Aligning Cancer Research Priorities in Europe with Recommendations for Conquering Cancer: A Comprehensive Analysis
Abstract
1. Introduction
Conquering Cancer: A Mission within Reach
2. Materials and Methods
2.1. A Survey of Patients and Citizens
- (a)
- Priority Areas for Cancer Research
- (b)
- Differences in Priorities Based on Cancer Type
- Factors influencing cancer development and risk;
- Cancer prevention and early detection;
- Cancer biology and therapeutic approaches;
- Aging and its intersections with cancer;
- Cancer complications and survivorship;
- Data generation and utilization in cancer research.
2.2. IQVIA Data on Single Biomarkers
- Single-Biomarker Test Access: This parameter measures the average proportion of laboratories offering each single-biomarker test, either in-house or through referral. The access levels are categorized as high (>75%), medium (50–75%), and low (<50%).
- Timing: Timing refers to the average time from the availability of medicines to the availability of single-biomarker tests. It categorizes countries as “on time” (test available around the time of medicine launch) or “late” (a lag from medicine availability to test availability, i.e., >1 year).
- Reimbursement: Reimbursement is based on the average proportion of tests reported to be covered by public reimbursement. It is categorized as high (>90%), medium (75–90%), or low (<75%).
- Order Rate: The order rate is calculated based on the average order rates across focus biomarkers (PD-L1, EGFR, BRCA* (breast), BRCA (ovarian), NTRK, HER-2, ALK, MMR/MSI, KRAS /NRAS, BRAF, and ROS1). It is categorized as high (>75%), medium (50–75%), or low (<50%).
- High: Countries with a score ranging from 2.5 to 3.0;
- Medium: Countries with a score in the range of 1.5 to <2.5;
- Low: Countries with a score in the range of 1.0 to <1.5.
2.3. Statistical Analysis
3. Results
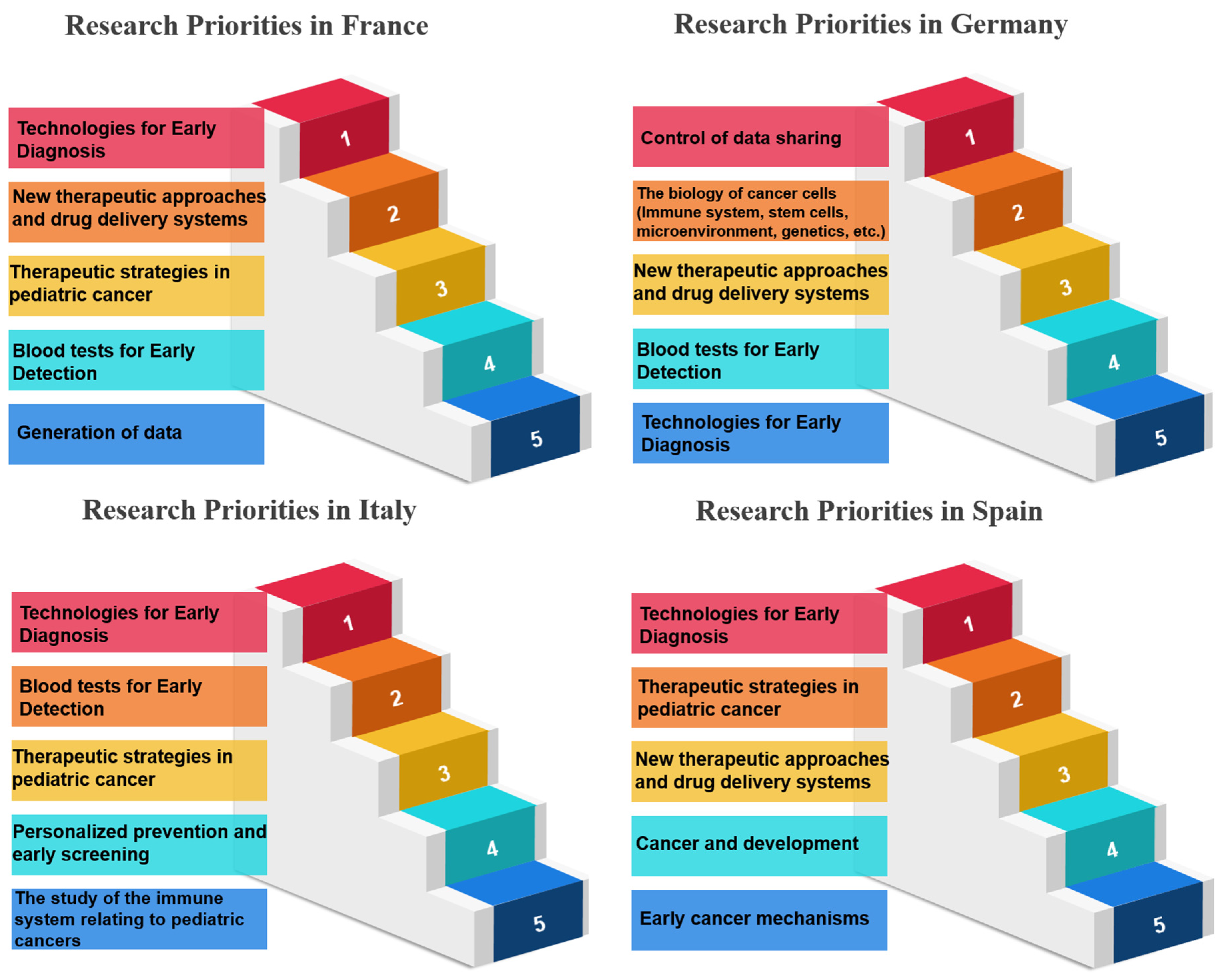
3.1. Research Priorities Based on Patients’ Responses—Countrywise
3.1.1. Correlation
3.1.2. Rank and Percentile Analysis
3.2. Research Priorities Based on Cancer Type
3.2.1. Correlation Analysis
3.2.2. Rank and Percentile Analysis
3.3. Correlation between Research Priorities and Access to Single Biomarkers
4. Discussion
4.1. Research Priorities
4.2. Correlations among Cancer Types and Research Priorities
4.2.1. Correlations among Cancer Types
4.2.2. Relation between Cancer Priorities and Access to Single-Biomarker Tests
4.3. Alignment with 13 Recommendations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Loud, J.T.; Murphy, J. Cancer Screening and Early Detection in the 21st Century. Semin. Oncol. Nurs. 2017, 33, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Crosby, D.; Bhatia, S.; Brindle, K.M.; Coussens, L.M.; Dive, C.; Emberton, M.; Esener, S.; Fitzgerald, R.C.; Gambhir, S.S.; Kuhn, P.; et al. Early detection of cancer. Science 2022, 375, eaay9040. [Google Scholar] [CrossRef] [PubMed]
- Batko, K.; Ślęzak, A. The use of Big Data Analytics in healthcare. J. Big Data 2022, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Europe’s Beating Cancer Plan. 2022. Available online: https://health.ec.europa.eu/system/files/2022-02/eu_cancer-plan_en_0.pdf (accessed on 3 November 2023).
