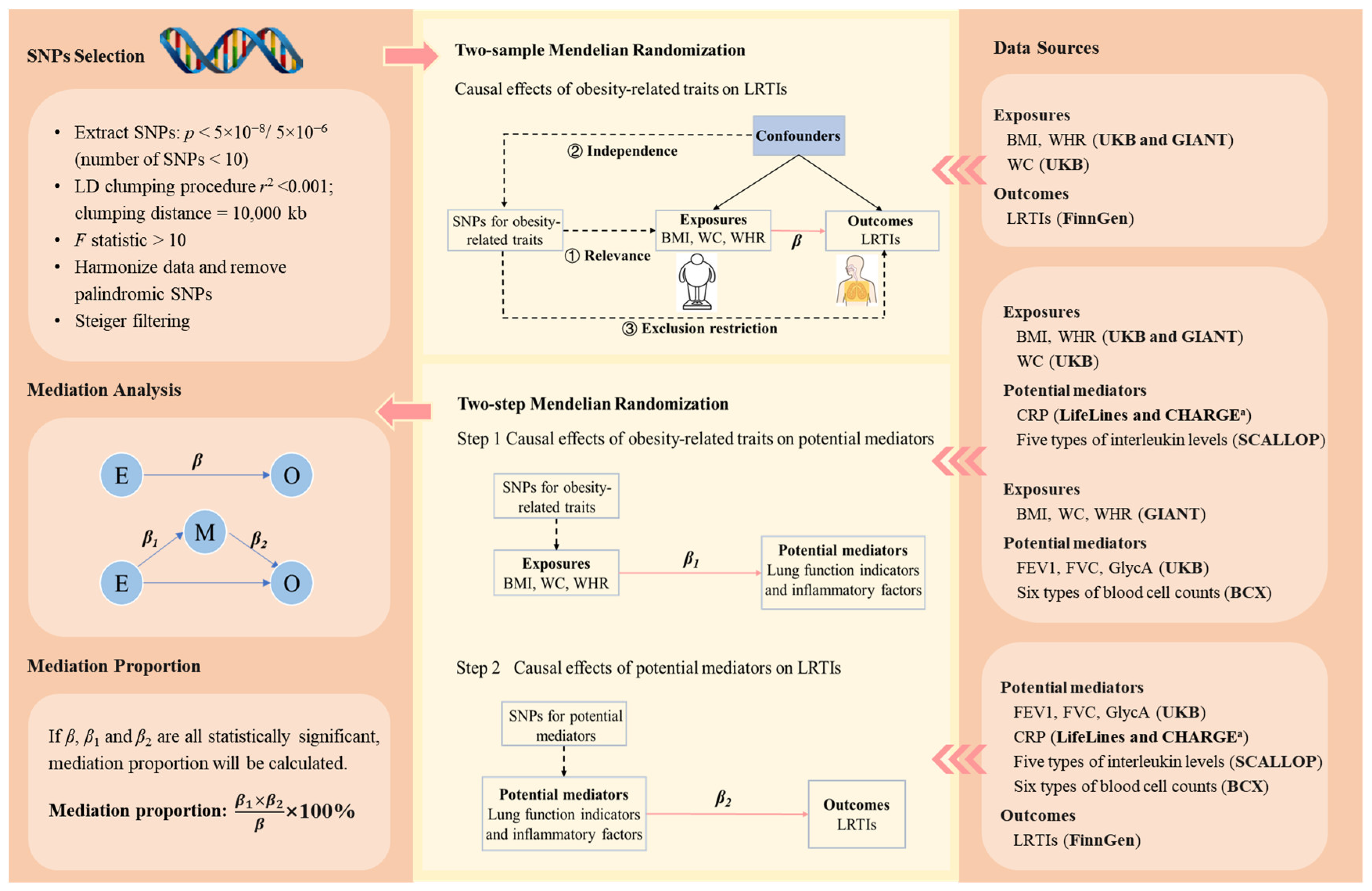
Figure 1.
The schematic diagram of the study design. The three core assumptions of Mendelian randomization are (1) relevance assumption: the genetic variants are strongly associated with the exposure of interest; (2) independence assumption: there are no (unmeasured) confounders (e.g., population structure and assortative mating) between the genetic variants and outcomes of interest; and (3) exclusion restriction criteria: there is no pathway between the genetic variants and the outcome other than via the exposure of interest. β is the change in the log-odds of a type of LRTI associated with a standard deviation (SD) increase in an obesity-related trait. represents the change in a potential mediator (in standard deviation unit, except for CRP, whose unit was one-unit natural-log-transformed CRP) associated with per SD increase in an obesity-related trait. represents the change in the log-odds of a type of LRTI associated with per SD increase in a potential mediator (except for CRP, whose unit was one-unit natural-log-transformed CRP). The bold texts in the brackets in the right panel indicate the data sources. a Genetic associations with CRP were obtained from a meta-analysis of genome-wide association studies provided by LifeLines Cohort Study and CHARGE Inflammation Working Group consortia. The five types of interleukin levels were interleukin-1-receptor antagonist, interleukin-6, interleukin-8, interleukin-18, and interleukin-27 levels. The six blood cell counts were white blood cell count, basophil count, eosinophil count, neutrophil count, monocyte count, and lymphocyte count. Abbreviations: SNPs, single-nucleotide polymorphisms; LRTIs, lower respiratory tract infection; BMI, body mass index; WC, waist circumference; WHR, waist-to-hip ratio; FEV1, forced expiratory volume in the first second; FVC, forced vital capacity; CRP, C-reactive protein; GlycA, glycoprotein acetyls levels; UKB, UK Biobank; GIANT, Genetic Investigation of ANthropometric Traits; BCX, Blood Cell Consortium; SCALLOP, Systematic and Combined Analysis of Olink Proteins; CHARGE, Cohorts for Heart and Aging Research in Genomic Epidemiology.

Table 1.
Details of data sources.
Genetic associations with forced expiratory volume in the first second (FEV1) and forced vital capacity (FVC) were extracted from a GWAS study of 321,047 individuals of European ancestry [27]. For interleukin-1-receptor antagonist (IL-1Ra), interleukin-6 (IL-6), interleukin-8 (IL-8), interleukin-18 (IL-18), and interleukin-27 (IL-27), we used the data from a GWAS meta-analysis of 21,758 European ancestry participants [28]. Genetic associations with C-reactive protein (CRP) were obtained from a GWAS meta-analysis including more than 200,000 participants of European ancestry [29]. For glycoprotein acetyls (GlycA), we extracted the data from a GWAS study conducted among 115,082 UK Biobank participants of European ancestry [30]. For white blood cell count, basophil count, eosinophil count, neutrophil count, monocyte count, and lymphocyte count, we obtained the data from a GWAS meta-analysis of 563,085 European ancestry participants (Figure 1 and Table 1) [31].
We extracted genetic associations for LRTIs from the latest publicly available GWAS summary statistics database provided by FinnGen (Figure 1, Table 1, and Supplementary Table S1) [32]. All GWAS summary statistics used in this study were from European populations to ensure consistency in the genetic background. Ethical approval and participant informed consent were not required, since published summary statistics of the GWAS were used in this study. This study followed the Strengthening the Reporting of Genetic Association Studies (STREGA) reporting guideline.
