Patient-Reported Outcomes of Depression and Fibromyalgia Symptoms Do Not Predict Non-Inflammatory versus Inflammatory Diagnoses at Initial Rheumatology Consultation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Objectives
2.2. Ethical Approval
2.3. Measurement of Primary and Secondary Variables
2.4. Statistical Analysis
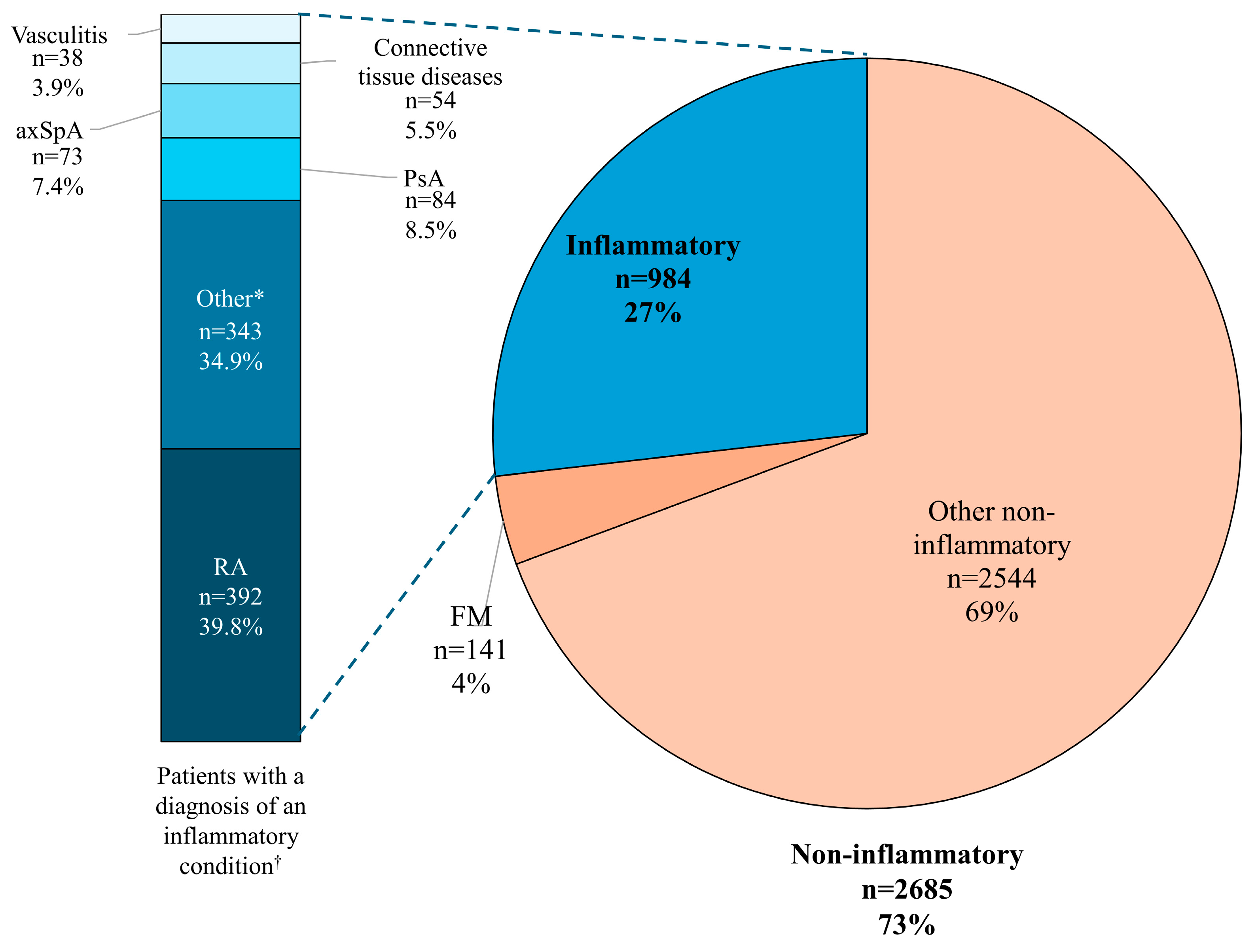
3. Results
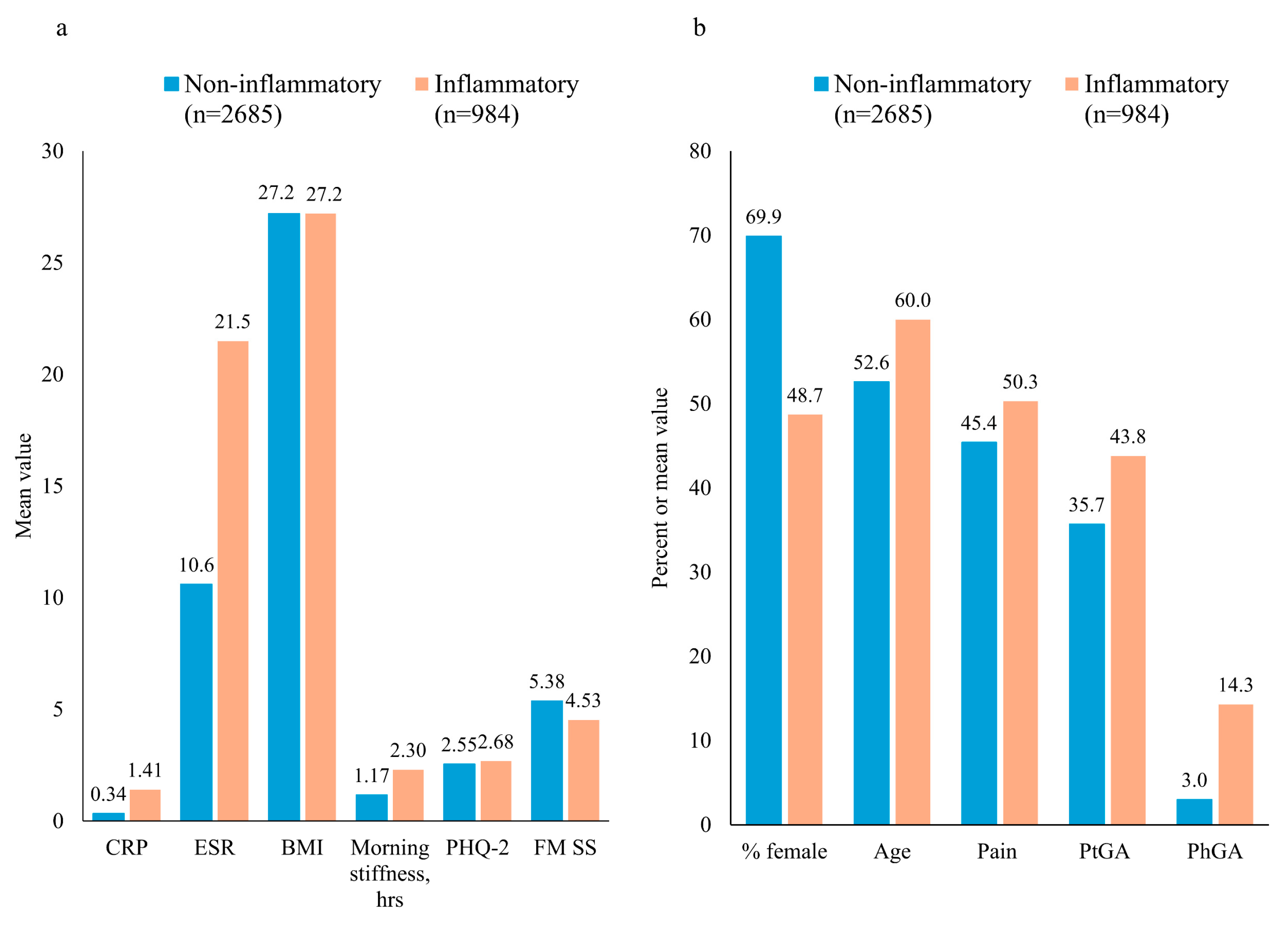
3.1. Patient Characteristics According to Non-Inflammatory/Inflammatory Diagnosis
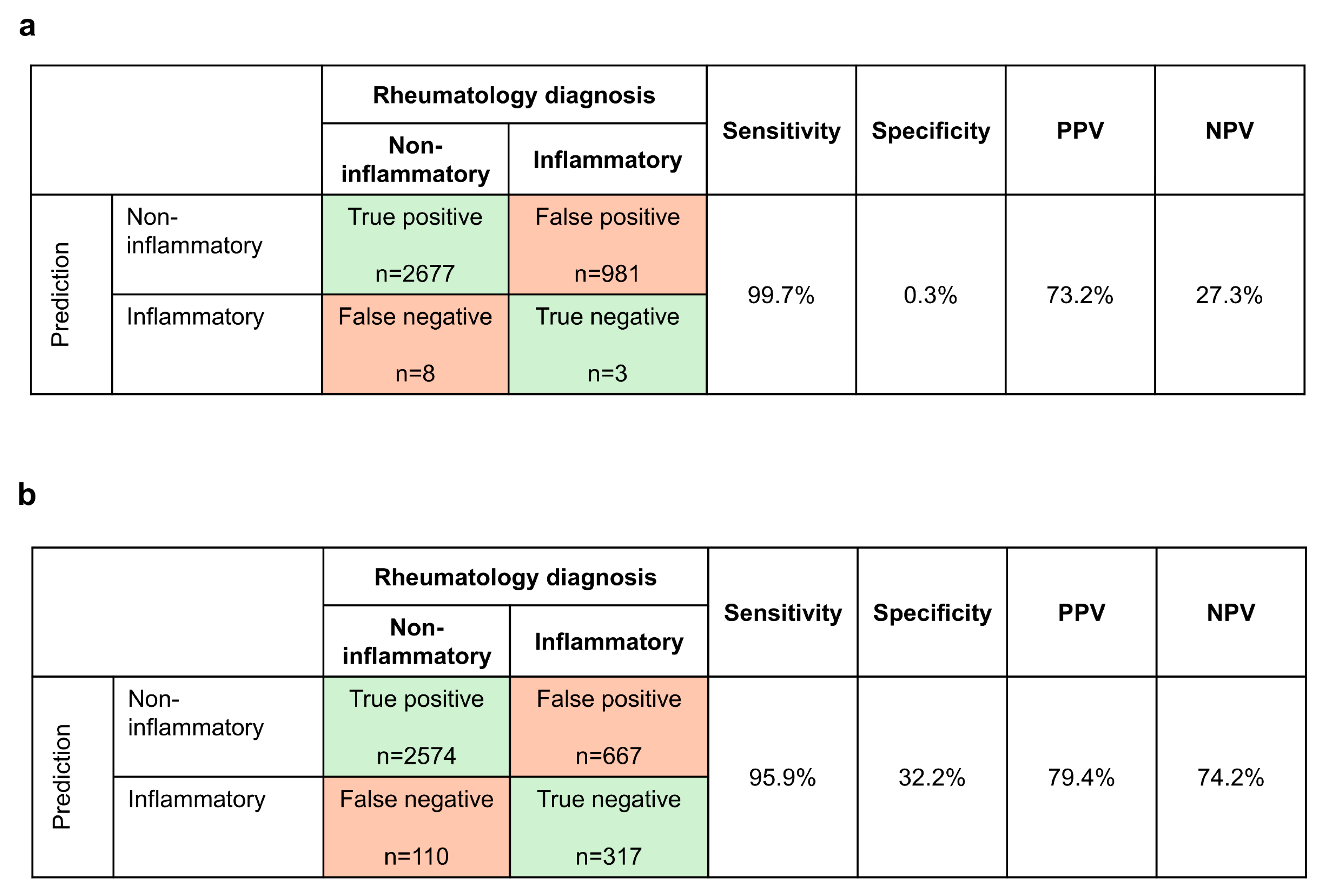
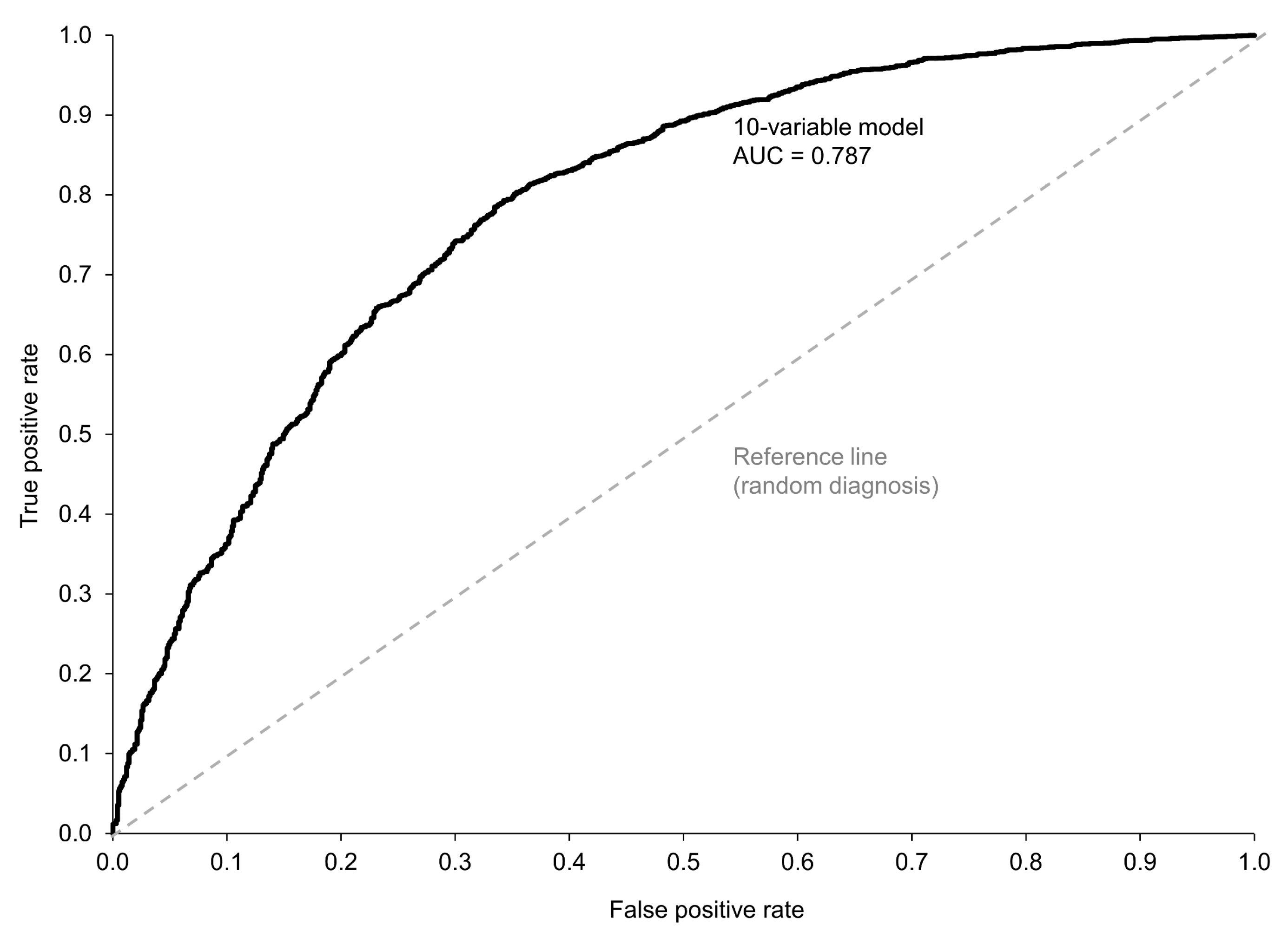
3.2. Prediction of Non-Inflammatory vs. Inflammatory Disease
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thoms, B.L.; Bonnell, L.N.; Tompkins, B.; Nevares, A.; Lau, C. Predictors of inflammatory arthritis among new rheumatology referrals: A cross-sectional study. Rheumatol. Adv. Pract. 2023, 7, rkad067. [Google Scholar] [CrossRef] [PubMed]
- Krusche, M.; Sewerin, P.; Kleyer, A.; Mucke, J.; Vossen, D.; Morf, H.; Rheumadocs und Arbeitskreis Junge Rheumatologie (AGJR). Facharztweiterbildung quo vadis? [Specialist training quo vadis?]. Z. Rheumatol. 2019, 78, 692–697. [Google Scholar] [CrossRef] [PubMed]
- Kuhlmann, E.; Bruns, L.; Hoeper, K.; Witte, T.; Ernst, D.; Jablonka, A. Fachkräfteentwicklung in der Rheumatologie: Ein berufsstruktureller Überblick und gesundheitspolitischer Weckruf [Health workforce development in rheumatology: A mapping exercise and wake-up call for health policy]. Z. Rheumatol. 2022, 81, 717–729. (In German) [Google Scholar] [CrossRef]
- Kulhawy-Wibe, S.C.; Widdifield, J.; Lee, J.J.Y.; Thome, C.; Yacyshyn, E.A.; Batthish, M.; Jerone, D.; Shupak, R.; Jilkine, K.; Purvis, J.; et al. Results From the 2020 Canadian Rheumatology Association’s Workforce and Wellness Survey. J. Rheumatol. 2022, 49, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Battafarano, D.F.; Ditmyer, M.; Bolster, M.B.; Fitzgerald, J.D.; Deal, C.; Bass, A.R.; Molina, R.; Erickson, A.R.; Hausmann, J.S.; Klein-Gitelman, M.; et al. 2015 American College of Rheumatology Workforce Study: Supply and demand projections of adult rheumatology workforce, 2015–2030. Arthritis Care Res. 2018, 70, 617–626. [Google Scholar] [CrossRef]