- Horgan, D.; Baird, A.M.; Middleton, M.; Mihaylova, Z.; Van Meerbeeck, J.P.; Vogel-Claussen, J.; Van Schil, P.E.; Malvehy, J.; Ascierto, P.A.; Dube, F.; et al. How Can the EU Beating Cancer Plan Help in Tackling Lung Cancer, Colorectal Cancer, Breast Cancer and Melanoma? Healthcare 2022, 10, 1618. [Google Scholar] [CrossRef]
- OECD. Private Health Insurance Spending. 2022. Available online: https://www.oecd.org/health/Spending-on-private-health-insurance-Brief-March-2022.pdf (accessed on 28 September 2023).
- Montagu, D. The Provision of Private Healthcare Services in European Countries: Recent Data and Lessons for Universal Health Coverage in Other Settings. Front. Public Health 2021, 9, 636750. [Google Scholar] [CrossRef] [PubMed]
- Ecrin, Funding for Multinational Clinical Trials. 2023. Available online: https://ecrin.org/funding-multinational-clinical-trials (accessed on 3 November 2023).
- Lalova, T.; Padeanu, C.; Negrouk, A.; Lacombe, D.; Geissler, J.; Klingmann, I.; Huys, I. Cross-Border Access to Clinical Trials in the EU: Exploratory Study on Needs and Reality. Front. Med. 2020, 7, 585722. [Google Scholar] [CrossRef]
- The Evolution of Biomarker Use in Clinical Trials for Cancer Research. Personalized Medicine Coalition. 2020. Available online: https://www.personalizedmedicinecoalition.org/Userfiles/PMC-Corporate/file/The_Evolution_of_Biomarker_Use_in_Clinical_Trials_for_Cancer_Treatments.pdf (accessed on 23 October 2023).
- Tsimberidou, A.M.; Fountzilas, E.; Nikanjam, M.; Kurzrock, R. Review of precision cancer medicine: Evolution of the treatment paradigm. Cancer Treat. Rev. 2020, 86, 102019. [Google Scholar] [CrossRef]
- Normanno, N.; Apostolidis, K.; Wolf, A.; Al Dieri, R.; Deans, Z.; Fairley, J.; Maas, J.; Martinez, A.; Moch, H.; Nielsen, S.; et al. Access and quality of biomarker testing for precision oncology in Europe. Eur. J. Cancer 2022, 176, 70–77. [Google Scholar] [CrossRef]
- Grill, C. Involving stakeholders in research priority setting: A scoping review. Res. Involv. Engagem. 2021, 7, 75. [Google Scholar] [CrossRef]
- European Commission. Pharmaceutical Strategy for Europe. 2020. Available online: https://health.ec.europa.eu/system/files/202011/pharmastrategy_consultationreport_en_0.pdf (accessed on 3 November 2023).
- Glasgow, R.E.; Brtnikova, M.; Dickinson, L.M.; Carroll, J.K.; Studts, J.L. Implementation strategies preferred by primary care clinicians to facilitate cancer prevention and control activities. J. Behav. Med. 2023, 46, 821–836. [Google Scholar] [CrossRef]
- Brtnikova, M.; Studts, J.L.; Robertson, E.; Dickinson, L.M.; Carroll, J.K.; Krist, A.H.; Cronin, J.T.; Glasgow, R.E. Priorities for improvement across cancer and noncancer related preventive services among rural and nonrural clinicians. BMC Prim. Care 2022, 23, 23. [Google Scholar] [CrossRef] [PubMed]
- Terry, R.F.; Charles, E.; Purdy, B.; Sanford, A. An analysis of research priority-setting at the World Health Organization—How mapping to a standard template allows for comparison between research priority-setting approaches. Health Res. Policy Syst. 2018, 16, 116. [Google Scholar] [CrossRef] [PubMed]
- European Commission, Directorate-General for Research and Innovation; Pita Barros, P.; Beets-Tan, R.; Chomienne, C.; Ghiorghiu, S.; Godfrey, F.; Ladenstein, R.; Leja, M.; Mäkelä, T.; Metspalu, A.; et al. Conquering Cancer—Mission Possible; Publications Office of the European Union: Luxembourg, 2020; Available online: https://data.europa.eu/doi/10.2777/045403 (accessed on 2 November 2023).