2.2. Genetic Instrument Selection
We selected genetic instrumental variables for obesity-related traits, lung function indicators, and inflammatory factors based on the following criteria (Figure 1) [20]. First, we extracted SNPs robustly associated with the exposures (p < 5 × 10−8; if the number of SNPs was less than 10, then we further released the threshold to p < 5 × 10−6) [33]. Second, the clumping process was conducted to select independent SNPs so as to reduce bias due to linkage disequilibrium (r2 < 0.001, clumping distance = 10,000 kb). Third, we excluded SNPs with an F < 10 to avoid weak instrumental variable bias. The calculation of F is detailed in Supplementary Method S1. Fourth, we harmonized exposure and outcome data, removing palindromic SNPs with a minor allele frequency greater than 0.42 as recommended by the “TwoSampleMR” R package [34]. Finally, we employed Steiger filtering to exclude SNPs that do not explain more variance in the exposure than the outcome [35]. The selected SNPs are listed in Supplementary Tables S2 and S3.
2.3. MR and Mediation Analysis
We conducted two-sample MR on (1) obesity-related traits and LRTIs, (2) obesity-related traits and potential mediators, and (3) potential mediators and LRTIs (Figure 1). We employed the inverse-variance weighted (IVW) in the main analysis and used weighted median, weighted mode, MR-Egger, and MR-Pleiotropy Residual Sum and Outlier (MR-PRESSO) in the sensitivity analyses (Supplementary Method S2). Cochran’s Q statistic was used to assess the heterogeneity of SNPs. If heterogeneity existed, the IVW random-effects model was employed. Conversely, a fixed-effects model was used. The MR-Egger intercept test was used to detect the presence of horizontal pleiotropy. The MR-PRESSO method was applied to identify possible outliers and correct the horizontal pleiotropy.
We applied the two-step MR to infer whether the considered lung function indicators and inflammatory factors mediate the associations between obesity-related traits and LRTIs (Figure 1). A factor was considered as a mediator of the association between an obesity-related trait and a type of LRTI if (1) the association between the obesity-related trait and the LRTI was statistically significant; (2) the association between the obesity-related trait and the potential mediator was statistically significant; (3) the association between the potential mediator and the LRTI was statistically significant; and (4) the 95% confidence interval (CI) of the proportion mediated did not contain zero. The proportion mediated was estimated as . is the estimated change in a potential mediator (in standard deviation unit, except for CRP, whose unit was one-unit natural-log-transformed CRP) associated with per standard deviation (SD) increase in an obesity-related trait; represents the estimated change in the log-odds of a type of LRTI associated with per SD increase in a potential mediator (except for CRP, whose unit was one-unit natural-log-transformed CRP); means the estimated change in the log-odds of a type of LRTI associated with per SD increase in an obesity-related trait. The 95% CI of the mediation proportion was estimated using the delta method [36].
All analyses were conducted with R software version 4.3.1 (R Foundation for Statistical Computing). Packages for MR analyses included “TwoSampleMR” and “MRPRESSO” [37,38]. Two-sided p < 0.05 was considered statistically significant.
3. Results
