Multi-Modal versus Uni-Modal Treatment for the Recovery of Lower Limb Motor Function in Patients after Stroke: A Systematic Review with Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Sources and Searches
2.2. Study Selection
2.3. Outcomes
2.4. Data Extraction and Management
2.5. Assessment of Risk of Bias in Included Studies
2.6. Measures of the Treatment Effect
2.7. Assessment of Heterogeneity
2.8. Data Synthesis
2.9. Subgroup Analysis
3. Results
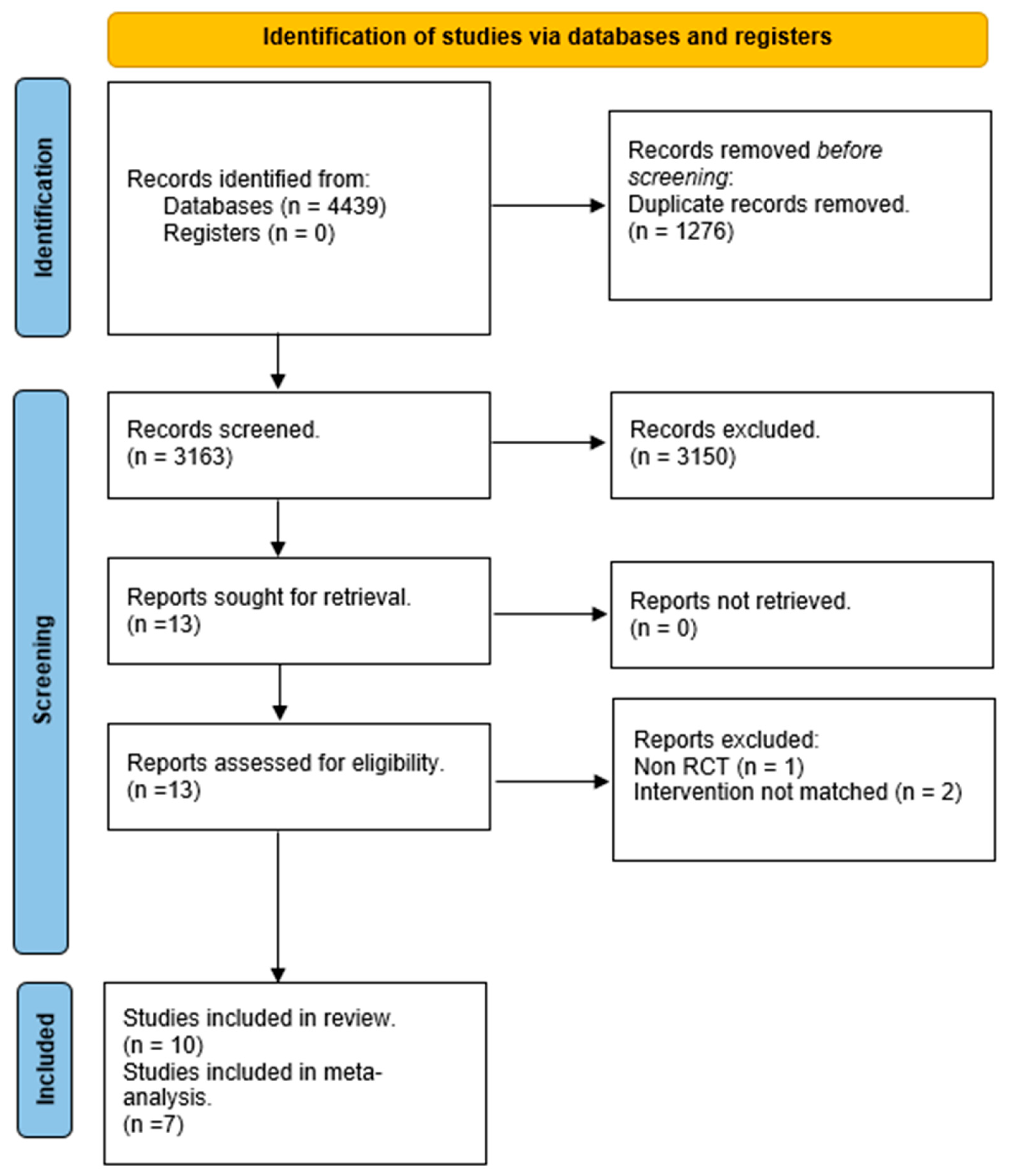
3.1. Included Studies
3.2. Excluded Studies
3.3. Risk of Bias in the Included Studies
- -
- Bias arising from the randomisation process: Six studies were assessed with a low risk of bias, as the authors described a correct randomisation process and, therefore, there were no differences between intervention groups related to this process. One study [20] was judged with a high risk of bias, as the participants were randomised according to clinical needs. Three studies [22,26,27] were judged with some concerns regarding the risk of bias, as no information was provided.