- Uncan.eu. 2023. Available online: https://uncan.eu/ (accessed on 31 October 2023).
- Solary, E.; Blanc, P.; Boutros, M.; Girvalaki, C.; Locatelli, F.; Medema, R.H.; Nagy, P.; Tabernero, J. UNCAN. eu, a European initiative to UNderstand CANcer. Cancer Discov. 2022, 12, 2504–2508. [Google Scholar] [CrossRef]
- Mukaka, M.M. Statistics corner: A guide to appropriate use of correlation coefficient in medical research. Malawi Med. J. 2012, 24, 69–71. [Google Scholar]
- Bornmann, L.; Williams, R. An evaluation of percentile measures of citation impact, and a proposal for making them better. Scientometrics 2020, 124, 1457–1478. [Google Scholar] [CrossRef]
- Haller, F.; Knopf, J.; Ackermann, A.; Bieg, M.; Kleinheinz, K.; Schlesner, M.; Moskalev, E.A.; Will, R.; Satir, A.A.; Abdelmagid, I.E.; et al. Paediatric and adult soft tissue sarcomas with NTRK1 gene fusions: A subset of spindle cell sarcomas unified by a prominent myopericytic/haemangiopericytic pattern. J. Pathol. 2016, 238, 700–710. [Google Scholar] [CrossRef]
- Erikson, C.; Salsberg, E.; Forte, G.; Bruinooge, S.; Goldstein, M.; Quinlan, R. Future supply and demand for oncologists: Challenges to assuring access to oncology services. J. Oncol. Pract. 2018, 14, e412–e421. [Google Scholar] [CrossRef]
- Snyder, M.; Bottiglieri, S.; Almhanna, K. The impact of primary tumor location on first-line bevacizumab or cetuximab in metastatic colorectal cancer: A Dutch population-based study. Cancer Med. 2018, 7, 5739–5747. [Google Scholar]
- Abbosh, C.; Birkbak, N.J.; Wilson, G.A.; Jamal-Hanjani, M.; Constantin, T.; Salari, R.; Le Quesne, J.; Moore, D.A.; Veeriah, S.; Rosenthal, R.; et al. Phylogenetic ctDNA analysis depicts early-stage lung cancer evolution. Nature 2017, 545, 446–451. [Google Scholar] [CrossRef]
- Kramer, I.; Lippert, H.; Bruns, C.J. Effective treatment and long-term survival after gastric cancer surgery in community hospitals. Eur. J. Surg. Oncol. 2018, 44, 713–718. [Google Scholar]
- Wang, R.C.; Wang, Z. Precision Medicine: Disease Subtyping and Tailored Treatment. Cancers 2023, 15, 3837. [Google Scholar] [CrossRef]
- Meacham, C.E.; Morrison, S.J. Tumour heterogeneity and cancer cell plasticity. Nature 2013, 501, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Visvader, J.E.; Lindeman, G.J. Cancer stem cells: Current status and evolving complexities. Cell Stem Cell. 2012, 10, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Heitzer, E.; Haque, I.S.; Roberts CE, S.; Speicher, M. Current and future perspectives of liquid biopsies in genomics-driven oncology. Nat. Rev. Genet. 2019, 20, 71–88. [Google Scholar] [CrossRef] [PubMed]
- Moyer, V.A. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann. Intern. Med. 2012, 157, 120–134. [Google Scholar] [CrossRef] [PubMed]
- Boccia, S.; Pastorino, R.; Ricciardi, G.; Adany, R.; Barnhoorn, F.; Boffetta, P.; Boretti, F.; Cornel, M.; De Vito, C.; Gray, M.; et al. Personalized medicine: A new option for early diagnosis and treatment of non-small cell lung cancer. Ther. Adv. Med. Oncol. 2016, 8, 179–187. [Google Scholar]
- Murphy, K.M.; Levis, M.; Hafez, M.J.; Geiger, T.; Cooper, L.C.; Smith, B.D.; Small, D.; Berg, K.D. Detection of FLT3 internal tandem duplication and D835 mutations by a multiplex polymerase chain reaction and capillary electrophoresis assay. J. Mol. Diagn. 2017, 19, 575–583. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Eccles, S.A.; Aboagye, E.O.; Ali, S.; Anderson, A.S.; Armes, J.; Berditchevski, F.; Blaydes, J.P.; Brennan, K.; Brown, N.J.; Bryant, H.E.; et al. Critical research gaps and translational priorities for the successful prevention and treatment of breast cancer. Breast Cancer Res. 2013, 15, R92. [Google Scholar] [CrossRef]
- Corner, J.; Wright, D.; Hopkinson, J.; Gunaratnam, Y.; McDonald, J.W.; Foster, C. The research priorities of patients attending UK cancer treatment centres: Findings from a modified nominal group study. Br. J. Cancer 2007, 96, 875–881. [Google Scholar] [CrossRef]
- Moore, C.M.; King, L.E.; Withington, J.; Amin, M.B.; Andrews, M.; Briers, E.; Chen, R.C.; Chinegwundoh, F.I.; Cooperberg, M.R.; Crowe, J.; et al. Best Current Practice and Research Priorities in Active Surveillance for Prostate Cancer—A Report of a Movember International Consensus Meeting. Eur. Urol. Oncol. 2023, 6, 160–182. [Google Scholar] [CrossRef]





