Traumatic Aneurysm Involving the Posterior Communicating Artery
Abstract
:1. Introduction
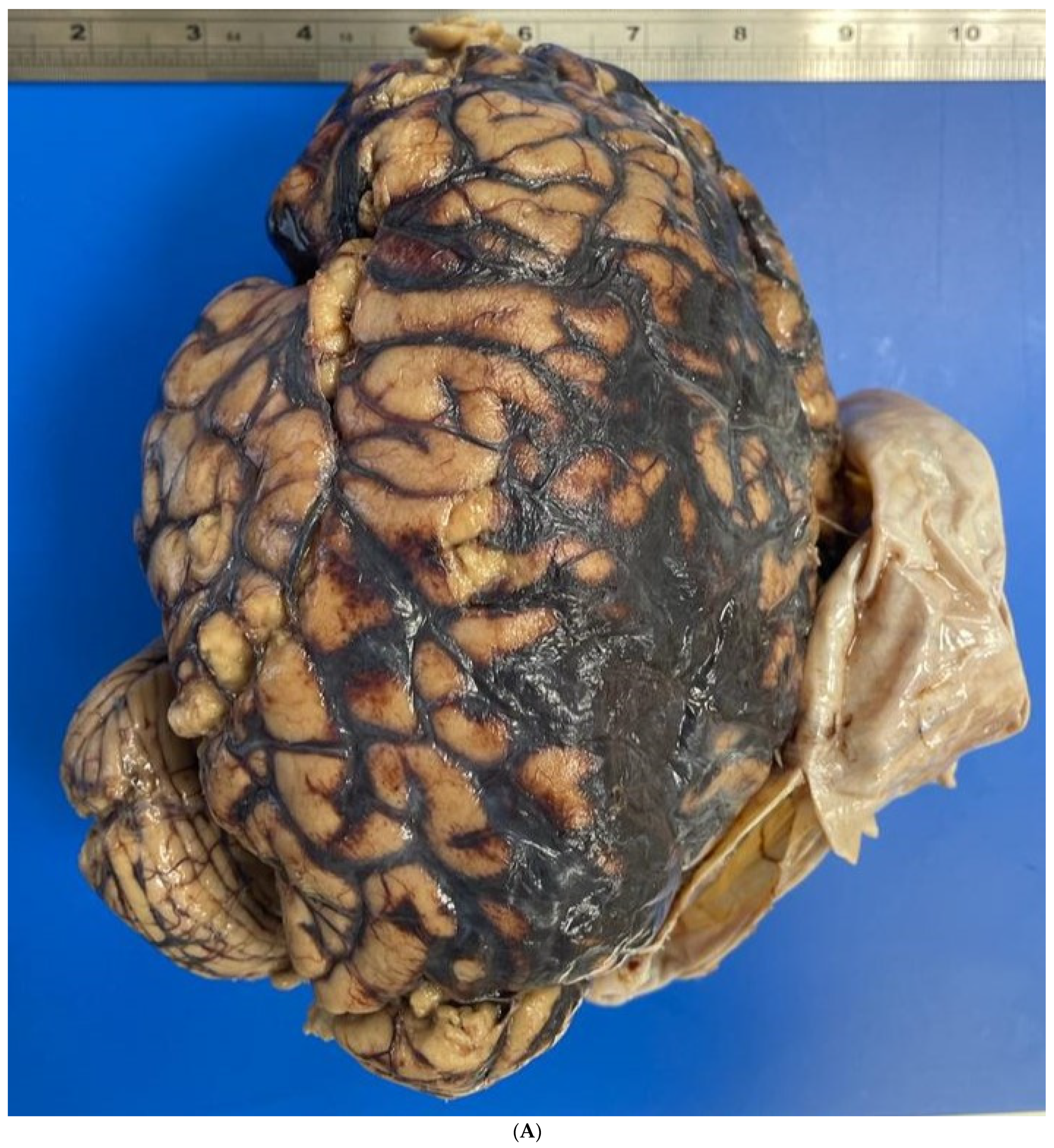
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Larson, P.S.; Reisner, A.; Morassutti, D.J.; Abdulhadi, B.; Harpring, J.E. Traumatic Intracranial Aneurysms. Neurosurg. Focus 2000, 8, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Dubey, A.; Sung, W.-S.; Chen, Y.-Y.; Amato, D.; Mujic, A.; Waites, P.; Erasmus, A.; Hunn, A. Traumatic Intracranial Aneurysm: A Brief Review. J. Clin. Neurosci. 2008, 15, 609–612. [Google Scholar] [CrossRef] [PubMed]
- Martin, E.M.; Hummelgard, A.B. Traumatic Aneurysms. J. Neurosci. Nurs. 1986, 18, 89–94. [Google Scholar] [CrossRef]
- Krauland, W.; Kugler, B.; Maxeiner, H. Aneurysms of the cerebral arteries at the base of the brain and trauma. Beitr. Gerichtl. Med. 1982, 40, 145–161. [Google Scholar] [PubMed]
- Froelich, J.J.; Neilson, S.; Peters-Wilke, J.; Dubey, A.; Thani, N.; Erasmus, A.; Carr, M.W.; Hunn, A.W.M. Size and Location of Ruptured Intracranial Aneurysms: A 5-Year Clinical Survey. World Neurosurg. 2016, 91, 260–265. [Google Scholar] [CrossRef]
- Holmes, B.; Harbaugh, R.E. Traumatic Intracranial Aneurysms: A Contemporary Review. J. Trauma 1993, 35, 855–860. [Google Scholar] [CrossRef]
- Vlak, M.H.M.; Rinkel, G.J.E.; Greebe, P.; van der Bom, J.G.; Algra, A. Trigger Factors and Their Attributable Risk for Rupture of Intracranial Aneurysms: A Case-Crossover Study. Stroke 2011, 42, 1878–1882. [Google Scholar] [CrossRef]
- Fisher: Forensic Pathology: A Handbook for Pathologists. Available online: https://scholar.google.com/scholar_lookup?journal=Am.+J.+Forensic+Med.+Pathol.&title=Forensic+Pathology,+A+handbook+for+pathologist.&author=R.S.+Fisher&author=C.S.+Petty&volume=1&issue=3&publication_year=2006&pages=286&doi=10.1097/00000433-198009000-00022& (accessed on 9 December 2023).
- Squier, W.; Mack, J. The Neuropathology of Infant Subdural Haemorrhage. Forensic Sci. Int. 2009, 187, 6–13. [Google Scholar] [CrossRef]
- Del Duca, F.; Maiese, A.; Spina, F.; Visi, G.; La Russa, R.; Santoro, P.; Pignotti, M.S.; Frati, P.; Fineschi, V. Idiopathic Pulmonary Hemorrhage in Infancy: A Case Report and Literature Review. Diagnostics 2023, 13, 1270. [Google Scholar] [CrossRef]
- Steinmann, J.; Hartung, B.; Bostelmann, R.; Kaschner, M.; Karadag, C.; Muhammad, S.; Li, L.; Büttner, A.; Petridis, A.K. Rupture of Intracranial Aneurysms in Patients with Blunt Head Trauma: Review of the Literature. Clin. Neurol. Neurosurg. 2020, 199, 106208. [Google Scholar] [CrossRef]