- -
- Bias due to deviations from the intended interventions: Eight studies had a low risk of bias in this domain. Moreover, one study [25] had a high risk of bias because the participants, carers, and therapists were aware of the intervention received, and the drop-out rate was high (13%). Finally, one study [20] did not provide information, resulting in some concerns about the risk of bias.
- -
- -
- Bias in measurement of the outcome: Five studies had a low risk of bias in this domain, whereas four studies [20,22,23,26] had a high risk of bias because the outcome assessor was not blinded or some outcome measures were collected only in the intervention group. One study [21] had some concerns about the risk of bias since the health professionals had free access to the subjects, making it difficult to guarantee the complete blinding of the evaluators.
- -
- Bias in the selection of the reported result: One study [24] had a low risk of bias since the data were in accordance with the pre-registered study protocol. Another study [11] had a high risk of bias because the reported results were not in accordance with the study protocol, whereas for the other eight studies, there were some concerns about the presence of risk of bias since no information about the study protocol was provided.
- -
3.4. Effects of Intervention
3.4.1. Effect of Multimodal Treatment on Endurance Compared to Unimodal and Usual Care Treatment
3.4.2. Effect of Multimodal Treatment on Knee-Extensor Muscle Strength Compared to Unimodal Treatment
3.4.3. Effect of Multimodal Treatment on Gait Speed Compared to Unimodal and No Treatment
3.4.4. Effect of Multimodal Treatment on Aerobic Capacity Compared to Unimodal Treatment
4. Discussion
Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saini, V.; Guada, L.; Yavagal, D.R. Global Epidemiology of Stroke and Access to Acute Ischemic Stroke Interventions. Neurology 2021, 97, S6–S16. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Brainin, M.; Norrving, B.; Martins, S.; Sacco, R.L.; Hacke, W.; Fisher, M.; Pandian, J.; Lindsay, P. World Stroke Organization (WSO): Global Stroke Fact Sheet 2022. Int. J. Stroke Off. J. Int. Stroke Soc. 2022, 17, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Belagaje, S.R. Stroke Rehabilitation. Continuum 2017, 23, 238–253. [Google Scholar] [CrossRef]
- Wade, D.T. Measurement in neurological rehabilitation. Curr. Opin. Neurol. Neurosurg. 1992, 5, 682–686. [Google Scholar] [PubMed]
- Katan, M.; Luft, A. Global Burden of Stroke. Semin. Neurol. 2018, 38, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Markus, H.S. Reducing disability after stroke. Int. J. Stroke Off. J. Int. Stroke Soc. 2022, 17, 249–250. [Google Scholar] [CrossRef] [PubMed]
- Broderick, P.; Horgan, F.; Blake, C.; Ehrensberger, M.; Simpson, D.; Monaghan, K. Mirror therapy for improving lower limb motor function and mobility after stroke: A systematic review and meta-analysis. Gait Posture 2018, 63, 208–220. [Google Scholar] [CrossRef]
- World Health Organization. International Classification of Functioning, Disability and Health; ICF: Lexington, KY, USA, 2001. [Google Scholar]
- Bergmann, J.; Krewer, C.; Bauer, P.; Koenig, A.; Riener, R.; Müller, F. Virtual reality to augment robot-assisted gait training in non-ambulatory patients with a subacute stroke: A pilot randomized controlled trial. Eur. J. Phys. Rehabil. Med. 2018, 54, 397–407. [Google Scholar] [CrossRef]
- Hatem, S.M.; Saussez, G.; Della Faille, M.; Prist, V.; Zhang, X.; Dispa, D.; Bleyenheuft, Y. Rehabilitation of Motor Function after Stroke: A Multiple Systematic Review Focused on Techniques to Stimulate Upper Extremity Recovery. Front. Hum. Neurosci. 2016, 10, 442. [Google Scholar] [CrossRef]