- Finckh, A.; Liang, M.H.; van Herckenrode, C.M.; de Pablo, P. Long-term impact of early treatment on radiographic progression in rheumatoid arthritis: A meta-analysis. Arthritis Rheum. 2006, 55, 864–872. [Google Scholar] [CrossRef]
- Kyburz, D.; Gabay, C.; Michel, B.A.; Finckh, A.; Physicians of SCQM-RA. The long-term impact of early treatment of rheumatoid arthritis on radiographic progression: A population-based cohort study. Rheumatology 2011, 50, 1106–1110. [Google Scholar] [CrossRef]
- Gwinnutt, J.M.; Symmons, D.P.M.; MacGregor, A.J. Twenty-year outcome and association between early treatment and mortality and disability in an inception cohort of patients with rheumatoid arthritis: Results from the Norfolk Arthritis Register. Arthritis Rheumatol. 2016, 69, 1566–1575. [Google Scholar] [CrossRef]
- Haroon, M.; Gallagher, P.; FitzGerald, O. Diagnostic delay of more than 6 months contributes to poor radiographic and functional outcome in psoriatic arthritis. Ann. Rheum. Dis. 2015, 74, 1045–1050. [Google Scholar] [CrossRef]
- Pang, H.Y.; Farrer, C.; Wu, W.; Gakhal, N.K. Quality of rheumatology care for patients with fibromyalgia and chronic pain syndromes. BMJ Open Qual. 2021, 1, e001061. [Google Scholar] [CrossRef]
- Tavares, R.; Huang, S.; Bykerk, V.P.; Bell, M.J. A parallel group cohort to determine the measurement properties of an early inflammatory arthritis detection tool. Rheumatology 2013, 52, 2077–2085. [Google Scholar] [CrossRef] [PubMed]
- Tavares, R.; Wells, G.A.; Bykerk, V.P.; Guillemin, F.; Tugwell, P.; Bell, M.J. Validation of a self-administered inflammatory arthritis detection tool for rheumatology triage. J. Rheumatol. 2013, 40, 417–424. [Google Scholar] [CrossRef] [PubMed]
- ten Brinck, R.M.; van Dijk, B.T.; van Steenbergen, H.W.; le Cessie, S.; Numans, M.E.; Hider, S.L.; Mallen, C.; van der Helm-van Mil, A. Development and validation of a clinical rule for recognition of early inflammatory arthritis. BMJ Open 2018, 8, e023552. [Google Scholar] [CrossRef]
