Health-Related Quality of Life in Long-Term Colorectal Cancer Survivors
Abstract
1. Introduction
2. Materials and Methods
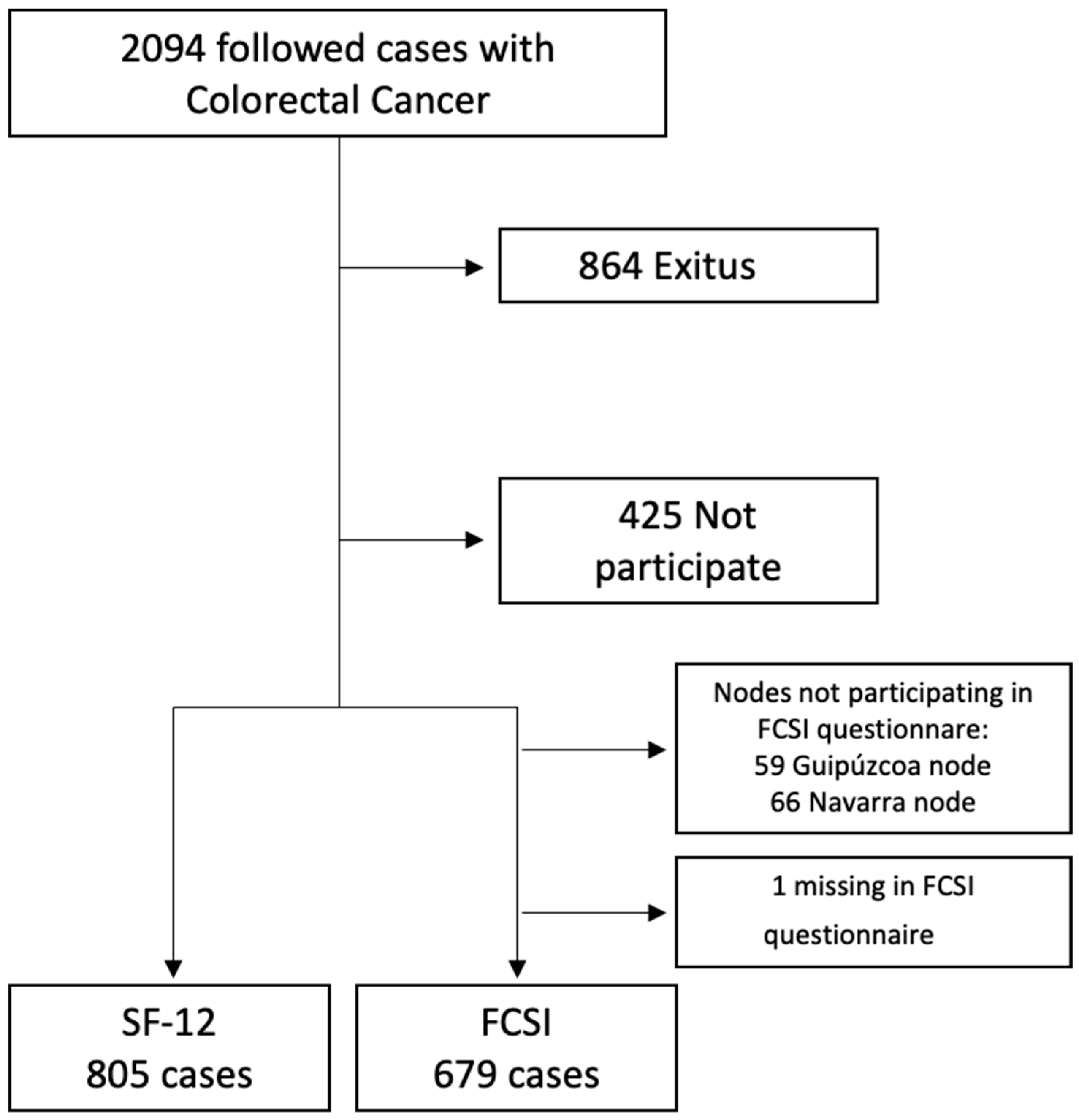
2.1. Study Design and Participants
2.2. Ethical Considerations
2.3. Health-Related Quality of Life
2.4. Study Variables
2.4.1. Independent Variables
2.4.2. Dependent Variables
2.5. Statistical Analysis
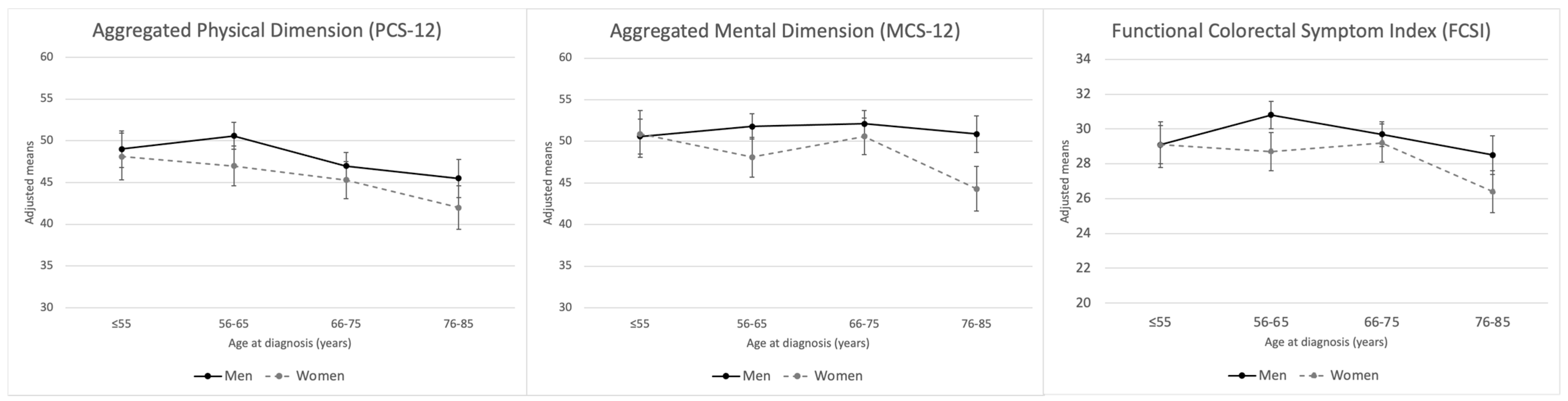
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Agency for Research on Cancer, World Health Organization. The Global Cancer Observatory. GLOBOCAN 2023. Colorectal Cancer; International Agency for Research on Cancer, World Health Organization: Lyon, France, 2023. [Google Scholar]
- Antoni, S.; Soerjomataram, I.; Møller, B.; Bray, F.; Ferlay, J. An assessment of GLOBOCAN methods for deriving national estimates of cancer incidence. Bull. World Health Organ. 2016, 94, 174–184. [Google Scholar] [CrossRef] [PubMed]
- SEOM: Sociedad Española de Oncología Médica. Las Cifras del Cáncer en España 2023. 2023. Available online: https://www.infocoponline.es/pdf/Las_cifras_del_Cancer_en_Espana_2023.pdf (accessed on 15 May 2024).
- Martín Sánchez, V.; Muinelo Voces, I.; Jorquera Plaza, F.; Molina de la Torre, A.J.; Olea, S.d.A.; Gómez, S.T.; López-Abente, G. Tendencia y distribución municipal de la incidencia de cáncer colorrectal en el área de salud de León (1994–2008). Gastroenterol. Hepatol. 2012, 35, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Centro Nacional de Epidemiología. Ariadna. 2023. Available online: https://www.isciii.es/QueHacemos/Servicios/VigilanciaSaludPublicaRENAVE/EnfermedadesCronicas/Paginas/Ariadna.aspx (accessed on 15 May 2024).
- Kim, K.; Kim, J.-S. Factors influencing health-related quality of life among Korean cancer survivors. Psychooncology 2017, 26, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Jansen, L.; Hermann, A.; Stegmaier, C.; Brenner, H.; Amdt, V. Health-related quality of life during the 10 years after diagnosis of colorectal cancer: A population-based study. J. Clin. Oncol. 2011, 29, 3263–3269. Available online: https://pubmed.ncbi.nlm.nih.gov/21768465/ (accessed on 15 May 2024). [CrossRef] [PubMed]
- Sánchez-Jiménez, A.; Cantarero-Villanueva, I.; Delgado-García, G.; Molina-Barea, R.; Fernández-Lao, C.; Galiano-Castillo, N.; Arroyo-Morales, M. Physical impairments and quality of life of colorectal cancer survivors: A case-control study. Eur. J. Cancer Care 2015, 24, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.L.; Hawkins, N.A.; Berkowitz, Z.; Li, C. Factors Associated with Health-Related Quality of Life Among Colorectal Cancer Survivors. Am. J. Prev. Med. 2015, 49, S518–S527. [Google Scholar] [CrossRef]