- Jørgensen, H.S.; Nakayama, H.; Raaschou, H.O.; Olsen, T.S. Recovery of walking function in stroke patients: The Copenhagen Stroke Study. Arch. Phys. Med. Rehabil. 1995, 76, 27–32. [Google Scholar] [CrossRef]
- Veldema, J.; Jansen, P. Resistance training in stroke rehabilitation: Systematic review and meta-analysis. Clin. Rehabil. 2020, 34, 1173–1197. [Google Scholar] [CrossRef] [PubMed]
- Wist, S.; Clivaz, J.; Sattelmayer, M. Muscle strengthening for hemiparesis after stroke: A meta-analysis. Ann. Phys. Rehabil. Med. 2016, 59, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Potempa, K.; Braun, L.T.; Tinknell, T.; Popovich, J. Benefits of aerobic exercise after stroke. Sports Med. 1996, 21, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Sharp, T.; Grandou, C.; Coutts, A.J.; Wallace, L. The Effects of High-Intensity Multimodal Training in Apparently Healthy Populations: A Systematic Review. Sports Med. Open 2022, 8, 43. [Google Scholar] [CrossRef]
- Winstein, C.J.; Stein, J.; Arena, R.; Bates, B.; Cherney, L.R.; Cramer, S.C.; Deruyter, F.; Eng, J.J.; Fisher, B.; Harvey, R.L.; et al. Guidelines for Adult Stroke Rehabilitation and Recovery: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2016, 47, e98–e169. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savovic, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Bowden, M.G.; Monsch, E.D.; Middleton, A.; Daughtry, C.; Powell, T.; Kraft, S.V. Lessons Learned: The Difficulties of Incorporating Intensity Principles Into Inpatient Stroke Rehabilitation. Arch. Rehabil. Res. Clin. Transl. 2020, 2, 100052. [Google Scholar] [CrossRef]
- Da Rosa Pinheiro, D.R.; Cabeleira, M.E.P.; da Campo, L.A.; Correa, P.S.; Blauth, A.; Cechetti, F. Effects of aerobic cycling training on mobility and functionality of acute stroke subjects: A randomized clinical trial. Neurorehabilitation 2021, 48, 39–47. [Google Scholar] [CrossRef]
- Marzolini, S.; Brooks, D.; Oh, P.; Jagroop, D.; MacIntosh, B.J.; Anderson, N.D.; Alter, D.; Corbett, D. Aerobic With Resistance Training or Aerobic Training Alone Poststroke: A Secondary Analysis From a Randomized Clinical Trial. Neurorehabilit. Neural Repair 2018, 32, 209–222. [Google Scholar] [CrossRef]
- Lee, M.J.; Kilbreath, S.L.; Singh, M.F.; Zeman, B.; Davis, G.M. Effect of progressive resistance training on muscle performance after chronic stroke. Med. Sci. Sports Exerc. 2010, 42, 23–34. [Google Scholar] [CrossRef]
- Lee, M.J.; Kilbreath, S.L.; Singh, M.F.; Zeman, B.; Lord, S.R.; Raymond, J.; Davis, G.M. Comparison of effect of aerobic cycle training and progressive resistance training on walking ability after stroke: A randomized sham exercise-controlled study. J. Am. Geriatr. Soc. 2008, 56, 976–985. [Google Scholar] [CrossRef]
- Lee, Y.H.; Park, S.H.; Yoon, E.S.; Lee, C.D.; Wee, S.O.; Fernhall, B.; Jae, S.Y. Effects of combined aerobic and resistance exercise on central arterial stiffness and gait velocity in patients with chronic poststroke hemiparesis. Am. J. Phys. Med. Rehabil. 2015, 94, 687–695. [Google Scholar] [CrossRef]
- Teixeira-Salmela, L.F.; Olney, S.J.; Nadeau, S.; Brouwer, B. Muscle strengthening and physical conditioning to reduce impairment and disability in chronic stroke survivors. Arch. Phys. Med. Rehabil. 1999, 80, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Jiang, Y.; Wei, Q.; Wang, B.; Ma, G. Intensive aerobic cycling training with lower limb weights in Chinese patients with chronic stroke: Discordance between improved cardiovascular fitness and walking ability. Disabil. Rehabil. 2012, 34, 1665–1671. [Google Scholar] [CrossRef] [PubMed]