With respect to the associations between obesity-related traits and LRTIs, elevated BMI and WC increased the risk of acute bronchitis and pneumonia. Likewise, enhanced BMI, WC, and WHR (odds ratio [OR] per SD = 1.14 [95% CI: 1.02, 1.27]) caused an increase in the risk of influenza. Nevertheless, BMI reduced the risk of bronchiectasis (Supplementary Tables S4 and S5).
Concerning the associations of obesity-related traits with lung function indicators, FEV1 and FVC decreased with BMI, WC, and WHR. Regarding the associations with inflammatory factors, CRP and IL-6 increased with BMI, WC, and WHR. GlycA went up with WC. At the same time, GlycA, IL-1Ra, and IL-18 augmented with WHR. By contrast, monocyte count declined with BMI (Supplementary Figure S1 and Tables S6 and S7).
With regard to the associations of lung function and inflammatory factors with LRTIs, increased FEV1 (OR per SD = 0.80 [95% CI: 0.73, 0.88]) and FVC (OR per SD = 0.87 [95% CI: 0.79, 0.95]) lowered the risk of acute bronchitis, while elevated CRP augmented the risk. Enhanced FEV1 reduced the risk of acute bronchiolitis. Concerning pneumonia, enhanced FEV1 (OR per SD = 0.93 [95% CI: 0.88, 0.98]) and FVC (OR per SD = 0.94 [95% CI: 0.88, 1.00]) lowered the infection risk, whereas elevated CRP, IL-6, and monocyte count magnified the risk of pneumonia (Supplementary Figure S2 and Tables S8 and S9).
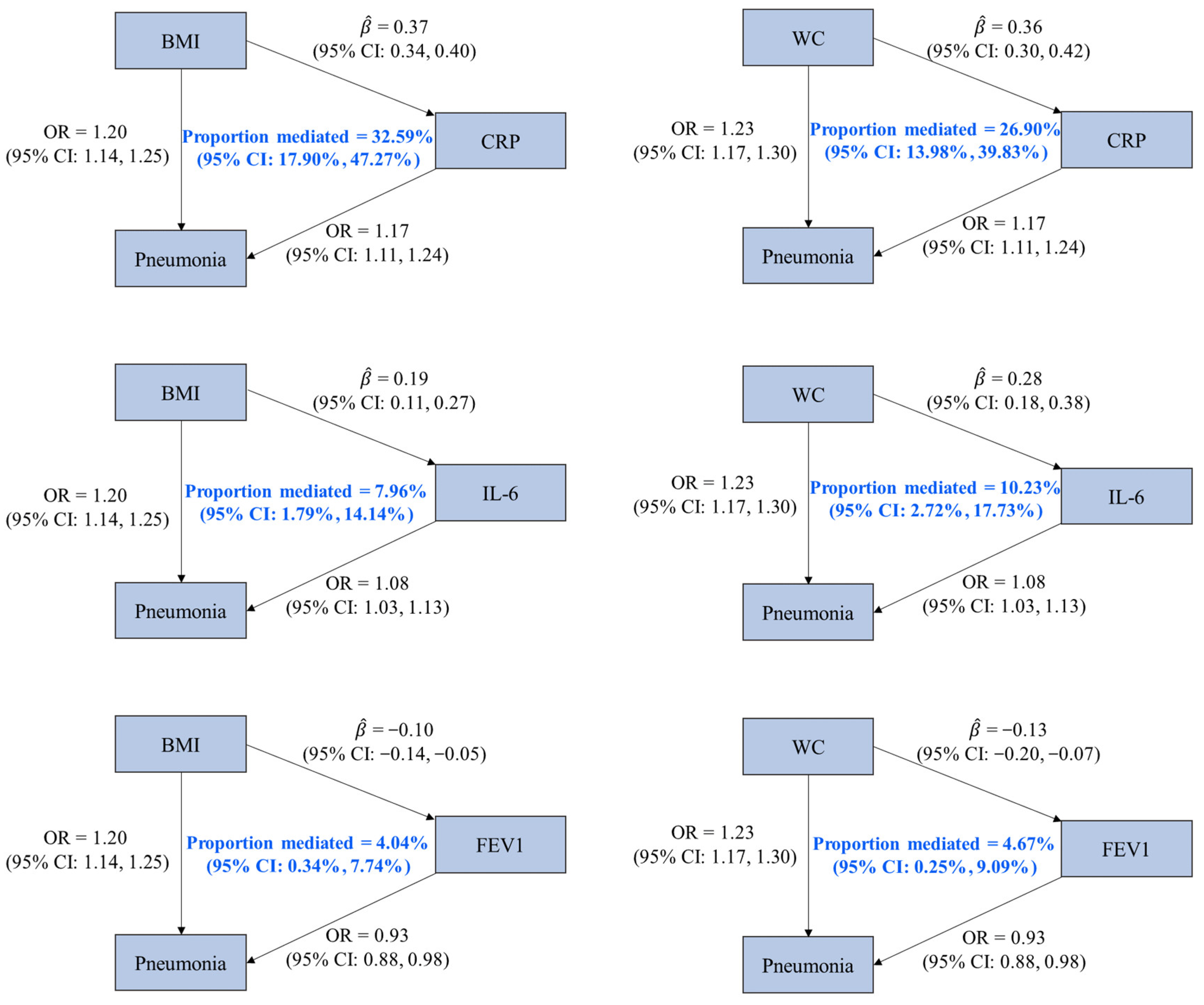
We identified three mediators (CRP, IL-6, and FEV1) of the associations of pneumonia with BMI and WC, given (1) the statistically significant effects of BMI and WC on pneumonia; (2) the associations of BMI and WC with the three factors; (3) the statistically significant effects of the three factors on pneumonia; and (4) the 95% CIs of mediation proportion that did not contain zero. It was estimated that CRP mediated 32.59% (95% CI: 17.90%, 47.27%) of the effect of BMI on pneumonia, which was higher than the proportion mediated by IL-6 (7.96% [95% CI: 1.79%, 14.14%]) and FEV1 (4.04% [95% CI: 0.34%, 7.74%]). Regarding the mediating pathways between WC and pneumonia, CRP, IL-6, and FEV1 mediated 26.90% (95% CI: 13.98%, 39.83%), 10.23% (95% CI: 2.72%, 17.73%), and 4.67% (95% CI: 0.25%, 9.09%) of the effect of WC on pneumonia, respectively (Figure 2 and Supplementary Table S10).
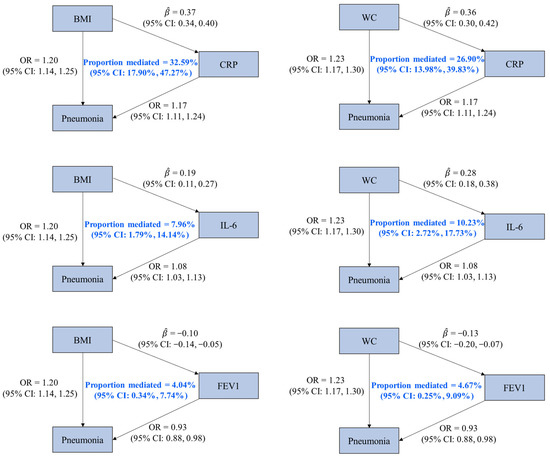
Figure 2.
Associations among mediators, obesity-related traits, and pneumonia with mediation proportions. OR is the odds ratio of pneumonia associated with per standard deviation (SD) increase in an obesity-related trait or a potential mediator (except for CRP, whose unit was one-unit natural-log-transformed CRP). represents the estimated change in a potential mediator (in one-unit natural-log-transformed CRP for CRP and in SD for others) associated with per SD increase in an obesity-related trait. Abbreviations: BMI, body mass index; WC, waist circumference; CRP, C-reactive protein; IL-6, interleukin-6; FEV1, forced expiratory volume in the first second; CI, confidence interval.
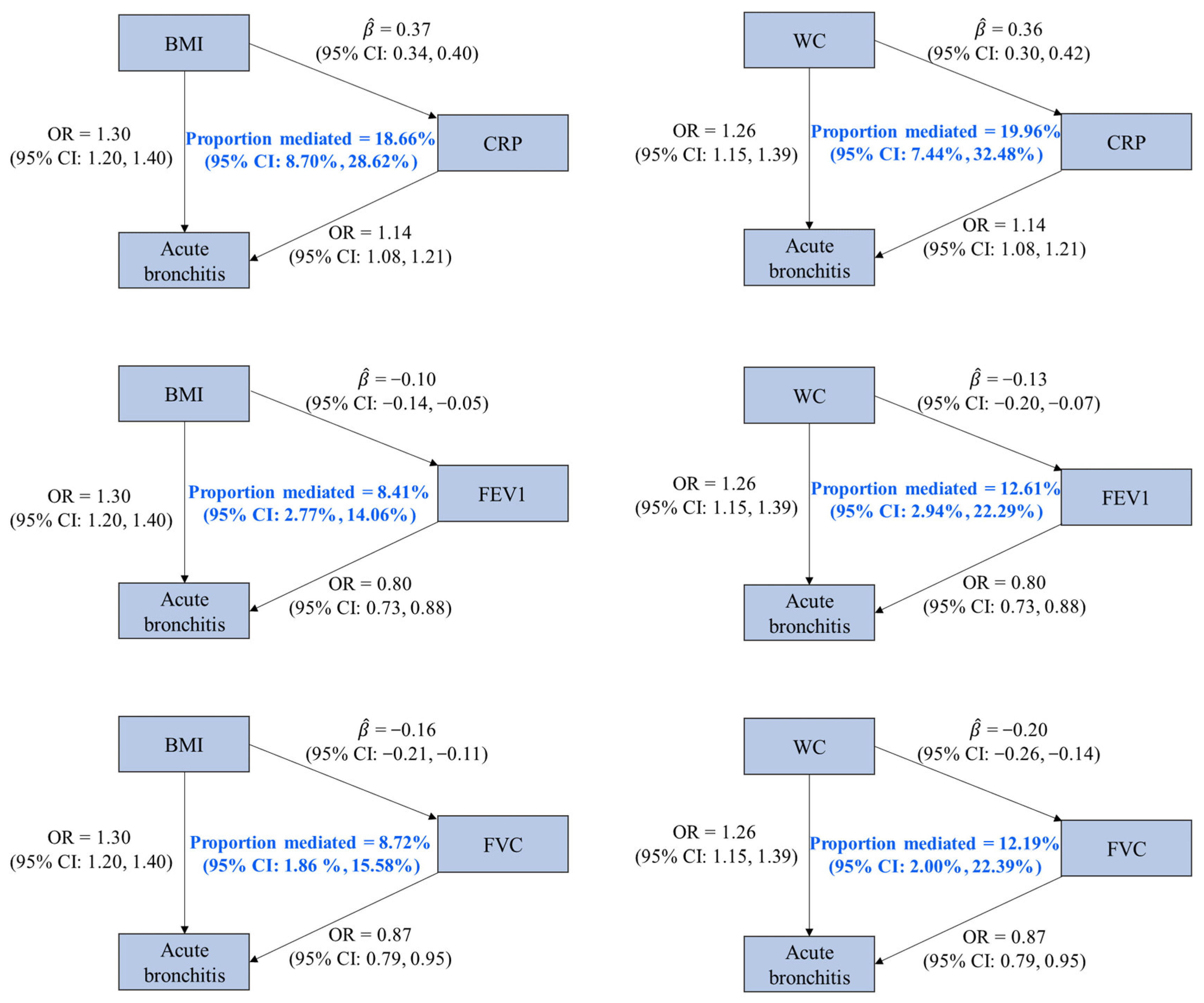
Based on the four criteria for the identification of a mediator, three factors (CRP, FVC, and FEV1) were identified as the mediators of the associations of BMI and WC on acute bronchitis. We found that 18.66% (95% CI: 8.70%, 28.62%), 8.72% (95% CI: 1.86%, 15.58%), and 8.41% (95% CI: 2.77%, 14.06%) of the effect of BMI on acute bronchitis were mediated by CRP, FVC, and FEV1, and these factors mediated 19.96% (95% CI: 7.44%, 32.48%), 12.19% (95% CI: 2.00%, 22.39%), and 12.61% (95% CI: 2.94%, 22.29%) of the effect of WC on acute bronchitis, respectively (Figure 3 and Supplementary Table S10).