| Recommendation | Short Description |
|---|---|
| 1. Launch UNCAN.eu—a European initiative to understand cancer | UNCAN.eu would integrate innovative models and technologies with longitudinal patient data, samples, and biomarkers to identify and translate to patients. |
| 2. Develop an EU-wide research program to identify (poly-)genic risk scores | The research program would promote the clinical validation of polygenic risk scores (PRS), educational activities on the clinical importance of polygenic risk to all citizens regardless of their age, and encourage public debate on their use and control. |
| 3. Support the development and implementation of effective cancer prevention strategies and policies within Member States and the EU | The Mission Board proposes a research program to identify strategies for cancer prevention and provide up-to-date knowledge to EU institutions and countries. |
| 4. Optimize existing screening programs and develop novel approaches for screening and early detection | The program will optimize existing screening programs, develop new approaches for early detection of cancers, and include individualized approaches to screening. |
| 5. Advance and implement personalized medicine approaches for all cancer patients in Europe | To increase its effectiveness, this recommendation encourages the optimization, implementation, and scaling of personalized medicine approaches for cancer. |
| 6. Develop an EU-wide research program on early diagnostics and minimally invasive treatment | Accurate diagnostic methods are important to detect tumors at an early stage, predict treatment response, and detect tumor regrowth. |
| 7. Develop an EU-wide research program and policy support to improve the quality of life of cancer patients and survivors, family members and carers, and all persons with an increased risk of cancer | Supportive policies need to be developed to identify and monitor physical and mental health problems among patients and survivors. |
| 8. Create a European Cancer Patient Digital Centre where cancer patients and survivors can deposit and share their data for personalized care | The proposal is to create the European Cancer Patient Digital Centre (ECPDC), a network where cancer patients and survivors can deposit their medical data in a standardized and ethical way. |
| 9. Achieve cancer health equity in the EU across the continuum of the disease | Policy support and interventions are necessary to address existing inequities across and within Member States. |
| 10. Set up a network of Comprehensive Cancer Infrastructures within and across all EU Member States to increase quality of research and care | EU citizens or cancer patients should have access to accredited Comprehensive Cancer Infrastructures (CCI) in their country (at least one CCI in each member state), albeit through a national access point to an accredited CCI in another country, if relevant. |
| 11. Childhood cancers and cancers in adolescents and young adults: cure more and cure better | Across Europe, cancer is the leading cause of death in children over one year of age. This population of cancer patients is characterized by several types of rare cancers, unique to them, with specific epidemiological, biological, and clinical features. |
| 12. Accelerate innovation and implementation of new technologies and create oncology-focused living labs to conquer cancer | The goal is to provide new ways for traditional and nontraditional innovators to contribute to cancer understanding, prevention, diagnosis and treatment, and quality life support. |
| 13. Transform cancer culture, communication, and capacity building | It is proposed to develop a coherent set of cross-sectoral activities to enable citizens, providers (including nurses, primary and other clinical doctors), researchers, other stakeholders (e.g., policy makers, health insurers, employers and trade unions), and communities within all Members States to think about cancer and challenge the culture of cancer in all its dimensions. |
| Measure ID | Pillar ID | Measure Name | Measure Description |
|---|---|---|---|
| 1 | 1 | Gut Microbiome and Dietary Impact | The last decade has brought us a greater understanding of the impact of our ‘diet’ on intestinal ‘microbiota’ (gut bacteria), and how changes in the ‘microbiota’ are associated with our health (cancer promotion and prevention). |