- Son, S.M.; Park, M.K.; Lee, N.K. Influence of Resistance Exercise Training to Strengthen Muscles across Multiple Joints of the Lower Limbs on Dynamic Balance Functions of Stroke Patients. J. Phys. Ther. Sci. 2014, 26, 1267–1269. [Google Scholar] [CrossRef] [PubMed]
- Vahlberg, B.; Lindmark, B.; Zetterberg, L.; Hellstrom, K.; Cederholm, T. Body composition and physical function after progressive resistance and balance training among older adults after stroke: An exploratory randomized controlled trial. Disabil. Rehabil. 2017, 39, 1207–1214. [Google Scholar] [CrossRef]
- Hill, T.R.; Gjellesvik, T.I.; Moen, P.M.; Tørhaug, T.; Fimland, M.S.; Helgerud, J.; Hoff, J. Maximal strength training enhances strength and functional performance in chronic stroke survivors. Am. J. Phys. Med. Rehabil. 2012, 91, 393–400. [Google Scholar] [CrossRef]
- Hyun, S.J.; Lee, J.; Lee, B.H. The Effects of Sit-to-Stand Training Combined with Real-Time Visual Feedback on Strength, Balance, Gait Ability, and Quality of Life in Patients with Stroke: A Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2021, 18, 12229. [Google Scholar] [CrossRef]
- Huang, W.Y.; Li, M.H.; Lee, C.H.; Tuan, S.H.; Sun, S.F.; Liou, I.H. Efficacy of lateral stair walking training in patients with chronic stroke: A pilot randomized controlled study. Gait Posture 2021, 88, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Hafer-Macko, C.E.; Ryan, A.S.; Ivey, F.M.; Macko, R.F. Skeletal muscle changes after hemiparetic stroke and potential beneficial effects of exercise intervention strategies. J. Rehabil. Res. Dev. 2008, 45, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Ivey, F.M.; Prior, S.J.; Hafer-Macko, C.E.; Katzel, L.I.; Macko, R.F.; Ryan, A.S. Strength Training for Skeletal Muscle Endurance after Stroke. J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Assoc. 2017, 26, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, S.M.; Ziai, W.C.; Cordonnier, C.; Dowlatshahi, D.; Francis, B.; Goldstein, J.N.; Hemphill, J.C., 3rd; Johnson, R.; Keigher, K.M.; Mack, W.J.; et al. 2022 Guideline for the Management of Patients With Spontaneous Intracerebral Hemorrhage: A Guideline From the American Heart Association/American Stroke Association. Stroke 2022, 53, e282–e361. [Google Scholar] [CrossRef] [PubMed]
- Teasell, R.; Salbach, N.M.; Foley, N.; Mountain, A.; Cameron, J.I.; Jong, A.d.; Acerra, N.E.; Bastasi, D.; Carter, S.L.; Fung, J.; et al. Canadian Stroke Best Practice Recommendations: Rehabilitation, Recovery, and Community Participation following Stroke. Part One: Rehabilitation and Recovery Following Stroke; 6th Edition Update 2019. Int. J. Stroke 2020, 15, 763–788. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.; Sinacore, D.R.; Host, H.H. The relationship of strength to function in the older adult. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 1995, 50, 55–59. [Google Scholar] [CrossRef]
- Severinsen, K.; Jakobsen, J.K.; Overgaard, K.; Andersen, H. Normalized muscle strength, aerobic capacity, and walking performance in chronic stroke: A population-based study on the potential for endurance and resistance training. Arch. Phys. Med. Rehabil. 2011, 92, 1663–1668. [Google Scholar] [CrossRef]
- Lamberti, N.; Straudi, S.; Malagoni, A.M.; Argiro, M.; Felisatti, M.; Nardini, E.; Zambon, C.; Basaglia, N.; Manfredini, F. Effects of low-intensity endurance and resistance training on mobility in chronic stroke survivors: A pilot randomized controlled study. Eur. J. Phys. Rehabil. Med. 2017, 53, 228–239. [Google Scholar] [CrossRef]