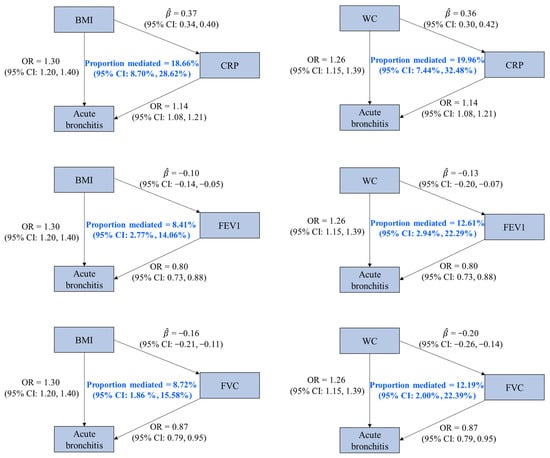
Figure 3.
Associations among mediators, obesity-related traits, and acute bronchitis with mediation proportions. OR is the odds ratio of acute bronchitis associated with per standard deviation (SD) increase in an obesity-related trait or a potential mediator (except for CRP, whose unit was one-unit natural-log-transformed CRP). represents the estimated change in a potential mediator (in one-unit natural-log-transformed CRP for CRP and in SD for others) associated with per SD increase in an obesity-related trait. Abbreviations: BMI, body mass index; WC, waist circumference; CRP, C-reactive protein; FEV1, forced expiratory volume in the first second; FVC, forced vital capacity; CI, confidence interval.
4. Discussion
In this study, a two-step, two-sample MR approach was applied to identify the mediators of the association between obesity and LRTIs from two lung function indicators and 13 inflammatory factors. We identified four factors, FEV1, FVC, CRP, and IL-6, that mediated the effects of obesity on LRTIs. We found that elevated BMI and WC increased the risk of acute bronchitis, influenza, and pneumonia, and elevated WHR increased the risk of influenza. The findings were in accordance with a previous MR study, which reported the associations of respiratory diseases with BMI and WC [12]. Regarding the associations between obesity-related traits and bronchiectasis, it was observed that increased BMI reduced the risk of bronchiectasis, while the effect of WHR was statistically non-significant. Fat-free mass depletion was considered as a risk factor for increased morbidity and mortality in bronchiectasis [39]. Weight loss and loss of muscle mass are possibly associated with decreased respiratory muscle strength and can lead to poorer lung function among bronchiectasis patients. Increasing fat-free mass, not fat, may reduce the risk of bronchiectasis [40,41]. BMI does not distinguish between fat and fat-free mass [42], whereas WHR reflects the distribution of fat in the body, which might explain the difference between BMI–bronchiectasis and WHR–bronchiectasis associations.
Our findings suggested that obesity decreased FEV1 and FVC. Consistently, a cohort study conducted among adults aged 18–30 years in the United States revealed that FEV1 decreased with weight gain, and participants with higher weight experienced a more apparent decline in FVC [43]. Another study indicated that FEV1 and FVC decreased with obesity indices (i.e., WC, waist height ratio, WHR, and body fat) in an older Chinese cohort [44]. Additionally, a study showed that the lung function indicators (i.e., FEV1 and FVC) in obese individuals (BMI ≥ 30 kg/m2) were significantly lower than those in non-obese individuals [45]. We further discovered that (1) FEV1 and FVC mediated the association of acute bronchitis with BMI and WC; and (2) FEV1 mediated the association of pneumonia with BMI and WC. A possible explanation is that, in obesity, the deposition of fat in the thoracic and abdominal cavities leads to significant changes in the mechanical characteristics of the lungs and chest wall, potentially resulting in decreased lung compliance, airway narrowing, and increased respiratory system resistance, manifested as decreased FEV1 and FVC [46,47]. These physiological changes can disrupt normal ventilation and may lead to the retention of respiratory contents, such as mucus and bacteria [48], thereby increasing the risk of acute bronchitis and pneumonia.
It was found that CRP and IL-6 increased with obesity-related traits. Consistent with our study, a prospective cohort study suggested that, for each 1 kg increase in body weight, the average increase in CRP and IL-6 was 0.08 mg/L and 0.04 pg/mL, respectively [49]. A meta-analysis comprising 51 cross-sectional studies also revealed that obesity was associated with an increased level of CRP [50]. Prior studies demonstrated that serum levels of IL-6 and CRP were significantly higher in obese subjects (BMI ≥ 30 kg/m2) than non-obese subjects [51,52]. Regarding the association of CRP and IL-6 with the risk of LRTIs, a cohort study indicated that elevated IL-6 levels in patients with chronic obstructive pulmonary disease increased the risk of pneumonia [53]; another study showed that long-term pneumonia risk was associated with inflammatory serum markers (i.e., CRP) [54]. In addition, a study showed that elevated serum IL-6 and CRP levels were closely associated with the severity of pneumonia in COVID-19 patients [55]. Interestingly, we observed that CRP mediated the association of obesity (i.e., increased BMI and WC) with acute bronchitis and pneumonia, while IL-6 mediated the effects of BMI and WC on pneumonia. Compared to IL-6, CRP exhibited a higher mediation proportion. IL-6 is a pro-inflammatory cytokine synthesized by adipose tissue, endothelial cells, macrophages, and lymphocytes. It can activate immune cells, promoting the occurrence of inflammatory responses [56]. CRP is an acute-phase protein mainly synthesized in liver cells under the induction of inflammatory cytokines, especially IL-6 [57]. In the state of obesity, there is a significant increase in the number and size of adipocytes. These active adipocytes release substantial cytokines, such as IL-6 and tumor necrosis factor-alpha, which stimulate the increase in serum CRP level and promote inflammation [58,59]. Additionally, immune cell infiltration, particularly macrophages, occurs in the obese state, releasing inflammatory mediators that enhance the inflammatory response [60]. IL-6 stimulates mucus production by pulmonary epithelial cells [61], and the excess mucus physically obstructs the airways, increasing airflow resistance [62] and thereby potentially elevating the risk of LRTIs. Persistently high levels of IL-6 and CRP can result in an exaggerated or dysregulated immune response, disrupting lung homeostasis and further increasing the risk of pulmonary infections [63,64]. Influenza, an important type of LRTI, imposes a heavy disease burden on the whole population [65]. However, this study did not identify mediators between obesity and influenza. Further endeavors are required to elucidate the mediating mechanisms between obesity and influenza.