| 2 | 1 | Metabolic Health and Physical Activity Influence | Studies have shown that lifestyle behaviors may impact metabolism and cancer risk. |
| 3 | 1 | Prolonged Inflammatory Responses | Studies have shown that inflammation that becomes chronic or lasts for too long is often associated with the development and progression of cancer. |
| 4 | 1 | Environmental Carcinogenic Factors | Studies have shown that some environmental factors, called also carcinogens, increase the risk of developing cancer. |
| 5 | 2 | Cancer Risk Reduction Strategies | By the use of chemo treatments, vaccines such as the human papillomavirus (HPV) vaccine (the immune system), and preventive drugs for certain cancer types. |
| 6 | 2 | Genetic and Epigenetic Cancer Influences | Studies have shown that cancers develop due to the accumulation of genetic (changes in the DNA sequence, some of which may be inherited) and epigenetic (changes not affecting the DNA sequence but its activity, that are noninherited) alterations. |
| 7 | 2 | Pre-Tumor Progression Phases | The development of cancer is a multistep process in which normal cells gradually become malignant through the progressive accumulation of molecular alterations. |
| 8 | 2 | Initial Cancer Development Phases | Cancer is a disease caused when cells divide uncontrollably and cooperate with other cells in their local environment, while fostering tumor progression. |
| 9 | 2 | Hematological Biomarkers for Early Detection | Specific blood tests are designed to identify tumor (bio)markers that may be found in the blood when some cancers are present before showing symptoms or being detected through conventional imaging approaches. |
| 10 | 2 | Advanced Early Cancer Diagnostic Technologies | Numerous cancer-associated deaths occur from cancers for which we do not screen. To overcome this, new scalable and cost-effective technologies are developed to allow for the detection and diagnosis of cancers at an earlier stage when these are more responsive to treatments. |
| 11 | 2 | Tailored Cancer Risk Management and Early Screening | Everybody does not have the same risk of developing a cancer. Careful analysis of individual risk factors to adapt prevention and systematic screening to the risk level would increase the rate of early diagnosis. |
| 12 | 2 | Hematological Assays for Treatment Responsiveness and Resistance | In the past two decades, specific tests have been developed to customize the treatment plan for a cancer patient according to the sensitivity and resistance patterns that can be monitored by analyzing the patient’s blood. |
| 13 | 3 | Cancer Cell Biology and Immune Microenvironment | Studies have shown that not all cancer cells are created equal, and they have the capacity to remodel the cells around them. There are intrinsic differences in the proliferative and invasive capacity of cancer cells within the same patient. Immune cells in their environment also acquire specific properties. |
| 14 | 3 | Innovative Anti-Cancer Therapies and Drug Delivery Methods | The development of more specific anticancer drugs, new types of biological and immune-mediated therapies, novel combinations of therapies with diverse mechanisms of action, and advanced drug delivery systems to target cancer cells more specifically have the potential to improve cancer treatment for patients and reduce long-term effects. |
| 15 | 3 | Hereditary Factors and Epigenetic Mechanisms in Pediatric Oncology | The contribution of nongenetic factors and the influence of the tissue environment remain poorly understood. |
| 16 | 3 | Oncogenesis and Growth Phases | The causes of the molecular changes during development that lead to cancer in children are mostly unknown. |
| 17 | 3 | Therapeutic Approaches for Pediatric Cancers | What is effective for an adult with cancer might not work for a pediatric cancer patient. Therefore, specific strategies to treat pediatric and adolescent cancer patients are needed. |
| 18 | 3 | Immunological Aspects in Pediatric Cancer | The immune system of children and adolescents is different from that of an adult. The efficiency of immunotherapy might vary depending on the age of the patient, and this needs to be better understood. |