- Balhareth, A.; Aldossary, M.Y.; McNamara, D. Impact of physical activity and diet on colorectal cancer survivors’ quality of life: A systematic review. World J. Surg. Oncol. 2019, 17, 153. [Google Scholar] [CrossRef]
- McDougall, J.A.; Blair, C.K.; Wiggins, C.L.; Goodwin, M.B.; Chiu, V.K.; Rajput, A.; Kinney, A.Y. Socioeconomic disparities in health-related quality of life among colorectal cancer survivors. J. Cancer Surviv. 2019, 13, 459–467. [Google Scholar] [CrossRef]
- Laghousi, D.; Jafari, E.; Nikbakht, H.; Nasiri, B.; Shamshirgaran, M.; Aminisani, N. Gender differences in health-related quality of life among patients with colorectal cancer. J. Gastrointest. Oncol. 2019, 10, 453–461. [Google Scholar] [CrossRef]
- van Veen, M.R.; Mols, F.; Bours, M.J.L.; Weijenberg, M.P.; Kampman, E.; Beijer, S. Adherence to the World Cancer Research Fund/American Institute for Cancer Research recommendations for cancer prevention is associated with better health-related quality of life among long-term colorectal cancer survivors: Results of the PROFILES registry. Support. Care Cancer 2019, 27, 4565–4574. [Google Scholar] [CrossRef]
- DuMontier, C.; Clough-Gorr, K.M.; Silliman, R.A.; Stuck, A.E.; Moser, A. Health-Related Quality of Life in a Predictive Model for Mortality in Older Breast Cancer Survivors. J. Am. Geriatr. Soc. 2018, 66, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Sandberg, E.A.; Thorat, T.; Neumann, P.J.; Chambers, J. Qaly Gains in Cancer Compared with Chronic Diseases. Value Health 2016, 19, A9–A10. [Google Scholar] [CrossRef]
- Stahlschmidt, R.; Ferracini, A.C.; de Souza, C.M.; de Medeiros, L.M.; Juliato CR, T.; Mazzola, P.G. Adherence and quality of life in women with breast cancer being treated with oral hormone therapy. Support. Care Cancer 2019, 27, 3799–3804. [Google Scholar] [CrossRef] [PubMed]
- Castaño-Vinyals, G.; Aragonés, N.; Pérez-Gómez, B.; Martín, V.; Llorca, J.; Moreno, V.; Altzibar, J.M.; Ardanaz, E.; Sanjosé, S.D.; Jiménez-Moleón, J.J.; et al. Population-based multicase-control study in common tumors in Spain (MCC-Spain): Rationale and study design. Gac. Sanit. 2015, 29, 308–315. [Google Scholar] [CrossRef]
- Ware, J.; Kosinski, M.; Turner-Bowker, D.; Gandek, B. How to score SF-12 items. SF-12 v2: How to Score Version 2 of the SF-12. Health Survey 2002, 29–38. [Google Scholar]
- Vilagut, G.; María Valderas, J.; Garin, O.; López-García, E.; Alonsoab, J.; Ferrer, M. Interpretación de los cuestionarios de salud SF-36 y SF-12 en España: Componentes físico y mental. Med. Clínica 2008, 130, 726–735. [Google Scholar] [CrossRef]