- Takayama, M.; Waters, B.; Fujii, H.; Hara, K.; Kashiwagi, M.; Matsusue, A.; Ikematsu, N.; Kubo, S.I. Subarachnoid hemorrhage in a Japanese cocaine abuser: Cocaine-related sudden death. Leg. Med. 2018, 32, 43–47. [Google Scholar] [CrossRef] [PubMed]
- McCormick, W.K. The Relationship of Closed-Head Trauma to Rupture of Saccular Intracranial Aneurysms. Am. J. Forensic Med. Pathol. 1980, 1, 223–226. [Google Scholar] [CrossRef]
- Skowronek, R.; Kobek, M.; Jankowski, Z.; Zielińska-Pająk, E.; Pałasz, A.; Pilch-Kowalczyk, J.; Kwarta, R.; Rygol, K.; Szczepański, M.; Chowaniec, C. Traumatic Basal Subarachnoid Haemorrhage or Ruptured Brain Aneurysm in 16-Year-Old Boy?—Case Report. Arch. Forensic Med. Criminol. 2016, 1, 32–40. [Google Scholar] [CrossRef]
- Palmieri, M.; Pesce, A.; Zancana, G.; Armocida, D.; Maiese, A.; Cirelli, C.; Santoro, A.; Frati, P.; Fineschi, V.; Frati, A. Post-Traumatic Intracranial Pseudo-Aneurysms of Posterior Circulation: A Comprehensive Review of an under-Diagnosed and Rare Entity. Neurosurg. Rev. 2022, 45, 1019–1029. [Google Scholar] [CrossRef] [PubMed]
- Bratzke, H.; Püschel, K.; Colmant, H.J. Zur Phänomenologie und Morphologie spontaner tödlicher Hirnaneurysmablutungen. Z. Rechtsmed. 1986, 96, 245–273. [Google Scholar] [CrossRef]
- Chen, H.-J.; Liang, C.-L.; Lu, K.; Lui, C.-C. Rapidly Growing Internal Carotid Artery Aneurysm After Amphetamine Abuse: Case Report. Am. J. Forensic Med. Pathol. 2003, 24, 32–34. [Google Scholar] [CrossRef] [PubMed]
- Stehbens, W.E. Etiology of Intracranial Berry Aneurysms. J. Neurosurg. 1989, 70, 823–831. [Google Scholar] [CrossRef]
- Juvela, S. Alcohol Consumption as a Risk Factor for Poor Outcome after Aneurysmal Subarachnoid Haemorrhage. BMJ 1992, 304, 1663–1667. [Google Scholar] [CrossRef]
- Juvela, S.; Hillbom, M.; Numminen, H.; Koskinen, P. Cigarette Smoking and Alcohol Consumption as Risk Factors for Aneurysmal Subarachnoid Hemorrhage. Stroke 1993, 24, 639–646. [Google Scholar] [CrossRef]
- Fuentes, A.M.; Stone McGuire, L.; Amin-Hanjani, S. Sex Differences in Cerebral Aneurysms and Subarachnoid Hemorrhage. Stroke 2022, 53, 624–633. [Google Scholar] [CrossRef]
- Davis, N.L.; Horan, P.M.; Romich, T.; Roman, J.L.; Lacy, J.M.; Catanese, C.A. Giant Berry Aneurysms in a Forensic Setting: A Series of 14 Cases. Am. J. Forensic Med. Pathol. 2010, 31, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Hamburger, C.; Schönberger, J.; Lange, M. Management and Prognosis of Intracranial Giant Aneurysms. A Report on 58 Cases. Neurosurg. Rev. 1992, 15, 97–103. [Google Scholar] [CrossRef]