| 19 | 3 | Maternal Factors and Pediatric Cancer Association | Epidemiological studies have suggested an association between maternal risk factors or exposure to carcinogens during pregnancy and pediatric cancer incidence. However, the precise factors and mechanisms involved remain unexplored. |
| 20 | 4 | Aging Factors and Cancer Susceptibility | The incidence of most cancers increases with age as, for most adults, age is associated with chronic conditions, decreased efficacy of the immune system, cumulative exposure to risk factors (carcinogens), and tissue aging with cell senescence. These events are causally associated with cancer. |
| 21 | 4 | Cellular Senescence in Cancer Biology | Aging is a complex phenomenon caused by the time-dependent loss of physiological organism functions, including those that protect from cancer development. |
| 22 | 4 | Aging and Carcinogenesis Relationship | Studies have shown that mechanisms of ageing are also found to occur in carcinogenesis. There is a need to better understand what aging and cancer development have in common and where the two processes diverge. |
| 23 | 4 | Aging Impact on Cancer Treatments | Various studies support the hypothesis that cancer and/or cancer treatment is associated with accelerated biological aging. These factors are key determinants of survivorship along with the long-term impact of cancer therapy on the biological aging of an individual. |
| 24 | 5 | Personal Adverse Events and Concurrent Medical Conditions in Cancer | In older patients affected by cancer, it is key to consider not only the characteristics of the tumor but to also pursue an integral geriatric assessment to systematically investigate factors that determine the patients’ well-being. In this context, research suggests that we may be able to measure a biological age, which will be more precise than civil age to guide therapeutic choices when treating cancer. |
| 25 | 5 | Treatment-Related Secondary Neoplasms | Although it happens infrequently, patients may develop a secondary cancer as a result of the treatment received to treat the primary cancer. |
| 26 | 5 | Persistent Immunological Consequences of Treatment | The effects of some cancer treatments can compromise properties of the immune system, rendering patients vulnerable to viral and bacterial infections or causing autoimmune conditions. |
| 27 | 5 | Reproductive Health Impact due to Cancer and Treatment | Cancer and its treatment can adversely impact reproductive function in both women and men. The effects of cancer treatment may lead to transient or permanent loss of fertility, sexual desire, and sexual function. |
| 28 | 5 | Cardiovascular, Respiratory, and Hormonal Health Impact due to Treatment | Both chemotherapy and radiation therapy to the chest can cause problems in the heart and lungs leading to potential cardiovascular and respiratory conditions that may be temporary or long-lasting. |
| 29 | 5 | Neurological Consequences of Cancer Treatments | Chemotherapy and radiation therapy can cause long-term side effects on the brain, spinal cord, and nerves, sometimes enhancing pain sensitivity. |
| 30 | 5 | Holistic Care for Cancer Survivors | For cancer survivors who are no longer in active treatment, their care needs include surveillance for recurrence, screening for the development of subsequent primary cancers, monitoring and intervention for the long-term and late physical and psychological effects of cancer and its treatment, and management of comorbid medical conditions, as well as routine preventive and primary care. |
| 31 | 6 | Data Generation in Oncological Research | The development of data that may guide more precise therapeutic choices and generate more efficacy in treating cancer patients. |
| 32 | 6 | Data Utilization for Informed Oncological Decision-making | Data whose analysis can inform precise disease diagnosis, their heterogeneity, the existence of constitutive predisposing factors, and the ability of the patient to support and favorably respond to a given therapy. |
| 33 | 6 | Data Collection and Analysis in Oncology | With the tools of data sciences, researchers can collect and analyze data to identify common mechanisms in a large series of patients with similar diseases. With data sciences, the higher the number of patients analyzed, the more precise the analysis. |