- Cella, D.; Hernandez, L.; Bonomi, A.E.; Corona, M.; Vaquero, M.; Shiomoto, G.; Baez, L. Spanish language translation and initial validation of the functional assessment of cancer therapy quality-of-life instrument. Med. Care 1998, 36, 1407–1418. [Google Scholar] [CrossRef]
- Ware, J.; Kosinski Keller, S.D. A 12-Item Short-Form Health Survey: Construction of Scales and Preliminary Tests of Reliability and Validity. Medical Care 1996, 34, 220–233. [Google Scholar] [CrossRef]
- Gandek, B.; Ware, J.E.; Aaronson, N.K.; Apolone, G.; Bjorner, J.B.; Brazier, J.E.; Bullinger, M.; Kaasa, S.; Leplege, A.; Prieto, L.; et al. Cross-validation of item selection and scoring for the SF-12 Health Survey in nine countries: Results from the IQOLA Project. International Quality of Life Assessment. J. Clin. Epidemiol. 1998, 51, 1171–1178. [Google Scholar] [CrossRef]
- Colwell, H.H.; Mathias, S.D.; Turner, M.P.; Lu, J.; Wright, N.; Peeters, M.; Cella, D.; Devercelli, G. Psychometric evaluation of the FACT Colorectal Cancer Symptom Index (FCSI-9): Reliability, validity, responsiveness, and clinical meaningfulness. Oncologist 2010, 15, 308–316. [Google Scholar] [CrossRef]
- Alonso-Molero, J.; Dierssen-Sotos, T.; Gomez-Acebo, I.; Fernandez de Larrea Baz, N.; Guevara, M.; Amiano, P.; Castaño-Vinyals, G.; Fernandez-Villa, T.; Moreno, V.; Bayo, J.; et al. Quality of life in a cohort of 1078 women diagnosed with breast cancer in spain: 7-year follow-up results in the MCC-Spain study. Int. J. Environ. Res. Public Health 2020, 17, 8411. [Google Scholar] [CrossRef]
- Storey, J.D.; Tibshirani, R. Statistical significance for genomewide studies. Proc. Natl. Acad. Sci. USA 2003, 100, 9440–9445. [Google Scholar] [CrossRef] [PubMed]
- Jansen, L.; Koch, L.; Brenner, H.; Arndt, V. Quality of life among long-term (≥5 years) colorectal cancer survivors: Systematic review. Eur. J. Cancer 2010, 46, 2879–2888. [Google Scholar] [CrossRef] [PubMed]
- Sharour, L.A.; Omari, O.A.; Malak, M.Z.; Salameh, A.B.; Yehia, D.; Subih, M.; Alrshoud, M. Using Mixed-Methods Research to Study Coping Strategies among Colorectal Cancer Patients. Asia Pac. J. Oncol. Nurs. 2019, 7, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Rinaldis, M.; Pakenham, K.I.; Lynch, B.M. Relationships between quality of life and finding benefits in a diagnosis of colorectal cancer. Br. J. Psychol. 2010, 101, 259–275. [Google Scholar] [CrossRef] [PubMed]
- Gellman, M.D.; Turner, J.R. (Eds.) Encyclopedia of Behavioral Medicine [Internet]; Springer: New York, NY, USA, 2013; Available online: http://link.springer.com/10.1007/978-1-4419-1005-9 (accessed on 15 May 2024).