- Fowler, J.; Fiani, B.; Quadri, S.A.; Cortez, V.; Frooqui, M.; Zafar, A.; Ahmed, F.S.; Ikram, A.; Ramachandran, A.; Siddiqi, J. Impact of Methamphetamine Abuse: A Rare Case of Rapid Cerebral Aneurysm Growth with Review of Literature. Case Rep. Neurol. Med. 2018, 2018, 1879329. [Google Scholar] [CrossRef]
- Mcevoy, N.D.; Kitchen, D.G.T.; Thom, A.W. Intracerebral Haemorrhage and Drug Abuse in Young Adults. Br. J. Neurosurg. 2000, 14, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Penn, D.L.; Witte, S.R.; Komotar, R.J.; Sander Connolly, E. The Role of Vascular Remodeling and Inflammation in the Pathogenesis of Intracranial Aneurysms. J. Clin. Neurosci. 2014, 21, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Büttner, A.; Mall, G.; Penning, R.; Sachs, H.; Weis, S. The Neuropathology of Cocaine Abuse. Leg. Med. 2003, 5, S240–S242. [Google Scholar] [CrossRef]
- Krauland, W. Die traumatische subarachnoidale Blutung. Z. Rechtsmed. 1981, 87, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Leestma, J.E. Forensic Neuropathology, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2014; pp. 549–552. [Google Scholar]
- Shibata, A.; Kamide, T.; Ikeda, S.; Yoshikawa, S.; Tsukagoshi, E.; Yonezawa, A.; Takeda, R.; Kikkawa, Y.; Kohyama, S.; Kurita, H. Clinical and Morphological Characteristics of Ruptured Small (<5 mm) Posterior Communicating Artery Aneurysms. Asian J. Neurosurg. 2021, 16, 335–339. [Google Scholar]
- Huhtakangas, J.; Lehecka, M.; Lehto, H.; Jahromi, B.R.; Niemelä, M.; Kivisaari, R. CTA analysis and assessment of morphological factors related to rupture in 413 posterior communicating artery aneurysms. Acta Neurochir. 2017, 159, 1643–1652. [Google Scholar] [CrossRef]
- Napoletano, G.; Port, A.; Büttner, A. Forensic implications of giant aneurysms: Two autopsy cases. Minerva Forensic Med. 2023, 143, 70–75. [Google Scholar] [CrossRef]
- Guo, S.; Wu, X. An unruptured posterior communicating artery aneurysm ruptured during angiography: A case report. Medicine 2019, 98, 17785. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, H.; Bai, R. Traumatic aneurysm on the posterior cerebral artery following blunt trauma in a 14-year-old girl: Case report. Neuropediatrics 2011, 42, 204–206. [Google Scholar] [CrossRef]