- Knitza, J.; Mohn, J.; Bergmann, C.; Kampylafka, E.; Hagen, M.; Bohr, D.; Morf, H.; Araujo, E.; Englbrecht, M.; Simon, D.; et al. Accuracy, patient-perceived usability, and acceptance of two symptom checkers (Ada and Rheport) in rheumatology: Interim results from a randomized controlled crossover trial. Arthritis Res. Ther. 2021, 23, 112. [Google Scholar] [CrossRef]
- Biln, N.K.; Bansback, N.; Shojania, K.; Puil, L.; Harrison, M. A scoping review of triage approaches for the referral of patients with suspected inflammatory arthritis, from primary to rheumatology care. Rheumatol. Int. 2024, ahead of print. [Google Scholar] [CrossRef]
- Feuchtenberger, M.; Nigg, A.P.; Kraus, M.R.; Schäfer, A. Rate of proven rheumatic diseases in a large collective of referrals to an outpatient rheumatology clinic under routine conditions. Clin. Med. Insights Arthritis Musculoskelet. Disord. 2016, 9, 181–187. [Google Scholar] [CrossRef] [PubMed]
- O’Neil, L.J.; Alpízar-Rodríguez, D.; Deane, K.D. Rheumatoid arthritis: The continuum of disease and strategies for prediction, early intervention, and prevention. J. Rheumatol. 2024, 51, 337–349. [Google Scholar] [CrossRef]
- Baymler Lundberg, A.S.; Esbensen, B.A.; Jensen, M.B.; Hauge, E.M.; Thurah, A. Facilitators and barriers in diagnosing rheumatoid arthritis as described by general practitioners: A Danish study based on focus group interviews. Scand. J. Prim. Health Care 2021, 39, 222–229. [Google Scholar] [CrossRef]
- Saraiva, L.; Duarte, C. Barriers to the diagnosis of early inflammatory arthritis: A literature review. Open Access Rheumatol. 2023, 15, 11–22. [Google Scholar] [CrossRef]
- Seifert, O.; Baerwald, C. Impact of fatigue on rheumatic diseases. Best. Pract. Res. Clin. Rheumatol. 2019, 33, 101435. [Google Scholar] [CrossRef]
- Sørensen, L.; Jensen, M.S.A.; Rathleff, M.S.; Holden, S. Comorbid insomnia, psychological symptoms and widespread pain among patients suffering from musculoskeletal pain in general practice: A cross-sectional study. BMJ Open 2019, 9, e031971. [Google Scholar] [CrossRef]
- D’Amuri, A.; Greco, S.; Pagani, M.; Presciuttini, B.; Ciaffi, J.; Ursini, F. Common non-rheumatic medical conditions mimicking fibromyalgia: A simple framework for differential diagnosis. Diagnostics 2024, 14, 1758. [Google Scholar] [CrossRef] [PubMed]