- Munari, D.; Pedrinolla, A.; Smania, N.; Picelli, A.; Gandolfi, M.; Saltuari, L.; Schena, F. High-intensity treadmill training improves gait ability, VO2peak and cost of walking in stroke survivors: Preliminary results of a pilot randomized controlled trial. Eur. J. Phys. Rehabil. Med. 2018, 54, 408–418. [Google Scholar] [CrossRef]
- Megna, M.; Marvulli, R.; Farì, G.; Gallo, G.; Dicuonzo, F.; Fiore, P.; Ianieri, G. Pain and Muscles Properties Modifications After Botulinum Toxin Type A (BTX-A) and Radial Extracorporeal Shock Wave (rESWT) Combined Treatment. Endocr. Metab. Immune Disord. Drug Targets 2019, 19, 1127–1133. [Google Scholar] [CrossRef]


| First Author | Groups | N | Dose of Interventions | Description of the Multimodal Treatment | Description of the Unimodal Treatment | Outcome Measures | Conclusions |
|---|---|---|---|---|---|---|---|
| Bowden, M.G. (2020) [20] |
|
|
| Walking gait intensity: 110–125% of SSWS (self-selected walking speed), strength intensity: 75% of 1RM (repetition maximum), and a cardiovascular training HR (heart rate) target: ranging from 60% to 80% of the maximum HR. | Usual care, defined as the physical therapy treatment normally provided at each individual facility. | TUG test (Timed Up and Go), 10MWT (10-metre walking test), 2MWT (2-minute walk test), 5xSTS test (five times Sit to Stand), Tinetti (POMA), FIM (Functional Independence Measure) | Both the intervention and control groups improved significantly in each outcome measure, but the change scores from admission to discharge were consistently larger in the intervention group for all variables except the 5xSTS. An increased intervention intensity during the inpatient rehabilitation stay was a simple way to maximise patient function. |
| da Rosa Pinheiro, D.R. (2021) [21] |
|
|
| One conventional physiotherapy session (5′ of stretch and strength exercises for biceps, triceps, quadriceps, hamstrings, gastrocnemius, 5′ of trunk control training and balance training, 5′ walking, and 5′ of breathing exercises) + one cycling session with an electric cycle ergometer (passive, active, and resistance exercises, with a biofeedback system for strength symmetry). | Conventional physiotherapy session (5′ of stretch and strength exercises for biceps, triceps, quadriceps, hamstrings, and gastrocnemius, 5′ of trunk control training and balance training, 5′ walking, and 5′ of breathing exercises). | Digital dynamometer (muscle strength), 10MWT, BBS (Berg Balance Scale), ICU Mobility Scale, Perme Score | Aerobic cycling training alongside conventional physiotherapy was effective in improving lower limb muscle strength, gait speed, balance, mobility and functionality. |
| Jin, H. (2012) [27] |
|
|
| Aerobic cycling training with a target aerobic intensity of 50–70% HRR (heart rate reserve). Initial low intensity (40–50% HRR) for 5′ to 10′ increased 5′ every 2 weeks; intensity increased by 5% HRR every 2 weeks. Added 3% of body weight only for the paretic limb, 6′–10′ pedaled and 2′–3′ of rest. | Low-intensity (20–30% HRR) overground walking training. | Isokinetic dynamometer (knee muscle strength), 6MWT (6-minute walking test), peak VO2, BBS, modified Ashworth scale | The intensive aerobic cycling training with lower limb weights improve both cardiovascular fitness and walking ability, but the enhancements in cardiovascular fitness induced with training were not associated with the increases in walking capacity. |
| Lee, M.J. (2010) [23] |
|
|