- Vilorio-Marqués, L.; Molina, A.J.; Tascón, C.D.; Cuenllas, B.Á.; Cañas, C.Á.; Martín, M.H.; Fernández-Villa, T.; Elosua, T.; Martín, V. Características clínicas, anatomopatológicas y moleculares en casos de cáncer colorrectal según localización tumoral y grado de diferenciación. Rev. Colomb. Cancerol. 2015, 19, 193–203. [Google Scholar] [CrossRef]
- Vissers, P.A.; Thong, M.S.; Pouwer, F.; Zanders, M.M.J.; Coebergh, J.W.W.; Van de Poll-Franse, L.V. The impact of comorbidity on Health-Related Quality of Life among cancer survivors: Analyses of data from the PROFILES registry. J. Cancer Surviv. 2013, 7, 602–613. [Google Scholar] [CrossRef]
- Adams, S.V.; Ceballos, R.; Newcomb, P.A. Quality of Life and Mortality of Long-Term Colorectal Cancer Survivors in the Seattle Colorectal Cancer Family Registry. PLoS ONE 2016, 11, e0156534. Available online: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0156534 (accessed on 15 May 2024). [CrossRef]
- Trentham-Dietz, A.; Remington, P.L.; Moinpour, C.M.; Hampton, J.M.; Sapp, A.L.; Newcomb, P.A. Health-related quality of life in female long-term colorectal cancer survivors. Oncologist 2003, 8, 342–349. [Google Scholar] [CrossRef]
- Krouse, R.S.; Herrinton, L.J.; Grant, M.; Wendel, C.S.; Green, S.B.; Mohler, M.J.; Baldwin, C.M.; McMullen, C.K.; Rawl, S.M.; Matayoshi, E.; et al. Health-related quality of life among long-term rectal cancer survivors with an ostomy: Manifestations by sex. J. Clin. Oncol. 2009, 27, 4664–4670. [Google Scholar] [CrossRef]
- Thong, M.S.Y.; Koch-Gallenkamp, L.; Jansen, L.; Bertram, H.; Eberle, A.; Holleczek, B.; Waldeyer-Sauerland, M.; Waldmann, A.; Zeissig, S.R.; Brenner, H.; et al. Age-specific health-related quality of life in long-term and very long-term colorectal cancer survivors versus population controls—A population-based study. Acta Oncol. 2019, 58, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Pate, A.; Lowery, J.; Kilbourn, K.; Blatchford, P.J.; McNulty, M.; Risendal, B. Quality of life and the negative impact of comorbidities in long-term colorectal cancer survivors: A population-based comparison. J. Cancer Surviv. 2020, 14, 653–659. [Google Scholar] [CrossRef] [PubMed]
| Quality of Life Questionnaire (n (%)) | ||||
|---|---|---|---|---|
| Men | Women | |||
| n = 486 | n = 319 | p-Value | ||
| Age at diagnosis | mean (sd) | 64.9 (9.8) | 65.4 (10.8) | <0.001 |
| Age | ≤55 * | 84 (17.3) | 68 (21.3) | 0.01 |
| 56–65 | 171 (35.2) | 84 (26.3) | ||
| 66–75 | 154 (31.7) | 96 (30.1) | ||
| 76–85 | 77 (15.8) | 71 (22.3) | ||
| Area | Asturias * | 14 (2.9) | 12 (3.8) | 0.39 |
| Barcelona | 125 (25.7) | 87 (27.3) | ||
| Cantabria | 33 (6.8) | 28 (8.8) | ||
| Granada | 15 (3.1) | 15 (4.7) | ||
| Guipúzcoa | 38 (7.8) | 21 (6.6) | ||
| Huelva | 21 (4.3) | 13 (4.1) | ||
| León | 107 (22.0) | 63 (19.8) | ||
| Madrid | 54 (11.1) | 40 (12.5) | ||
| Murcia | 5 (1.0) | 2 (0.6) | ||
| Navarra | 49 (10.1) | 17 (5.3) | ||