- Schubiner, H.; Lowry, W.J.; Heule, M.; Ashar, Y.K.; Lim, M.; Mekaru, S.; Kitts, T.; Lumley, M.A. Application of a clinical approach to diagnosing primary pain: Prevalence and correlates of primary back and neck pain in a community physiatry Clinic. J. Pain 2024, 25, 672–681. [Google Scholar] [CrossRef]
- Poddighe, D.; Castelli, L.; Marseglia, G.L.; Bruni, P. A sudden onset of a pseudo-neurological syndrome after HPV-16/18 AS04-adjuvated vaccine: Might it be an autoimmune/inflammatory syndrome induced by adjuvants (ASIA) presenting as a somatoform disorder? Immunol. Res. 2014, 60, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Duffield, S.J.; Miller, N.; Zhao, S.; Goodson, N.J. Concomitant fibromyalgia complicating chronic inflammatory arthritis: A systematic review and meta-analysis. Rheumatology 2018, 57, 1453–1460. [Google Scholar] [CrossRef]
- Bair, M.J.; Krebs, E.E. Fibromyalgia. Ann. Intern. Med. 2020, 172, ITC33–ITC48. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.A.; Goldenberg, D.L.; Katz, R.S.; Mease, P.; Russell, A.S.; Russell, I.J.; Winfield, J.B.; Yunus, M.B. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res. 2010, 62, 600–610. [Google Scholar] [CrossRef]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.A.; Goldenberg, D.L.; Häuser, W.; Katz, R.S.; Mease, P.; Russell, A.S.; Russell, I.J.; Winfield, J.B. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: A modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. J. Rheumatol. 2011, 38, 1113–1122. [Google Scholar] [CrossRef] [PubMed]
- Bucourt, E.; Martaillé, V.; Goupille, P.; Joncker-Vannier, I.; Huttenberger, B.; Réveillère, C.; Mulleman, D.; Courtois, A.R. A comparative study of fibromyalgia, rheumatoid arthritis, spondyloarthritis, and Sjögren’s syndrome; Impact of the disease on quality of life, psychological adjustment, and use of coping strategies. Pain Med. 2021, 22, 372–381. [Google Scholar] [CrossRef]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B. The Patient Health Questionnaire-2: Validity of a two-item depression screener. Med. Care 2003, 41, 1284–1292. [Google Scholar] [CrossRef]