| Progressive resistance training (PRT) consisting of two sets of eight repetitions at 50% of 1RM to start, then progressive to 80% + Aerobic cycle training consisting of 30′ of isokinetic leg cycling at 50% of VO2 peak to start, then progressive to 85%. | Sham PRT consisting of bilateral leg exercises using the same resistance of a training machine, but without any resistance other than the weight of the bar or gravity + sham cycling consisting of 30′ on motorised leg-passive cycling without any voluntary contraction. | Dynamometer (maximal force muscle), W (maximal muscle power), 1 TM, repetitions (muscle endurance) | Individuals who undertook PRT improved their muscle performance such as strength, peak power, and muscle endurance measures in both the affected and nonaffected lower limbs. |
| Lee, M.J. (2008) [24] |
|
|
| Included 30′ cycling with motomed set at 40 rev/min and HR 50% of VO2 peak for 1–2 weeks, increased to 70% by week 4. After cycling, there is sham resistance training with two sets of eight repetitions for each exercise. PRT with pneumatic resistance equipment, two sets of eight repetitions unilaterally at 50% of baseline 1RM and progression to 80% by week 2. | Sham PRT consisting of bilateral leg exercises using the same resistance of a training machine but without any resistance other than the weight of the bar or gravity + sham cycling consisting of 30′ on motorised leg-passive cycling without any voluntary contraction. | Gait velocity, 6MWT, 10MWT, peak of HR and VO2, 1RM, dynamometer (muscle strength), SF-36 Questionnaire | Single-modality exercises targeted at existing impairments did not optimally address the functional deficits of walking but did ameliorate the underlying impairments. The underlying cardiovascular and musculoskeletal impairments were significantly modifiable years after stroke with targeted robust exercise. |
| Lee, Y.H. (2015) [25] |
|
|
| Each exercise intervention comprised a 5′ warm-up (standardised whole-body stretching, light walking, 10 stretching movement), a 30′ aerobic exercise (walking exercise, 10′ fast walking on a sloping way, 10′ walking in up-stairs), a 20′ resistance exercise (using elastic bands, lunges, squats, hip flexion/extension, hip abduction/adduction, knee flexion/extension, shoulder abduction/adduction, shoulder flexion/extension, and abdominal crunch/back extension), and a 5′ cool down (standardised whole-body stretching, light walking). AN exercise intensity target was established (60–70 HRR). | Unsystematic physical activities, no exercise intervention. They were asked to continue their normal daily activities. | TUG test, 6MWT, 10MWT, grip strength, CS30 test (30′′ Chair-Stand), CSR (Chair Sit and Reach), FRT (Functional Reach Test) | The combined aerobic and resistance exercise program significantly reduced central arterial stiffness and increased gait velocity in patients with chronic poststroke hemiparesis. |
| Marzolini, S. (2018) [22] |
|
|
| Aerobic training (walking with stationary recumbent/upright cycling) + resistance training (multi-joint and single-joint exercises. One to two sets with 10/11 exercises: lunge, squat, abdominal curl-up, heel raise, bicep curl, supine triceps extension, affected-side hip flexion/extension, affected-side ankle dorsiflexion, single-limb knee extension, and flexion. Initially 50% or 60% 1RM then 70%). | Aerobic training (walking with stationary recumbent/upright cycling). | Sit-to-stand, 6MWT, stair climbing performance, VO2 peak, muscular strength | Despite the lack of advantage in 6MWT, combined training enhanced stroke recovery by improving components of cardiorespiratory fitness, muscular strength, and muscle mass accretion. |