- Takahashi, A.; Kamiyama, H.; Imamura, H.; Kitagawa, M.; Abe, H. “True” posterior communicating artery aneurysm—Report of two cases. Neurol. Med. Chir. 1992, 32, 338–341. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Li, Z.; Song, L.; Han, T.; Feng, Q.; Guo, Y.; Xu, J.; He, M.; You, C. Increased Apoptosis and Cysteinyl Aspartate Specific Protease-3 Gene Expression in Human Intracranial Aneurysm. J. Clin. Neurosci. 2007, 14, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Zhang, H.; Su, J. MicroRNA-29a Contributes to Intracranial Aneurysm by Regulating the Mitochondrial Apoptotic Pathway. Mol. Med. Rep. 2018, 18, 2945–2954. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Tian, H.; Jin, F.; Zhang, X.; Su, S.; Liu, Y.; Wen, Z.; He, X.; Li, X.; Duan, C. CypD Induced ROS Output Promotes Intracranial Aneurysm Formation and Rupture by 8-OHdG/NLRP3/MMP9 Pathway. Redox Biol. 2023, 67, 102887. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Liu, Y.; Li, C.; Qu, X.; Zheng, G.; Zhang, Q.; Pan, Z.; Wang, Y.; Rong, J. microRNA-331-3p Maintains the Contractile Type of Vascular Smooth Muscle Cells by Regulating TNF-α and CD14 in Intracranial Aneurysm. Neuropharmacology 2020, 164, 107858. [Google Scholar] [CrossRef]
- Manetti, A.C.; Maiese, A.; Baronti, A.; Mezzetti, E.; Frati, P.; Fineschi, V.; Turillazzi, E. MiRNAs as New Tools in Lesion Vitality Evaluation: A Systematic Review and Their Forensic Applications. Biomedicines 2021, 9, 1731. [Google Scholar] [CrossRef]
- Maiese, A.; Manetti, A.C.; Santoro, P.; Del Duca, F.; De Matteis, A.; Turillazzi, E.; Frati, P.; Fineschi, V. FOXO3 Depletion as a Marker of Compression-Induced Apoptosis in the Ligature Mark: An Immunohistochemical Study. Int. J. Mol. Sci. 2023, 24, 1396. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Napoletano, G.; Di Fazio, N.; Delogu, G.; Del Duca, F.; Maiese, A. Traumatic Aneurysm Involving the Posterior Communicating Artery. Healthcare 2024, 12, 192. https://doi.org/10.3390/healthcare12020192
Napoletano G, Di Fazio N, Delogu G, Del Duca F, Maiese A. Traumatic Aneurysm Involving the Posterior Communicating Artery. Healthcare. 2024; 12(2):192. https://doi.org/10.3390/healthcare12020192
Chicago/Turabian StyleNapoletano, Gabriele, Nicola Di Fazio, Giuseppe Delogu, Fabio Del Duca, and Aniello Maiese. 2024. "Traumatic Aneurysm Involving the Posterior Communicating Artery" Healthcare 12, no. 2: 192. https://doi.org/10.3390/healthcare12020192
APA StyleNapoletano, G., Di Fazio, N., Delogu, G., Del Duca, F., & Maiese, A. (2024). Traumatic Aneurysm Involving the Posterior Communicating Artery. Healthcare, 12(2), 192. https://doi.org/10.3390/healthcare12020192