- Mandrekar, J.N. Receiver operating characteristic curve in diagnostic test assessment. J. Thorac. Oncol. 2010, 5, 1315–1316. [Google Scholar] [CrossRef]
- Burgers, L.E.; Siljehult, F.; Ten Brinck, R.M.; van Steenbergen, H.W.; Landewé, R.B.M.; Rantapää-Dahlqvist, S.; van der Helm-van Mil, A.H.M. Validation of the EULAR definition of arthralgia suspicious for progression to rheumatoid arthritis. Rheumatology 2017, 56, 2123–2128. [Google Scholar] [CrossRef] [PubMed]
- Shadick, N.A.; Cook, N.R.; Karlson, E.W.; Ridker, P.M.; Maher, N.E.; Manson, J.E.; Buring, J.E.; Lee, I.M. C-reactive protein in the prediction of rheumatoid arthritis in women. Arch. Intern. Med. 2006, 166, 2490–2494. [Google Scholar] [CrossRef] [PubMed]
- Aho, K.; Palosuo, T.; Knekt, P.; Alha, P.; Aromaa, A.; Heliövaara, M. Serum C-reactive protein does not predict rheumatoid arthritis. J. Rheumatol. 2000, 27, 1136–1138. [Google Scholar] [PubMed]
- van Nies, J.A.; Alves, C.; Radix-Bloemen, A.L.; Gaujoux-Viala, C.; Huizinga, T.W.; Hazes, J.M.; Brouwer, E.; Fautrel, B.; van der Helm-van Mil, A.H. Reappraisal of the diagnostic and prognostic value of morning stiffness in arthralgia and early arthritis: Results from the Groningen EARC, Leiden EARC, ESPOIR, Leiden EAC and REACH. Arthritis Res. Ther. 2015, 17, 108. [Google Scholar] [CrossRef]
- Krijbolder, D.I.; Wouters, F.; van Mulligen, E.; van der Helm-van Mil, A.H.M. Morning stiffness precedes the development of rheumatoid arthritis and associates with systemic and subclinical joint inflammation in arthralgia patients. Rheumatology 2022, 61, 2113–2118. [Google Scholar] [CrossRef]
- Bennett, R.M.; Jones, J.; Turk, D.C.; Russell, I.J.; Matallana, L. An internet survey of 2596 people with fibromyalgia. BMC Musculoskelet. Disord. 2006, 8, 27. [Google Scholar] [CrossRef]
- van de Stadt, L.A.; Haugen, I.K.; Felson, D.; Kloppenburg, M. Prolonged morning stiffness is common in hand OA and does not preclude a diagnosis of hand osteoarthritis. Osteoarthr. Cartil. 2023, 31, 529–533. [Google Scholar] [CrossRef]
- ten Brinck, R.M.; van Steenbergen, H.W.; van Delft, M.A.M.; Verheul, M.K.; Toes, R.E.M.; Trouw, L.A.; van der Helm-van Mil, A.H.M. The risk of individual autoantibodies, autoantibody combinations and levels for arthritis development in clinically suspect arthralgia. Rheumatology 2017, 56, 2145–2153. [Google Scholar] [CrossRef]