| Son, S.M. (2014) [28] |
|
|
| Joint mobilization, muscle strengthening, balance training, resistance exercise training in a sitting position with a leg press (three sets—8 to 10 repetitions at 70% of 1RM). | Joint mobilization, muscle strengthening, and balance training. | BBS, TUG test, A-P (antero, posterior), M-L (medio, lateral) sway distances | Training involving muscle strength across multiple joints was an effective intervention for an improvement in the dynamic balance function of stroke patients. |
| Teixeira-Salmela, L.F. (1999) [26] |
|
|
| Each supervised training session included: 5′ to 10′ warm-up (calisthenics, mild exercises, ROM exercises), aerobic exercises (stepping or cycling with a HR 70% target), strength training, cool-down with relax, and strenght exercises. | No intervention. | Isokinetic peak, gait speed, stair climbing, HAP (Human Activity Profile), NHP (Nottingham Health Profile) | The 10-week combined program of muscle strengthening and physical conditioning resulted in gains in all measures of impairment and disability. These gains were not associated with measurable changes in spasticity in either the quadriceps or ankle plantarflexors. |
| Vahlberg, B. (2017) [29] |
|
|
| PRB (Progressive Resistance Balance) training including 10′ warm-up (stationary cycling or walking), 45′ circuit class, and 20′ motivational session (discussions on issues and goals). Exercises followed HIFE (high-intensity functional exercise) program and consisted of lower limb strength and balance exercises, such as rising from a seated position and squats in parallel or walking stance or walking on a soft surface. | Usual care, individuals were encouraged to continue their regular activities. | PASE (Physical Activity Scale for the Elderly), 6MWT, BBS, SPPB (Short Physical Performance Battery), SPMSQ (Short Portable Mental Status Questionnaire), CRS (disease core risk), cholesterol HDL/LDL, BMI | Three-month progressive resistance and balance training was associated with reduced fat mass, which was related to improvements in walking capacity in older adults approximately one year after stroke. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lando, A.; Cacciante, L.; Mantineo, A.; Baldan, F.; Pillastrini, P.; Turolla, A.; Pregnolato, G. Multi-Modal versus Uni-Modal Treatment for the Recovery of Lower Limb Motor Function in Patients after Stroke: A Systematic Review with Meta-Analysis. Healthcare 2024, 12, 189. https://doi.org/10.3390/healthcare12020189
Lando A, Cacciante L, Mantineo A, Baldan F, Pillastrini P, Turolla A, Pregnolato G. Multi-Modal versus Uni-Modal Treatment for the Recovery of Lower Limb Motor Function in Patients after Stroke: A Systematic Review with Meta-Analysis. Healthcare. 2024; 12(2):189. https://doi.org/10.3390/healthcare12020189
Chicago/Turabian StyleLando, Alex, Luisa Cacciante, Alessio Mantineo, Francesca Baldan, Paolo Pillastrini, Andrea Turolla, and Giorgia Pregnolato. 2024. "Multi-Modal versus Uni-Modal Treatment for the Recovery of Lower Limb Motor Function in Patients after Stroke: A Systematic Review with Meta-Analysis" Healthcare 12, no. 2: 189. https://doi.org/10.3390/healthcare12020189
APA StyleLando, A., Cacciante, L., Mantineo, A., Baldan, F., Pillastrini, P., Turolla, A., & Pregnolato, G. (2024). Multi-Modal versus Uni-Modal Treatment for the Recovery of Lower Limb Motor Function in Patients after Stroke: A Systematic Review with Meta-Analysis. Healthcare, 12(2), 189. https://doi.org/10.3390/healthcare12020189