It is expected that targeting the disruption of pathways mediated by FVC, FEV1, CRP, and IL-6 can reduce the adverse impact of obesity on acute bronchitis and pneumonia, thereby alleviating the burden of LRTIs. Lung function tests aid in early detection and can inform management against lung function decline. Physical activity would help maintain or improve lung function [66]. Caloric restriction diet intervention is an option for the targeted population since it can effectively decrease CRP and IL-6 levels [67]. Furthermore, it is suggested to advocate diets rich in whole grains, dietary fiber, vegetables, fruits, fish, marine n-3 fatty acids, polyunsaturated fatty acids, vitamin C, vitamin E, and carotenoids for the targeted population, as previous studies have indicated that these diets can alleviate systemic inflammation [68].
Our study has several limitations. First, the GWAS data were derived from the European population, restricting the generalization of the findings to individuals of diverse ethnic backgrounds. Second, there was bias due to horizontal pleiotropy when assessing the association between WHR and acute bronchitis as well as the association between WHR and pneumonia, which prevented us from further exploring the potential mediation mechanisms. Third, the numbers of acute bronchiolitis and bronchiectasis cases were relatively small, possibly due to the lack of hospitalization records for the patients with mild symptoms, potentially resulting in insufficient statistical power. Fourth, we did not investigate the nonlinear association between obesity-related traits and LRTIs, since the validity of the nonlinear MR method is currently unclear [69]. Finally, we did not conduct subgroup analyses by age, sex, and obesity status; other potential mediators (e.g., presence of pathogenic bacteria or specific antibodies, neutrophil-to-lymphocyte ratio [70,71]) and the outcome of respiratory failure [72] were not examined in this study due to the lack of corresponding GWAS data. Further efforts with individual-level data are warranted to address these issues.
5. Conclusions
CRP, IL-6, and FEV1 play crucial intermediary roles in the association of obesity with pneumonia, and CRP, FVC, and FEV1 mediate the effect of obesity on acute bronchitis. Health interventions linked to reducing inflammation and maintaining normal lung function could help mitigate the risk of obesity-related LRTIs. Further efforts are warranted to identify other important mediators.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/healthcare12181882/s1. Method S1: The calculation of the F statistic; Method S2: Two-sample Mendelian randomization inverse-variance weighted; Figure S1: Associations between obesity-related traits and potential mediators estimated using the inverse-variance weighted two-sample Mendelian randomization; Figure S2: Associations between potential mediators and LRTIs estimated using the inverse-variance weighted two-sample Mendelian randomization; Table S1: ICD-10 codes for lower respiratory tract infections in this study; Table S2: Characteristics of the genetic instrument variables for the obesity-related traits at the genome-wide significance level; Table S3: Characteristics of the genetic instrument variables for the potential mediators at the genome-wide significance level; Table S4: Effects of obesity-related traits on lower respiratory tract infections estimated using different methods; Table S5: Results of sensitivity analyses for the effects of obesity-related traits on lower respiratory tract infections; Table S6: Effects of obesity-related traits on potential mediators estimated using different methods; Table S7: Results of sensitivity analyses for the effects of obesity-related traits on potential mediators; Table S8: Effects of potential mediators on lower respiratory tract infections estimated using different methods; Table S9: Results of sensitivity analyses for the effects of potential mediators on lower respiratory tract infections; Table S10: Results of mediation analyses. References [73,74,75,76] are cited in the Supplementary Materials.
Author Contributions
Concept and design: X.M. and L.L.; Acquisition, analysis, or interpretation of data: X.M. and P.-P.Z.; Drafting of the manuscript: X.M.; Critical review of the manuscript for important intellectual content: X.M., P.-P.Z., Q.Y., Y.S., C.-Q.O. and L.L.; Statistical analysis: X.M. and P.-P.Z.; Administrative, technical, or material support: X.M. and Q.Y.; Supervision: C.-Q.O. and L.L.; L.L. had full access to all of the data in this study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Guangdong Basic and Applied Basic Research Foundation [2024A1515011957] and Science and Technology Plan Project of Guangzhou [2024A04J4864].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets analyzed in the current study are available in the IEU OpenGwas platform (https://gwas.mrcieu.ac.uk/ [accessed on 1 January 2024]), the GIANT consortium (https://portals.broadinstitute.org/collaboration/giant/index.php/GIANT_consortium_data_files [accessed on 1 January 2024]), and the FinnGen consortium (https://www.finngen.fi/en [accessed on 1 January 2024]).
Acknowledgments
We thank the OpenGWAS website, UK Biobank, FinnGen consortium, Blood Cell consortium, Systematic and Combined Analysis of Olink Proteins consortium, and GIANT consortium for providing public datasets. We also thank the participants and investigators involved in these GWAS studies.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
| MR | Mendelian randomization |
| GWAS | Genome-wide association studies |
| SNPs | Single-nucleotide polymorphisms |
| MR-PRESSO | MR-pleiotropy residual sum and outlier |
| IVW | Inverse-variance weighted |
| UKB | UK Biobank |
| GIANT | Genetic Investigation of Anthropometric Traits |
| LRTIs | Lower respiratory tract infections |
| BMI | Body mass index |
| WC | Waist circumference |
| WHR | Waist-to-hip ratio |
| FEV1 | Forced expiratory volume in the first second |
| FVC | Forced vital capacity |
| IL-1Ra | Interleukin-1-receptor antagonist |
| IL-6 | Interleukin-6 |
| IL-8 | Interleukin-8 |
| IL-18 | Interleukin-18 |
| IL-27 | Interleukin-27 |
| CRP | C-reactive protein |
| GlycA | Glycoprotein acetyls |
| CI | Confidence interval |
| SD | Standard deviation |
| OR | Odds ratio |
References
- Murdoch:, D.R.; Howie, S.R.C. The global burden of lower respiratory infections: Making progress, but we need to do better. Lancet Infect. Dis. 2018, 18, 1162–1163. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 LRI Collaborators. Age-sex differences in the global burden of lower respiratory infections and risk factors, 1990–2019: Results from the Global Burden of Disease Study 2019. Lancet Infect. Dis. 2022, 22, 1626–1647. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. The Top 10 Causes of Death. Available online: https://www.who.int/en/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 20 January 2024).