| Valencia | 25 (5.1) | 21 (6.6) | ||
| Education level | Primary education or less * | 310 (63.8) | 226 (70.9) | 0.11 |
| Secondary education | 120 (24.7) | 62 (19.4) | ||
| University | 56 (11.5) | 31 (9.7) | ||
| Civil status | Single * | 24 (4.9) | 26 (8.2) | <0.001 |
| Married or Living with a partner | 445 (91.6) | 211 (66.1) | ||
| Widow | 17 (3.5) | 82 (25.7) | ||
| Smoking | Non-smoker and former at diagnosis * | 123 (25.3) | 233 (73.0) | <0.001 |
| Smoker at diagnosis | 363 (74.7) | 86 (27.0) | ||
| BMI | 17.5–25 * | 125 (25.7) | 129 (40.4) | <0.001 |
| 25–29.9 | 235 (48.4) | 124 (38.9) | ||
| ≥30 | 126 (25.9) | 66 (20.7) | ||
| Family history of colon cancer | None * | 336 (69.1) | 206 (64.6) | 0.18 |
| Some degree family history of colon cancer | 150 (30.9) | 113 (35.4) | ||
| Tumour size | T0 * | 33 (6.8) | 19 (6.0) | 0.91 |
| T1 | 35 (7.2) | 26 (8.2) | ||
| T2 | 87 (17.9) | 49 (15.4) | ||
| T3 | 270 (55.6) | 181 (56.7) | ||
| T4 | 47 (9.7) | 33 (10.3) | ||
| Missing | 14 (2.9) | 11 (3.5) | ||
| Node infiltration | N0 * | 324 (66.7) | 215 (67.4) | 0.94 |
| N1 | 109 (22.4) | 67 (21.0) | ||
| N2 | 37 (7.6) | 27 (8.5) | ||
| Missing | 16 (3.3) | 10 (3.1) | ||
| Metastasis | M0 * | 451 (92.8) | 305 (95.6) | 0.11 |
| M1 | 25 (5.1) | 7 (2.2) | ||
| Missing | 10 (2.1) | 7 (2.2) | ||
| Complete clinical remission | No * | 481 (99.0) | 319 (100) | 0.07 |
| Yes | 5 (1.0) | 0 | ||
| Missing | 0 | 0 | ||
| Recurrence | No * | 441 (90.7) | 302 (94.7) | 0.04 |
| Yes | 45 (9.3) | 17 (5.3) | ||
| Missing | 0 | 0 | ||
| TNM pathological stage | 0 * | 26 (5.4) | 16 (5.0) | 0.48 |
| I | 97 (20.0) | 65 (20.4) | ||
| II | 183 (37.7) | 125 (39.2) | ||
| III | 130 (26.8) | 89 (27.9) | ||
| IV | 25 (5.1) | 7 (2.2) | ||
| Missing | 25 (5.1) | 17 (5.33) | ||
| Histological grade | Well differentiated * | 155 (31.9) | 94 (29.5) | 0.72 |
| Moderately differentiated | 237 (48.8) | 168 (52.7) | ||
| Poorly differentiated | 48 (9.9) | 27 (8.5) | ||
| Missing | 46 (9.5) | 30 (9.4) | ||
| Histological type | Adenocarcinoma * | 446 (91.8) | 291 (91.2) | 0.79 |
| Other | 24 (4.9) | 18 (5.6) | ||
| Missing | 16 (3.3) | 10 (3.1) | ||
| Chemotherapy | No * | 176 (36.2) | 149 (46.7) | 0.004 |
| Yes | 299 (61.5) | 168 (52.7) | ||
| Missing | 11 (2.3) | 2 (0.6) | ||
| Radiotherapy | No * | 322 (66.3) | 241 (75.6) | 0.02 |
| Yes | 132 (27.2) | 62 (19.4) | ||
| Missing | 32 (6.6) | 16 (5.0) | ||
| Surgery Type | Radical * | 448 (92.2) | 292 (91.5) | 0.76 |
| Palliative | 20 (4.1) | 12 (3.8) | ||
| Missing | 18 (3.7) | 15 (4.7) | ||
| SF-12 | PCS-12 (mean (sd)) | 48.4 (10.3) | 45.6 (11.0) | <0.001 |
| MCS-12 (mean (sd)) | 51.5 (9.7) | 48.6 (11.4) | <0.001 | |
| FCSI | mean (sd) | 29.8 (4.7) | 28.4 (4.9) | <0.001 |
| Physical Component Summary (PCS-12) | |||||||
|---|---|---|---|---|---|---|---|
| Total Sample | Men | Women | |||||
| (n = 805) | (n = 486) | (n = 319) | |||||