| 34 | 6 | Data Quality Assurance in Oncological Studies | The efficacy of data sciences requires data standardization and interoperability to be reused by multiple teams asking complementary questions. |
| 35 | 6 | Regulated Sharing of Patient Data for Oncology Research | Patient data sharing requires strict regulation to protect privacy (anonymization). While such regulation is mandatory, it must also be organized in a manner that favors rather than prevents patient data sharing at the European level to support cancer research. |
| BE | BG | FR | DE | GR | HU | IT | LU | NL | PT | RO | SK | ES | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BE | 1.00 | ||||||||||||
| BG | 0.58 *,# | 1.00 | |||||||||||
| FR | 0.78 | 0.62 | 1.00 | ||||||||||
| DE | 0.73 | 0.56 | 0.86 | 1.00 | |||||||||
| GR | 0.59 | 0.57 | 0.74 | 0.62 | 1.00 | ||||||||
| HU | 0.67 | 0.72 | 0.66 | 0.69 | 0.73 | 1.00 | |||||||
| IT | 0.84 | 0.67 | 0.79 | 0.73 | 0.79 | 0.80 | 1.00 | ||||||
| LU | 0.75 | 0.48 | 0.85 | 0.77 | 0.62 | 0.50 | 0.77 | 1.00 | |||||
| NL | 0.69 | 0.63 | 0.81 | 0.69 | 0.53 | 0.65 | 0.67 | 0.64 | 1.00 | ||||
| PT | 0.78 | 0.70 | 0.80 | 0.79 | 0.72 | 0.83 | 0.91 | 0.70 | 0.73 | 1.00 | |||
| RO | 0.60 | 0.63 | 0.54 | 0.50 | 0.57 | 0.73 | 0.68 | 0.46 | 0.53 | 0.62 | 1.00 | ||
| SK | 0.80 | 0.55 | 0.82 | 0.81 | 0.67 | 0.62 | 0.79 | 0.79 | 0.69 | 0.71 | 0.64 | 1.00 | |
| ES | 0.79 | 0.47 | 0.75 | 0.75 | 0.66 | 0.75 | 0.88 | 0.75 | 0.65 | 0.85 | 0.68 | 0.76 | 1.00 |
| Meas. * | FR ** | # Rank | % *** | Meas. | DE ** | Rank | % | Meas. | IT ** | Rank | % | Meas. | ES ** | Rank | % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10 | 68.4 | 1 | 100.0 | 35 | 72.6 | 1 | 100.0 | 10 | 62.4 | 1 | 100.0 | 10 | 78.2 | 1 | 100.0 |
| 14 | 64.7 | 2 | 94.1 | 13 | 69.5 | 2 | 94.1 | 9 | 56.5 | 2 | 97.0 | 17 | 77.3 | 2 | 97.0 |
| 17 | 64.7 | 2 | 94.1 | 14 | 69.5 | 2 | 94.1 | 17 | 55.0 | 3 | 94.1 | 9 | 73.9 | 3 | 94.1 |
| 9 | 64.0 | 4 | 91.1 | 9 | 68.4 | 4 | 88.2 | 11 | 53.0 | 4 | 91.1 | 16 | 67.2 | 4 | 91.1 |
| 31 | 61.0 | 5 | 85.2 | 10 | 68.4 | 4 | 88.2 | 18 | 52.1 | 5 | 88.2 | 8 | 66.4 | 5 | 88.2 |
| 3 | 26.5 | 31 | 8.8 | 19 | 36.8 | 31 | 11.7 | 23 | 32.5 | 31 | 11.7 | 22 | 39.5 | 31 | 11.7 |
| 22 | 26.5 | 31 | 8.8 | 1 | 33.7 | 32 | 8.8 | 1 | 30.5 | 32 | 8.8 | 1 | 38.7 | 32 | 8.8 |
| 1 | 25.0 | 33 | 5.8 | 27 | 32.6 | 33 | 5.8 | 20 | 29.6 | 33 | 5.8 | 2 | 38.7 | 32 | 5.8 |
| 20 | 23.5 | 34 | 2.9 | 20 | 31.6 | 34 | 2.9 | 22 | 28.4 | 34 | 2.9 | 27 | 37.8 | 34 | 2.9 |
| 21 | 21.3 | 35 | 0.0 | 21 | 30.5 | 35 | 0.0 | 21 | 27.8 | 35 | 0.0 | 23 | 37.0 | 35 | 0.0 |
| Meas * | BE ** | # Rank | % *** | Meas. | BG ** | Rank | % | Meas. | GR ** | Rank | % | Meas. | HU ** | Rank | % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 17 | 71.2 | 1 | 100.0 | 30 | 67.9 | 1 | 100.0 | 10 | 74.3 | 1 | 100.0 | 10 | 58.9 | 1 | 100.0 |
| 9 | 66.1 | 2 | 97.0 | 28 | 62.5 | 2 | 97.0 | 11 | 71.4 | 2 | 97.0 | 17 | 54.6 | 2 | 97.0 |
| 4 | 61.0 | 3 | 94.1 | 10 | 60.7 | 3 | 79.4 | 4 | 68.6 | 3 | 91.1 | 30 | 52.5 | 3 | 94.1 |
| 14 | 61.0 | 3 | 91.1 | 14 | 60.7 | 3 | 79.4 | 17 | 65.7 | 4 | 91.1 | 9 | 51.8 | 4 | 88.2 |
| 18 | 61.0 | 3 | 88.2 | 25 | 60.7 | 3 | 79.4 | 9 | 62.9 | 5 | 88.2 | 7 | 50.4 | 5 | 88.2 |
| 23 | 22.0 | 31 | 11.7 | 6 | 30.4 | 31 | 11.7 | 25 | 40.0 | 29 | 5.8 | 27 | 33.3 | 31 | 8.8 |
| 24 | 22.0 | 31 | 8.8 | 2 | 28.6 | 32 | 2.9 | 2 | 37.1 | 32 | 5.8 | 23 | 32.6 | 32 | 8.8 |
| 20 | 20.3 | 33 | 5.8 | 22 | 26.8 | 33 | 2.9 | 22 | 37.1 | 32 | 5.8 | 6 | 31.2 | 33 | 2.9 |
| 22 | 20.3 | 33 | 2.9 | 21 | 25.0 | 34 | 2.9 | 3 | 34.3 | 34 | 2.9 | 2 | 30.5 | 34 | 2.9 |
| 21 | 16.9 | 35 | 0.0 | 23 | 23.2 | 35 | 0.0 | 1 | 31.4 | 35 | 0.0 | 1 | 25.5 | 35 | 0.0 |
| Meas. * | LU ** | # Rank | % *** | Meas. | NL ** | Rank | % | Meas. | PT ** | Rank | % | Meas. | RO ** | Rank | % | Meas. | SK ** | Rank | % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10 | 76.3 | 1 | 100.0 | 30 | 60.7 | 1 | 100.0 | 10 | 81.4 | 1 | 100.0 | 10 | 69.7 | 1 | 100.0 | 10 | 73.0 | 1 | 100.0 |
| 34 | 76.3 | 1 | 97.0 | 14 | 59.0 | 2 | 97.0 | 17 | 80.4 | 2 | 97.0 | 11 | 69.7 | 1 | 97.0 | 4 | 71.4 | 2 | 97.0 |
| 4 | 68.4 | 3 | 91.1 | 10 | 57.4 | 3 | 94.1 | 18 | 76.5 | 3 | 94.1 | 30 | 66.7 | 3 | 91.1 | 14 | 68.3 | 3 | 94.1 |
| 9 | 68.4 | 3 | 91.1 | 12 | 57.4 | 3 | 91.1 | 11 | 73.5 | 4 | 91.1 | 31 | 66.7 | 3 | 91.1 | 9 | 65.1 | 4 | 91.1 |