- GBD 2016 Lower Respiratory Infections Collaborators. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect. Dis. 2018, 18, 1191–1210. [Google Scholar] [CrossRef] [PubMed]
- Roth, D.E.; Caulfield, L.E.; Ezzati, M.; Black, R.E. Acute lower respiratory infections in childhood: Opportunities for reducing the global burden through nutritional interventions. Bull. World Health Organ. 2008, 86, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Yin, P.; Brauer, M.; Cohen, A.J.; Wang, H.; Li, J.; Burnett, R.T.; Stanaway, J.D.; Causey, K.; Larson, S.; Godwin, W.; et al. The effect of air pollution on deaths, disease burden, and life expectancy across China and its provinces, 1990–2017: An analysis for the Global Burden of Disease Study 2017. Lancet Planet Health 2020, 4, e386–e398. [Google Scholar] [CrossRef]
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef]
- News Medical Life Science. Alarming Projection: By 2035, More than Half of the Global Population Will Be Obese. Available online: https://www.news-medical.net/news/20230302/Alarming-Projection-By-2035-more-than-half-of-the-global-population-will-be-obese.aspx (accessed on 20 January 2024).
- Zhao, X.; Gang, X.; He, G.; Li, Z.; Lv, Y.; Han, Q.; Wang, G. Obesity increases the severity and mortality of influenza and COVID-19: A systematic review and meta-analysis. Front. Endocrinol. 2020, 11, 595109. [Google Scholar] [CrossRef]
- Baik, I.; Curhan, G.C.; Rimm, E.B.; Bendich, A.; Willett, W.C.; Fawzi, W.W. A prospective study of age and lifestyle factors in relation to community-acquired pneumonia in US men and women. Arch. Intern. Med. 2000, 160, 3082–3088. [Google Scholar] [CrossRef]
- Lee, Y.L.; Chen, Y.C.; Chen, Y.A. Obesity and the occurrence of bronchitis in adolescents. Obesity 2013, 2, E149–E153. [Google Scholar] [CrossRef]
- Yang, W.; Yang, Y.; Guo, Y.; Guo, J.; Ma, M.; Han, B. Obesity and risk for respiratory diseases: A Mendelian randomization study. Front. Endocrinol. 2023, 14, 1197730. [Google Scholar] [CrossRef]
- Butler-Laporte, G.; Harroud, A.; Forgetta, V.; Richards, J.B. Elevated body mass index is associated with an increased risk of infectious disease admissions and mortality: A mendelian randomization study. Clin. Microbiol. Infect. 2021, 27, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Sood, T.; Perrot, N.; Chong, M.; Mohammadi-Shemirani, P.; Mushtaha, M.; Leong, D.; Rangarajan, S.; Hess, S.; Yusuf, S.; Gerstein, H.C.; et al. Biomarkers associated with severe COVID-19 among populations with high cardiometabolic risk: A 2-sample Mendelian randomization study. JAMA Netw. Open 2023, 6, e2325914. [Google Scholar] [CrossRef] [PubMed]
- Medical News Today. Why Is the Hip-Waist Ratio Important? Available online: https://www.medicalnewstoday.com/articles/319439 (accessed on 20 January 2024).
- Cabrera-Mendoza, B.; Wendt, F.R.; Pathak, G.A.; De Angelis, F.; De Lillo, A.; Koller, D.; Polimanti, R. The association of obesity-related traits on COVID-19 severity and hospitalization is affected by socio-economic status: A multivariable Mendelian randomization study. Int. J. Epidemiol. 2022, 51, 1371–1383. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wen, J.; Wan, X.; Luo, P. The causal relationship between air pollution, obesity, and COVID-19 risk: A large-scale genetic correlation study. Front. Endocrinol. 2023, 14, 1221442. [Google Scholar] [CrossRef] [PubMed]
- Ji, P.; Zhu, J.; Zhong, Z.; Li, H.; Pang, J.; Li, B.; Zhang, J. Association of elevated inflammatory markers and severe COVID-19: A meta-analysis. Medicine 2020, 99, e23315. [Google Scholar] [CrossRef]
- Ponti, G.; Maccaferri, M.; Ruini, C.; Tomasi, A.; Ozben, T. Biomarkers associated with COVID-19 disease progression. Crit. Rev. Clin. Lab. Sci. 2020, 57, 389–399. [Google Scholar] [CrossRef]
- Sanderson, E.; Glymour, M.M.; Holmes, M.V.; Kang, H.; Morrison, J.; Munafò, M.R.; Palmer, T.; Schooling, C.M.; Wallace, C.; Zhao, Q.; et al. Mendelian randomization. Nat. Rev. Methods Primers 2022, 2, 6. [Google Scholar] [CrossRef]
- Ponsford, M.J.; Gkatzionis, A.; Walker, V.M.; Grant, A.J.; Wootton, R.E.; Moore, L.S.P.; Fatumo, S.; Mason, A.M.; Zuber, V.; Willer, C.; et al. Cardiometabolic traits, sepsis, and severe COVID-19: A Mendelian randomization investigation. Circulation 2020, 142, 1791–1793. [Google Scholar] [CrossRef]
- Richardson, T.G.; Fang, S.; Mitchell, R.E.; Holmes, M.V.; Smith, G.D. Evaluating the effects of cardiometabolic exposures on circulating proteins which may contribute to severe SARS-CoV-2. eBioMedicine 2021, 64, 103228. [Google Scholar] [CrossRef]
- Khan, I.; Chong, M.; Le, A.; Mohammadi-Shemirani, P.; Morton, R.; Brinza, C.; Kiflen, M.; Narula, S.; Akhabir, L.; Mao, S.; et al. Surrogate adiposity markers and mortality. JAMA Netw. Open 2023, 6, e2334836. [Google Scholar] [CrossRef]
- Pulit, S.L.; Stoneman, C.; Morris, A.P.; Wood, A.R.; Glastonbury, C.A.; Tyrrell, J.; Yengo, L.; Ferreira, T.; Marouli, E.; Ji, Y.; et al. Meta-analysis of genome-wide association studies for body fat distribution in 694 649 individuals of European ancestry. Hum. Mol. Genet. 2019, 28, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Locke, A.E.; Kahali, B.; Berndt, S.I.; Justice, A.E.; Pers, T.H.; Day, F.; Powell, C.; Vedantam, S.; Buchkovich, M.L.; Yang, J.; et al. Genetic studies of body mass index yield new insights for obesity biology. Nature 2015, 518, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Shungin, D.; Winkler, T.W.; Croteau-Chonka, D.C.; Ferreira, T.; Locke, A.E.; Mägi, R.; Strawbridge, R.J.; Pers, T.H.; Fischer, K.; Justice, A.E.; et al. New genetic loci link adipose and insulin biology to body fat distribution. Nature 2015, 518, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Shrine, N.; Guyatt, A.L.; Erzurumluoglu, A.M.; Jackson, V.E.; Hobbs, B.D.; Melbourne, C.A.; Batini, C.; Fawcett, K.A.; Song, K.; Sakornsakolpat, P.; et al. New genetic signals for lung function highlight pathways and chronic obstructive pulmonary disease associations across multiple ancestries. Nat. Genet. 2019, 51, 1067. [Google Scholar] [CrossRef]