- Boeren, A.M.P.; Oei, E.H.G.; van der Helm-van Mil, A.H.M. The value of MRI for detecting subclinical joint inflammation in clinically suspect arthralgia. RMD Open 2022, 8, e002128. [Google Scholar] [CrossRef]
- Ohrndorf, S.; Glimm, A.M.; Ammitzbøll-Danielsen, M.; Ostergaard, M.; Burmester, G.R. Fluorescence optical imaging: Ready for prime time? RMD Open 2021, 7, e001497. [Google Scholar] [CrossRef]
- Garcia-Montoya, L.; Kang, J.; Duquenne, L.; Di Matteo, A.; Nam, J.L.; Harnden, K.; Chowdhury, R.; Mankia, K.; Emery, P. Factors associated with resolution of ultrasound subclinical synovitis in anti-CCP-positive individuals with musculoskeletal symptoms: A UK prospective cohort study. Lancet Rheumatol. 2024, 6, e72–e80. [Google Scholar] [CrossRef] [PubMed]
- Di Matteo, A.; Corradini, D.; Mankia, K. What is the value of ultrasound in individuals ‘at-risk’ of rheumatoid arthritis who do not have clinical synovitis? Healthcare 2021, 9, 752. [Google Scholar] [CrossRef]
- Kleykamp, B.A.; Ferguson, M.C.; McNicol, E.; Bixho, I.; Arnold, L.M.; Edwards, R.R.; Fillingim, R.; Grol-Prokopczyk, H.; Turk, D.C.; Dworkin, R.H. The prevalence of psychiatric and chronic pain comorbidities in fibromyalgia: An ACTTION systematic review. Semin. Arthritis Rheum. 2021, 51, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard, S.B.; Esbensen, B.A.; Klausen, J.M.; Glintborg, B.; Lau, L.; Yilmaz Jantzen, C.; Aadahl, M.; Fevejle Cromhout, P.; de Thurah, A. Prevalence of anxiety and depression and the association with self-management behaviour in >12 000 patients with inflammatory rheumatic disease: A cross-sectional nationwide study. RMD Open 2024, 10, e003412. [Google Scholar] [CrossRef]
- Ruschak, I.; Montesó-Curto, P.; Rosselló, L.; Aguilar Martín, C.; Sánchez-Montesó, L.; Toussaint, L. Fibromyalgia syndrome pain in men and women: A scoping review. Healthcare 2023, 11, 223. [Google Scholar] [CrossRef]