| Mean (95% CI) | p-Value (q-Values) | Mean (95% CI) | p-Value (q-Value) | Mean (95% CI) | p-Value (q-Value) | ||
| Sex | Men * | 48.1 (45.1–53.1) | <0.001 (0.007) | ||||
| Women | 45.7 (44.6–46.9) | ||||||
| Age at diagnosis | beta (95% CI) | −0.19 (−0.27–(−0.12)) | <0.001 (<0.001) | −0.18 (−0.31–(−0.06)) | 0.004 (0.003) | −0.18 (−0.31–(−0.06)) | 0.004 (0.021) |
| Age | ≤55 * | 48.9 (47.2–50.6) | <0.001 (<0.001) | 49.0 (46.8–51.2) | 0.001 (0.009) | 48.1 (45.3–50.9) | 0.01 (0.006) |
| 56–65 | 49.3 (48.0–50.6) | 50.6 (49.0–52.1) | 47.0 (44.6–49.4) | ||||
| 66–75 | 46.3 (45.0–47.5) | 47.0 (45.4–48.6) | 45.3 (43.1–47.6) | ||||
| 76–85 | 43.9 (42.2–45.6) | 45.5 (43.2–47.8) | 42.0 (39.4–44.7) | ||||
| Area | Asturias * | 49.1 (45.1–53.1) | 0.003 (0.02) | 47.5 (42.2–52.8) | 0.003 (0.02) | 50.7 (44.3–57.0) | 0.53 (0.51) |
| Barcelona | 47.7 (46.3–49.2) | 49.1 (47.2–52.8) | 45.8 (43.3–48.2) | ||||
| Cantabria | 48.5 (45.9–51.2) | 50.0 (46.5–53.5) | 46.7 (42.5–50.8) | ||||
| Granada | 43.4 (45.9–51.2) | 44.6 (39.4–49.8) | 42.7 (37.1–48.3) | ||||
| Guipúzcoa | 47.6 (44.9–50.4) | 47.7 (44.4–51.0) | 47.7 (42.8–52.6) | ||||
| Huelva | 42.6 (39.1–47.1) | 42.3 (37.9–46.6) | 43.5 (37.4–48.3) | ||||
| León | 48.9 (47.3–50.5) | 50.1 (48.2–52.1) | 46.7 (43.9–49.5) | ||||
| Madrid | 46.5 (44.3–48.6) | 47.7 (45.0–50.5) | 44.3 (40.9–47.8) | ||||
| Murcia | 38.5 (30.8–46.2) | 39.0 (30.2–47.9) | 37.6 (22.4–52.8) | ||||
| Navarra | 47.8 (45.2–50.3) | 50.3 (47.4–53.2) | 42.5 (37.2–47.8) | ||||
| Smoking | Non-smoker and former at diagnosis * | 48.0 (46.8–49.2) | 0.13 (0.29) | 49.9 (48.1–51.7) | 0.06 (0.18) | 45.8 (44.3–47.2) | 0.76 (0.58) |
| Smoker at diagnosis | 46.7 (45.7–47.7) | 47.8 (46.8–48.9) | 45.2 (42.6–47.9) | ||||
| Yes | 43.9 (41.3–46.5) | 43.9 (40.9–46.8) | 45.4 (40.0–50.7) | ||||
| Missing | - | - | - | ||||
| Surgery Type | Radical * | 47.0 (46.3–47.8) | 0.01 (0.06) | 48.1 (47.2–49.0) | 0.13 (0.29) | 45.5 (44.2–46.7) | 0.20 (0.36) |
| Palliative | 52.9 (49.1–56.7) | 52.8 (48.0–57.5) | 51.4 (44.7–58.1) | ||||
| Mental Component Summary (MCS-12) | |||||||
|---|---|---|---|---|---|---|---|
| Total Sample | Men | Women | |||||
| (n = 805) | (n = 486) | (n = 319) | |||||
| Mean (95% CI) | p-Value (q-Value) | Mean (95% CI) | p-Value (q-Value) | Mean (95% CI) | p-Value (q-Value) | ||
| Sex | Men * | 51.5 (50.5–52.4) | <0.001 | ||||
| Women | 48.8 (47.6–49.9) | (0.004) | |||||
| Age at diagnosis | beta (95% CI) | −0.04 (−0.11–0.04) | 0.33 (0.58) | 0.05 (−0.04–0.14) | 0.29 (0.30) | −0.18 | 0.007 (0.01) |
| Age | ≤55 * | 50.7 (49.1–52.4) | 0.02 (0.06) | 50.6 (48.5–52.7) | 0.63 (0.53) | 50.9 (48.1–53.7) | 0.002 (0.01) |
| 56–65 | 50.5 (49.2–51.7) | 51.8 (50.3–53.3) | 48.1 (45.7–50.5) | ||||
| 66–75 | 51.5 (50.2–52.7) | 52.1 (50.5–53.6) | 50.6 (48.4–52.9) | ||||
| 76–85 | 48.1 (46.4–49.8) | 50.9 (48.7–53.1) | 44.3 (41.6–47.0) | ||||
| Area | Asturias * | 47.8 (43.8–51.7) | <0.001 | 49.4 (44.4–54.5) | 0.01 (0.04) | 44.2 (37.8–50.7) | 0.001 (0.008) |