- Folkersen, L.; Gustafsson, S.; Wang, Q.; Hansen, D.H.; Hedman, Å.K.; Schork, A.; Page, K.; Zhernakova, D.V.; Wu, Y.; Peters, J.; et al. Genomic and drug target evaluation of 90 cardiovascular proteins in 30,931 individuals. Nat. Metab. 2020, 2, 11351148. [Google Scholar] [CrossRef]
- Ligthart, S.; Vaez, A.; Võsa, U.; Stathopoulou, M.G.; de Vries, P.S.; Prins, B.P.; Van der Most, P.J.; Tanaka, T.; Naderi, E.; Rose, L.M.; et al. Genome analyses of >200,000 individuals identify 58 loci for chronic inflammation and highlight pathways that link inflammation and complex disorders. Am. J. Hum. Genet. 2018, 103, 691–706. [Google Scholar] [CrossRef]
- Richardson, T.G.; Leyden, G.M.; Wang, Q.; Bell, J.A.; Elsworth, B.; Davey Smith, G.; Holmes, M.V. Characterising metabolomic signatures of lipid-modifying therapies through drug target Mendelian randomisation. PLoS Biol. 2022, 20, e3001547. [Google Scholar] [CrossRef] [PubMed]
- Vuckovic, D.; Bao, E.L.; Akbari, P.; Lareau, C.A.; Mousas, A.; Jiang, T.; Chen, M.H.; Raffield, L.M.; Tardaguila, M.; Huffman, J.E.; et al. The polygenic and monogenic basis of blood traits and diseases. Cell 2020, 182, 1214–1231.e11. [Google Scholar] [CrossRef]
- Kurki, M.I.; Karjalainen, J.; Palta, P.; Sipilä, T.P.; Kristiansson, K.; Donner, K.M.; Reeve, M.P.; Laivuori, H.; Aavikko, M.; Kaunisto, M.A.; et al. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature 2023, 615, E19. [Google Scholar] [CrossRef]
- Choi, K.W.; Stein, M.B.; Nishimi, K.M.; Ge, T.; Coleman, J.R.I.; Chen, C.Y.; Ratanatharathorn, A.; Zheutlin, A.B.; Dunn, E.C.; 23andMe Research Team; et al. An exposure-wide and Mendelian randomization approach to identifying modifiable factors for the prevention of depression. Am. J. Psychiatry 2020, 177, 944–954. [Google Scholar] [CrossRef]
- Hemani, G. Harmonise Data. Available online: https://mrcieu.github.io/TwoSampleMR/articles/harmonise.html (accessed on 20 January 2024).
- Hemani, G.; Tilling, K.; Davey Smith, G. Orienting the causal relationship between imprecisely measured traits using GWAS summary data. PLoS Genet 2017, 13, e1007081. [Google Scholar]
- Cox, C. Delta method. In Encyclopedia of Biostatistics; 2005; pp. 1125–1127. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/0470011815.b2a15029 (accessed on 20 January 2024).
- Hemani, G.; Zheng, J.; Elsworth, B.; Wade, K.H.; Haberland, V.; Baird, D.; Laurin, C.; Burgess, S.; Bowden, J.; Langdon, R.; et al. The MR-Base platform supports systematic causal inference across the human phenome. eLife 2018, 7, e34408. [Google Scholar] [CrossRef] [PubMed]
- Verbanck, M.; Chen, C.Y.; Neale, B.; Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 2018, 50, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Olveira, G.; Olveira, C.; Gaspar, I.; Porras, N.; Martín-Núñez, G.; Rubio, E.; Colomo, N.; Rojo-Martínez, G.; Soriguer, F. Fat-free mass depletion and inflammation in patients with bronchiectasis. J. Acad. Nutr. Diet. 2012, 112, 1999–2006. [Google Scholar] [CrossRef]
- Lee, J.M.; Lee, S.A.; Han, C.H.; Lee, S.M.; Kim, C.J.; Lee, S.C.; Park, S.C. Body mass index as a predictor of mortality in bronchiectasis: A nationwide population-based study. Respir. Med. 2021, 180, 106370. [Google Scholar] [CrossRef]
- Zheng, H.Y.; Li, M.Q. Relationship between body mass index and lung function and disease severity in patients with bronchiectasis. Mod. Instrum. Med. Treat. 2018, 24, 92–94. (In Chinese) [Google Scholar]
- Merchant, R.A.; Seetharaman, S.; Au, L.; Wong, M.W.K.; Wong, B.L.L.; Tan, L.F.; Chen, M.Z.; Ng, S.E.; Soong, J.T.Y.; Hui, R.J.Y.; et al. Relationship of fat mass index and fat free mass index with body mass index and association with function, cognition and carcopenia in pre-frail older adults. Front. Endocrinol. 2021, 12, 765415. [Google Scholar] [CrossRef]
- Thyagarajan, B.; Jacobs, D.R., Jr.; Apostol, G.G.; Smith, L.J.; Jensen, R.L.; Crapo, R.O.; Barr, R.G.; Lewis, C.E.; Williams, O.D. Longitudinal association of body mass index with lung function: The CARDIA study. Respir. Res. 2008, 9, 31. [Google Scholar] [CrossRef]
- Pan, J.; Xu, L.; Lam, T.H.; Jiang, C.Q.; Zhang, W.S.; Jin, Y.L.; Zhu, F.; Zhu, T.; Thomas, G.N.; Cheng, K.K.; et al. Association of adiposity with pulmonary function in older Chinese: Guangzhou biobank cohort study. Respir. Med. 2017, 132, 102–108. [Google Scholar] [CrossRef]
- Mala, K. Comparative Study of Pulmonary Function Tests in Obese and Non Obese Male Individuals. Ph.D. Thesis, Rajiv Gandhi University of Health Sciences, Bidar, India, 2017. [Google Scholar]
- Salome, C.M.; King, G.G.; Berend, N. Physiology of obesity and effects on lung function. J. Appl. Physiol. 2010, 108, 206–211. [Google Scholar] [CrossRef]
- Dixon, A.E.; Peters, U. The effect of obesity on lung function. Expert. Rev. Respir. Med. 2018, 12, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Lempesis, I.G.; Georgakopoulou, V.E. Implications of obesity and adiposopathy on respiratory infections; focus on emerging challenges. World J. Clin. Cases 2023, 11, 2925–2933. [Google Scholar] [CrossRef] [PubMed]
- Fransson, E.I.; Batty, G.D.; Tabák, A.G.; Brunner, E.J.; Kumari, M.; Shipley, M.J.; Singh-Manoux, A.; Kivimäki, M. Association between change in body composition and change in inflammatory markers: An 11-year follow-up in the Whitehall II study. J. Clin. Endocrinol. Metab. 2010, 95, 5370–5374. [Google Scholar] [CrossRef]
- Choi, J.; Joseph, L.; Pilote, L. Obesity and C-reactive protein in various populations: A systematic review and meta-analysis. Obes. Rev. 2013, 14, 232–244. [Google Scholar] [CrossRef] [PubMed]