- Chmielewski, G.; Majewski, M.S.; Kuna, J.; Mikiewicz, M.; Krajewska-Włodarczyk, M. Fatigue in inflammatory joint diseases. Int. J. Mol. Sci. 2023, 24, 12040. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Choy, E. Non-inflammatory pain in inflammatory arthritis. Rheumatology 2023, 62, 2360–2365. [Google Scholar] [CrossRef]
- Desthieux, C.; Hermet, A.; Granger, B.; Fautrel, B.; Gossec, L. Patient-physician discordance in global assessment in rheumatoid arthritis: A systematic literature review with meta-analysis. Arthritis Care Res. 2016, 68, 1767–1773. [Google Scholar] [CrossRef]
- Lindström Egholm, C.; Krogh, N.S.; Pincus, T.; Dreyer, L.; Ellingsen, T.; Glintborg, B.; Kowalski, M.R.; Lorenzen, T.; Madsen, O.R.; Nordin, H.; et al. Discordance of global assessments by patient and physician is higher in female than in male patients regardless of the physician’s sex: Data on patients with rheumatoid arthritis, axial spondyloarthritis, and psoriatic arthritis from the DANBIO Registry. J. Rheumatol. 2015, 42, 1781–1785. [Google Scholar] [CrossRef]
- Khan, N.A.; Spencer, H.J.; Abda, E.; Aggarwal, A.; Alten, R.; Ancuta, C.; Andersone, D.; Bergman, M.; Craig-Muller, J.; Detert, J.; et al. Determinants of discordance in patients’ and physicians’ rating of rheumatoid arthritis disease activity. Arthritis Care Res. 2012, 64, 206–214. [Google Scholar] [CrossRef]
- Ward, M.M.; Guthrie, L.C.; Dasgupta, A. Direct and indirect determinants of the patient global assessment in rheumatoid arthritis: Differences by level of disease activity. Arthritis Care Res. 2017, 69, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Challa, D.N.; Kvrgic, Z.; Cheville, A.L.; Crowson, C.S.; Bongartz, T.; Mason, T.G., 2nd; Matteson, E.L.; Michet, C.J., Jr.; Persellin, S.T.; Schaffer, D.E.; et al. Patient-provider discordance between global assessments of disease activity in rheumatoid arthritis: A comprehensive clinical evaluation. Arthritis Res. Ther. 2017, 19, 212. [Google Scholar] [CrossRef] [PubMed]
- Feuchtenberger, M.; Kovacs, M.; Nigg, A.; Schäfer, A. Prioritising appointments by telephone interview: Duration from symptom onset to appointment request predicts likelihood of inflammatory rheumatic diseases. J. Clin. Med. 2024, 13, 4551. [Google Scholar] [CrossRef]