| Barcelona | 49.3 (47.9–50.7) | (<0.001) | 50.0 (48.3–51.7) | 48.5 (46.0–50.9) | |||
| Cantabria | 53.2 (50.6–55.8) | 52.8 (49.5–56.2) | 52.9 (48.6–57.1) | ||||
| Granada | 46.6 (42.9–50.3) | 48.4 (43.5–53.4) | 44.8 (39.1–50.5) | ||||
| Guipúzcoa | 51.3 (48.6–54.0) | 52.1 (49.0–55.2) | 50.3 (45.3–55.3) | ||||
| Huelva | 43.5 (40.0–47.0) | 45.7 (41.6–49.9) | 39.6 (33.4–45.8) | ||||
| León | 52.5 (51.0–54.1) | 53.3 (51.5–55.2) | 51.5 (48.7–54.4) | ||||
| Madrid | 48.6 (46.5–50.7) | 51.2 (48.6–53.8) | 44.6 (41.1–48.1) | ||||
| Murcia | 44.9 (37.3–52.4) | 47.7 (39.2–56.1) | 39.1 (23.6–54.5) | ||||
| Navarra | 50.9 (48.4–53.4) | 52.8 (50.0–55.5) | 47.5 (42.1–52.8) | ||||
| Valencia | 55.2 (52.2 (58.2) | 56.1 (52.3–59.9) | 53.4 (48.4–58.3) | ||||
| Education level | Primary education or less * | 49.9 (49.0–50.8) | 0.22 (0.38) | 50.8 (49.7–51.9) | 0.09 (0.26) | 48.6 (47.1–50.1) | 0.40 (0.44) |
| Secondary education | 51.4 (49.9–53.0) | 52.5 (50.8–54.3) | 49.8 (46.9–52.7) | ||||
| University | 51.0 (48.8–53.2) | 53.4 (50.8–55.9) | 46.4 (42.3–50.5) | ||||
| Recurrence | No * | 50.5 (49.8–51.3) | 0.13 (0.29) | 51.8 (50.9–52.7) | 0.04 (0.13) | 48.6 (47.3–49.8) | 0.77 (0.58) |
| Yes | 48.5 (45.9–51.0) | 48.7 (45.9–51.5) | 49.4 (44.0–54.8) | ||||
| Functional Assessment of Cancer Therapy Colorectal Symptom Index (FCSI) | |||||||
|---|---|---|---|---|---|---|---|
| Total Sample | Men | Women | |||||
| (n = 679) | (n = 398) | (n = 281) | |||||
| Mean (95% CI) | p-value (q-value) | Mean (95% CI) | p-value (q-value) | Mean (95% CI) | p-value (q-value) | ||
| Sex | Men * | 29.7 (29.3–30.2) | <0.001 | ||||
| Women | 28.4 (27.9–29.0) | (0.004) | |||||
| Age at diagnosis | beta (95% CI) | −0.05 (−0.08–0.56) | 0.01 (0.05) | −0.03 (−0.08–0.01) | 0.16 (0.32) | −0.07 (−0.13–(−0.01)) | 0.02 (0.07) |
| Age | ≤55 * | 29.1 (28.2–29.9) | <0.001 | 29.1 (28.0–30.2) | 0.005 (0.02) | 29.1 (27.8–30.4) | 0.003 (0.02) |
| 56–65 | 30.0 (29.4–30.7) | (0.002) | 30.8 (30.0–31.6) | 28.7 (27.6–29.8) | |||
| 66–75 | 29.5 (28.9–30.1) | 29.7 (29.0–30.5) | 29.2 (28.1–30.2) | ||||
| 76–85 | 27.6–26.8–28.4) | 28.5 (27.4–29.6) | 26.4 (25.2–27.6) | ||||
| Area | Asturias * | 29.4 (27.6–31.2) | <0.001 | 29.7 (27.8–32.1) | 0.001 | 28.6 (25.8–31.4) | 0.01 (0.06) |
| Barcelona | 28.8 (28.2–29.5) | (<0.001) | 29.4 (28.5–30.2) | (0.009) | 28.0 (27.0–29.1) | ||
| Cantabria | 30.0 (28.8–31.2) | 30.5 (28.9–32.1) | 29.2 (27.3–31.0) | ||||
| Granada | 27.0 (25.3–28.6) | 27.0 (24.6–29.4) | 27.1 (24.7–29.6) | ||||
| Guipúzcoa | - | - | - | ||||
| Huelva | 26.0 (24.4–27.6) | 26.2 (24.2–28.3) | 25.9 (23.2–28.6) | ||||
| León | 30.5 (29.8–31.2) | 30.8 (29.9–31.7) | 30.2 (29.0–31.4) | ||||
| Madrid | 28.8 (27.9–29.8) | 30.3 (29.0–31.5) | 26.8 (25.2–28.3) | ||||
| Murcia | 26.5 (23.0–29.9) | 26.6 (22.6–30.7) | 26.5 (19.8–33.2) | ||||
| Navarra | - | - | - | ||||