- Derosa, G.; Fogari, E.; D’Angelo, A.; Bianchi, L.; Bonaventura, A.; Romano, D.; Maffioli, P. Adipocytokine levels in obese and non-obese subjects: An observational study. Inflammation 2013, 36, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Khaodhiar, L.; Ling, P.R.; Blackburn, G.L.; Bistrian, B.R. Serum levels of interleukin-6 and C-reactive protein correlate with body mass index across the broad range of obesity. JPEN J. Parenter. Enteral Nutr. 2004, 28, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, M.; Dahl, M.; Lange, P.; Vestbo, J.; Nordestgaard, B.G. Inflammatory biomarkers and comorbidities in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2012, 186, 982–988. [Google Scholar] [CrossRef]
- Lee, M.M.; Zuo, Y.; Steiling, K.; Mizgerd, J.P.; Kalesan, B.; Walkey, A.J. Clinical risk factors and blood protein biomarkers of 10-year pneumonia risk. PLoS ONE 2024, 19, e0296139. [Google Scholar] [CrossRef]
- Sun, H.; Guo, P.; Zhang, L.; Wang, F. Serum Interleukin-6 Concentrations and the Severity of COVID-19 Pneumonia: A Retrospective Study at a Single Center in Bengbu City, Anhui Province, China, in January and February 2020. Med. Sci. Monit. 2020, 26, e926941. [Google Scholar] [CrossRef]
- Scheller, J.; Chalaris, A.; Schmidt-Arras, D.; Rose-John, S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta 2011, 1813, 878–888. [Google Scholar] [CrossRef]
- Sproston, N.R.; Ashworth, J.J. Role of C-Reactive protein at sites of inflammation and infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-Alaniz, M.H.; Takada, J.; Alonso-Vale, M.I.; Lima, F.B. Adipose tissue as an endocrine organ: From theory to practice. J. Pediatr. 2007, 83 (Suppl. S5), S192–S203. [Google Scholar] [CrossRef]
- Visser, M.; Bouter, L.M.; McQuillan, G.M.; Wener, M.H.; Harris, T.B. Elevated C-reactive protein levels in overweight and obese adults. JAMA 1999, 282, 2131–2135. [Google Scholar] [CrossRef]
- Johnson, A.R.; Milner, J.J.; Makowski, L. The inflammation highway: Metabolism accelerates inflammatory traffic in obesity. Immunol. Rev. 2012, 249, 218–238. [Google Scholar] [CrossRef]
- Neveu, W.A.; Allard, J.B.; Dienz, O.; Wargo, M.J.; Ciliberto, G.; Whittaker, L.A.; Rincon, M. IL-6 is required for airway mucus production induced by inhaled fungal allergens. J. Immunol. 2009, 183, 1732–1738. [Google Scholar] [CrossRef]
- Agrawal, A.; Rengarajan, S.; Adler, K.B.; Ram, A.; Ghosh, B.; Fahim, M.; Dickey, B.F. Inhibition of mucin secretion with MARCKS-related peptide improves airway obstruction in a mouse model of asthma. J. Appl. Physiol. 2007, 102, 399–405. [Google Scholar] [CrossRef]
- Dawson, R.E.; Jenkins, B.J.; Saad, M.I. IL-6 family cytokines in respiratory health and disease. Cytokine 2021, 143, 155520. [Google Scholar] [CrossRef] [PubMed]
- Kony, S.; Zureik, M.; Driss, F.; Neukirch, C.; Leynaert, B.; Neukirch, F. Association of bronchial hyperresponsiveness and lung function with C-reactive protein (CRP): A population based study. Thorax 2004, 59, 892–896. [Google Scholar] [CrossRef]
- GBD 2017 Influenza Collaborators. Mortality, morbidity, and hospitalisations due to influenza lower respiratory tract infections, 2017: An analysis for the Global Burden of Disease Study 2017. Lancet Respir. Med. 2019, 7, 69–89. [Google Scholar] [CrossRef]
- Cheng, Y.J.; Macera, C.A.; Addy, C.L.; Sy, F.S.; Wieland, D.; Blair, S.N. Effects of physical activity on exercise tests and respiratory function. Br. J. Sports Med. 2003, 37, 521–528. [Google Scholar] [CrossRef]
- Imayama, I.; Ulrich, C.M.; Alfano, C.M.; Wang, C.; Xiao, L.; Wener, M.H.; Campbell, K.L.; Duggan, C.; Foster-Schubert, K.E.; Kong, A.; et al. Effects of a caloric restriction weight loss diet and exercise on inflammatory biomarkers in overweight/obese postmenopausal women: A randomized controlled trial. Cancer Res. 2012, 72, 2314–2326. [Google Scholar] [CrossRef] [PubMed]
- Asoudeh, F.; Fallah, M.; Aminianfar, A.; Djafarian, K.; Shirzad, N.; Clark, C.C.T.; Larijani, B.; Esmaillzadeh, A. The effect of Mediterranean diet on inflammatory biomarkers and components of metabolic syndrome in adolescent girls. J. Endocrinol. Invest. 2024, 47, 257. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, F.W.; Hughes, D.A.; Spiller, W.; Tilling, K.; Smith, G.D. Non-linear Mendelian randomization: Detection of biases using negative controls with a focus on BMI, Vitamin D and LDL cholesterol. Eur. J. Epidemiol. 2024, 39, 451–465. [Google Scholar] [CrossRef] [PubMed]
- de Jager, C.P.; Wever, P.C.; Gemen, E.F.; Kusters, R.; van Gageldonk-Lafeber, A.B.; van der Poll, T.; Laheij, R.J. The neutrophil-lymphocyte count ratio in patients with community-acquired pneumonia. PLoS ONE 2012, 7, e46561. [Google Scholar] [CrossRef]
- Cataudella, E.; Giraffa, C.M.; Di Marca, S.; Pulvirenti, A.; Alaimo, S.; Pisano, M.; Terranova, V.; Corriere, T.; Ronsisvalle, M.L.; Di Quattro, R.; et al. Neutrophil-to-lymphocyte ratio: An emerging marker predicting prognosis in elderly adults with community-acquired pneumonia. J. Am. Geriatr. Soc. 2017, 65, 1796–1801. [Google Scholar] [CrossRef]
- Regolo, M.; Sorce, A.; Vaccaro, M.; Colaci, M.; Stancanelli, B.; Natoli, G.; Motta, M.; Isaia, I.; Castelletti, F.; Giangreco, F.; et al. Assessing humoral immuno-inflammatory pathways associated with respiratory failure in COVID-19 patients. J. Clin. Med. 2023, 12, 4057. [Google Scholar] [CrossRef]
- Palmer, T.M.; Lawlor, D.A.; Harbord, R.M.; Sheehan, N.A.; Tobias, J.H.; Timpson, N.J.; Davey Smith, G.; Sterne, J.A. Using multiple genetic variants as instrumental variables for modifiable risk factors. Stat. Methods Med. Res. 2012, 21, 223–242. [Google Scholar] [CrossRef]
- Bowden, J.; Davey Smith, G.; Haycock, P.C.; Burgess, S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet. Epidemiol. 2016, 40, 304–314. [Google Scholar] [CrossRef]
- Burgess, S.; Thompson, S.G. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur. J. Epidemiol. 2017, 32, 377–389. [Google Scholar] [CrossRef]
- Hartwig, F.P.; Davey Smith, G.; Bowden, J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int. J. Epidemiol. 2017, 46, 1985–1998. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).