| 11 | 68.4 | 3 | 79.4 | 29 | 52.5 | 5 | 88.2 | 14 | 73.5 | 4 | 88.2 | 9 | 63.6 | 5 | 79.4 | 11 | 61.9 | 5 | 88.2 |
| 27 | 31.6 | 31 | 8.8 | 22 | 27.9 | 28 | 11.7 | 2 | 44.1 | 31 | 11.7 | 22 | 48.5 | 28 | 8.8 | 27 | 31.7 | 29 | 11.7 |
| 21 | 28.9 | 32 | 8.8 | 23 | 27.9 | 28 | 5.8 | 3 | 44.1 | 31 | 5.8 | 27 | 48.5 | 28 | 8.8 | 21 | 30.2 | 32 | 5.8 |
| 22 | 28.9 | 32 | 2.9 | 27 | 27.9 | 28 | 5.8 | 20 | 43.1 | 33 | 5.8 | 2 | 45.5 | 33 | 2.9 | 24 | 30.2 | 32 | 5.8 |
| 23 | 28.9 | 32 | 2.9 | 20 | 26.2 | 34 | 2.9 | 21 | 43.1 | 33 | 2.9 | 35 | 45.5 | 33 | 2.9 | 22 | 25.4 | 34 | 2.9 |
| 20 | 23.7 | 35 | 0.0 | 24 | 21.3 | 35 | 0.0 | 1 | 41.2 | 35 | 0.0 | 23 | 42.4 | 35 | 0.0 | 23 | 17.5 | 35 | 0.0 |
| PC | BC | OGC | LC | CC | |
|---|---|---|---|---|---|
| PC | 1 | ||||
| BC | 0.80 *,# | 1 | |||
| OGC | 0.70 | 0.77 | 1 | ||
| LC | 0.69 | 0.87 | 0.82 | 1 | |
| CC | 0.79 | 0.79 | 0.64 | 0.75 | 1 |
| Meas * | PC ** | # Rank | % *** | Meas | BC | Rank | % | Meas | OGC | Rank | % | Meas | LC | Rank | % | Meas | CC | Rank | % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 9 | 64.2 | 1 | 1.00 | 10 | 62.3 | 1 | 1.00 | 9 | 67.7 | 1 | 1.00 | 10 | 78.7 | 1 | 1.00 | 32 | 70.0 | 1 | 1.00 |
| 10 | 64.2 | 1 | 0.97 | 14 | 57.1 | 2 | 0.94 | 10 | 64.5 | 2 | 0.97 | 14 | 76.0 | 2 | 0.97 | 4 | 66.0 | 2 | 0.97 |
| 35 | 62.3 | 3 | 0.94 | 30 | 57.1 | 2 | 0.94 | 4 | 58.1 | 3 | 0.94 | 13 | 73.3 | 3 | 0.94 | 10 | 64.0 | 3 | 0.85 |
| 16 | 60.4 | 4 | 0.88 | 17 | 56.1 | 4 | 0.91 | 14 | 54.8 | 4 | 0.91 | 11 | 69.3 | 4 | 0.91 | 14 | 64.0 | 3 | 0.85 |
| 17 | 60.4 | 4 | 0.88 | 12 | 55.5 | 5 | 0.88 | 12 | 51.6 | 5 | 0.88 | 32 | 68.0 | 5 | 0.88 | 31 | 64.0 | 3 | 0.85 |
| 20 | 30.2 | 31 | 0.12 | 23 | 33.7 | 31 | 0.12 | 29 | 32.3 | 27 | 0.12 | 20 | 33.3 | 31 | 0.09 | 1 | 34.0 | 31 | 0.09 |
| 23 | 28.3 | 32 | 0.06 | 1 | 32.5 | 32 | 0.06 | 21 | 29.0 | 32 | 0.09 | 22 | 33.3 | 31 | 0.09 | 2 | 34.0 | 31 | 0.09 |
| 24 | 28.3 | 32 | 0.06 | 20 | 32.5 | 32 | 0.06 | 27 | 25.8 | 33 | 0.06 | 2 | 32.0 | 33 | 0.06 | 20 | 32.0 | 33 | 0.03 |
| 3 | 26.4 | 34 | 0.03 | 21 | 32.2 | 34 | 0.03 | 1 | 22.6 | 34 | 0.00 | 27 | 30.7 | 34 | 0.03 | 22 | 32.0 | 33 | 0.03 |
| 21 | 24.5 | 35 | 0.00 | 22 | 31.3 | 35 | 0.00 | 23 | 22.6 | 34 | 0.00 | 1 | 22.7 | 35 | 0.00 | 21 | 30.0 | 35 | 0.00 |
| Pillars | Availability | Timing | Reimbursement | Order Rate |
|---|---|---|---|---|
| Factors Influencing Cancer Development and Risk | −0.21 *,# | −0.52 | 0.026 | −0.06 |
| Cancer Prevention and Early Detection | 0.21 | −0.10 | 0.11 | 0.18 |
| Cancer Biology and Therapeutic Approaches | 0.34 | 0.04 | 0.27 | 0.32 |
| Aging and its Intersections with Cancer | 0.03 | −0.13 | −0.22 | −0.01 |
| Cancer Complications and Survivorship | −0.04 | −0.26 | −0.36 | −0.12 |
| Data Generation and Utilization in Cancer Research | 0.18 | −0.15 | 0.56 | 0.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horgan, D.; Bulcke, M.V.d.; Malapelle, U.; Normanno, N.; Capoluongo, E.D.; Prelaj, A.; Rizzari, C.; Stathopoulou, A.; Singh, J.; Kozaric, M.; et al. Aligning Cancer Research Priorities in Europe with Recommendations for Conquering Cancer: A Comprehensive Analysis. Healthcare 2024, 12, 259. https://doi.org/10.3390/healthcare12020259
Horgan D, Bulcke MVd, Malapelle U, Normanno N, Capoluongo ED, Prelaj A, Rizzari C, Stathopoulou A, Singh J, Kozaric M, et al. Aligning Cancer Research Priorities in Europe with Recommendations for Conquering Cancer: A Comprehensive Analysis. Healthcare. 2024; 12(2):259. https://doi.org/10.3390/healthcare12020259
Chicago/Turabian StyleHorgan, Denis, Marc Van den Bulcke, Umberto Malapelle, Nicola Normanno, Ettore D. Capoluongo, Arsela Prelaj, Carmelo Rizzari, Aliki Stathopoulou, Jaya Singh, Marta Kozaric, and et al. 2024. "Aligning Cancer Research Priorities in Europe with Recommendations for Conquering Cancer: A Comprehensive Analysis" Healthcare 12, no. 2: 259. https://doi.org/10.3390/healthcare12020259
APA StyleHorgan, D., Bulcke, M. V. d., Malapelle, U., Normanno, N., Capoluongo, E. D., Prelaj, A., Rizzari, C., Stathopoulou, A., Singh, J., Kozaric, M., Dube, F., Ottaviano, M., Boccia, S., Pravettoni, G., Cattaneo, I., Malats, N., Buettner, R., Lekadir, K., de Lorenzo, F., ... De Maria, R. (2024). Aligning Cancer Research Priorities in Europe with Recommendations for Conquering Cancer: A Comprehensive Analysis. Healthcare, 12(2), 259. https://doi.org/10.3390/healthcare12020259