| Valencia | 30.0 (28.6–31.4) | 30.4 (28.6–32.2) | 29.2 (27.0–31.3) | ||||
| Civil status | Single * | 28.1 (26.7–29.5) | 0.24 (0.38) | 27.6 (25.4–29.7) | 0.10 (0.27) | 28.3 (26.3–30.3) | 0.89 (0.62) |
| Married or Living with a partner | 29.3 (28.9–29.7) | 29.8 (29.4–30.3) | 28.5 (27.8–29.2) | ||||
| Widow | 29.0 (27.9–30.1) | 30.7 (28.3–33.1) | 28.2 (27.0–29.4) | ||||
| Smoking | Non-smoker and former at diagnosis * | 29.8 (29.2–30.3) | 0.02 (0.07) | 30.4 (29.5–31.3) | 0.14 (0.30) | 28.7 (28.1–29.4) | 0.10 (0.27) |
| Smoker at diagnosis | 28.7 (28.2–29.3) | 29.6 (29.1–30.1) | 27.4 (26.1–28.7) | ||||
| Metastasis | M0 * | 29.1 (28.7–29.5) | 0.07 (0.21) | 29.6 (29.2–30.1) | 0.08 (0.23) | 28.3 (27.8–28.9) | 0.57 (0.53) |
| M1 | 30.8 (29.0–32.7) | 31.4 (29.3–33.6) | 30.1 (25.8–34.4) | ||||
| Recurrence | No * | 28.4 (29.0–29.8) | <0.001 | 30.0 (29.6–30.5) | 0.005 (0.005) | 28.5 (27.9–29.1) | 0.23 (0.38) |
| Yes | 27.0 (25.8–28.3) | (0.004) | 27.3 (25.9–28.8) | 26.9 (24.4–29.4) | |||
| Radiotherapy | No * | 29.5 (29.0–29.9) | 0.11 (0.28) | 30.0 (29.5–30.6) | 0.33 (0.41) | 28.7 (28.0–29.3) | 0.08 (0.23) |
| Yes | 28.6 (27.8–29.3) | 29.3 (28.5–30.2) | 27.1 (25.8–28.4) | ||||
| Surgery Type | Radical * | 29.1 (28.7–29.4) | 0.06 (0.19) | 29.7 (29.2–30.1) | 0.35 (0.43) | 28.3 (27.7–28.8) | 0.26 (0.38) |
| Palliative | 30.9 (29.2–32.7) | 31.0 (28.8–33.2) | 30.6 (27.7–33.6) | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcos-Delgado, A.; Martín-Sánchez, V.; Molina-Barceló, A.; Alonso-Molero, J.; Pérez-Gómez, B.; Pollán, M.; Aragonés, N.; Ederra-Sanza, M.; Fernández-Tardón, G.; Binefa, G.; et al. Health-Related Quality of Life in Long-Term Colorectal Cancer Survivors. Healthcare 2024, 12, 1917. https://doi.org/10.3390/healthcare12191917
Marcos-Delgado A, Martín-Sánchez V, Molina-Barceló A, Alonso-Molero J, Pérez-Gómez B, Pollán M, Aragonés N, Ederra-Sanza M, Fernández-Tardón G, Binefa G, et al. Health-Related Quality of Life in Long-Term Colorectal Cancer Survivors. Healthcare. 2024; 12(19):1917. https://doi.org/10.3390/healthcare12191917
Chicago/Turabian StyleMarcos-Delgado, Alba, Vicente Martín-Sánchez, Ana Molina-Barceló, Jessica Alonso-Molero, Beatriz Pérez-Gómez, Marina Pollán, Nuria Aragonés, María Ederra-Sanza, Guillermo Fernández-Tardón, Gemma Binefa, and et al. 2024. "Health-Related Quality of Life in Long-Term Colorectal Cancer Survivors" Healthcare 12, no. 19: 1917. https://doi.org/10.3390/healthcare12191917
APA StyleMarcos-Delgado, A., Martín-Sánchez, V., Molina-Barceló, A., Alonso-Molero, J., Pérez-Gómez, B., Pollán, M., Aragonés, N., Ederra-Sanza, M., Fernández-Tardón, G., Binefa, G., Moreno, V., Barrios-Rodríguez, R., Amiano, P., Huerta, J. M., Teso, E. P., Alguacil, J., Castaño-Vinyals, G., Kogevinas, M., & Molina de la Torre, A. J. (2024). Health-Related Quality of Life in Long-Term Colorectal Cancer Survivors. Healthcare, 12(19), 1917. https://doi.org/10.3390/healthcare12191917