- Gilvaz, V.J.; Reginato, A.M. Artificial intelligence in rheumatoid arthritis: Potential applications and future implications. Front. Med. 2023, 10, 1280312. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Rheumatology Diagnosis | |
|---|---|---|
| Non-Inflammatory (n = 2685) | Inflammatory (n = 984) | |
| Female, n (%) | 1876 (69.87) | 479 (48.69) |
| Age, years | 52.6 (14.9) | 60.0 (15.3) |
| Body mass index, kg/m2 | 27.2 (5.8) | 27.2 (5.2) |
| Morning stiffness, h | 1.17 (3.5) | 2.30 (5.3) |
| CRP, mg/dL | 0.34 (0.67) | 1.41 (2.32) |
| ESR, mm/h | 10.6 (10.0) a | 21.5 (19.2) |
| Vitamin D, ng/mL | 26.9 (14.0) | 25.5 (12.7) |
| TSH, μU/mL | 1.62 (1.10) | 1.68 (1.20) |
| PhGA (VAS) | 3.0 (7.1) b | 14.3 (16.9) |
| PtGA (VAS) | 35.7 (28.4) | 43.8 (30.9) |
| Pain (VAS) | 45.4 (26.7) | 50.3 (29.2) |
| PHQ-2 | 2.55 (1.84) | 2.68 (1.88) |
| FM SS | 5.38 (3.12) | 4.53 (2.95) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schäfer, A.; Kovacs, M.S.; Nigg, A.; Feuchtenberger, M. Patient-Reported Outcomes of Depression and Fibromyalgia Symptoms Do Not Predict Non-Inflammatory versus Inflammatory Diagnoses at Initial Rheumatology Consultation. Healthcare 2024, 12, 1948. https://doi.org/10.3390/healthcare12191948
Schäfer A, Kovacs MS, Nigg A, Feuchtenberger M. Patient-Reported Outcomes of Depression and Fibromyalgia Symptoms Do Not Predict Non-Inflammatory versus Inflammatory Diagnoses at Initial Rheumatology Consultation. Healthcare. 2024; 12(19):1948. https://doi.org/10.3390/healthcare12191948
Chicago/Turabian StyleSchäfer, Arne, Magdolna Szilvia Kovacs, Axel Nigg, and Martin Feuchtenberger. 2024. "Patient-Reported Outcomes of Depression and Fibromyalgia Symptoms Do Not Predict Non-Inflammatory versus Inflammatory Diagnoses at Initial Rheumatology Consultation" Healthcare 12, no. 19: 1948. https://doi.org/10.3390/healthcare12191948
APA StyleSchäfer, A., Kovacs, M. S., Nigg, A., & Feuchtenberger, M. (2024). Patient-Reported Outcomes of Depression and Fibromyalgia Symptoms Do Not Predict Non-Inflammatory versus Inflammatory Diagnoses at Initial Rheumatology Consultation. Healthcare, 12(19), 1948. https://doi.org/10.3390/healthcare12191948






