Hospital–Provider Company Network for Home Non-Invasive Ventilation: A Feasibility Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
- Inclusion criteria encompassed consecutive patients diagnosed with chronic respiratory failure (CRF) proposed for the NIV program between 1 June 2022 and 30 August 2023, exhibiting persistent PaCO2 values > 46 mmHg within one month post exacerbation/hospitalization. Additional criteria included at least one hospitalization in the prior year and/or a minimum of two severe exacerbations.
- Exclusion criteria comprised individuals already on NIV, those with significant comorbidities such as congestive heart failure and neurological diseases, pure apnea sleep syndrome, and those unwilling or unable to provide informed consent.
2.3. Intervention Programs
- Telemonitoring using AirView™ for Ventilation (RESMED, ResMed Inc., San Diego, CA, USA): A cloud-based system facilitating efficient management of patients with respiratory insufficiency. It enables prompt access to patient data, allows the sharing of clinical insights among healthcare professionals, and permits remote adjustments of therapy when necessary. The platform wirelessly connects with ventilators, providing dynamic visualization of therapy data for individual programs.
- Customizable Care Plans: Tailored plans addressing individual patient needs.
- Home Visits: Personalized visits to the patient’s residence.
- Digital Communication: Utilization of messaging and video calls.
2.4. Hospital Phase
2.5. Home Phase
- Airview online platform at least three times a week to monitor patients’ progress.
- Routine calls (every 15 days). Additional calls were conducted in cases of technical requirements or poor adherence.
- Two mandatory home visits (one in the first week after returning home and one at the end of the 3 months) and extra visits based on patients’ needs (ranging from 1 to 4 visits).
- Changes to the NIV settings after feedback from the chest physician.
- Extra instrumental examinations such as arterial blood gases (ABG) and night pulsed oxygen (O2) saturation carried out at home by PC nurses and technicians. Final report was filled in by the doctor affiliated with the PC.
- Regular feedback to the hospital pulmonologist and PC technicians sharing all relevant data.
2.6. Measurements
- Sample characteristics collection;
- Recruitment capability (number of patients included/patients who fulfilled inclusion criteria);
- Acceptability (number of drop-outs, severe side effects within 3 months, hospitalizations, and survival since NIV initiation, and patient satisfaction);
- Evaluation of resources and suitability of procedures (time and dedication by each professional and the mean personnel cost/patient).
- Only at baseline (T0):
- At T0, after 1 week (T1) and after 3 months (T3):
- At T0 and T3:
- At T1 and T3:
- Only at T3:
3. Results
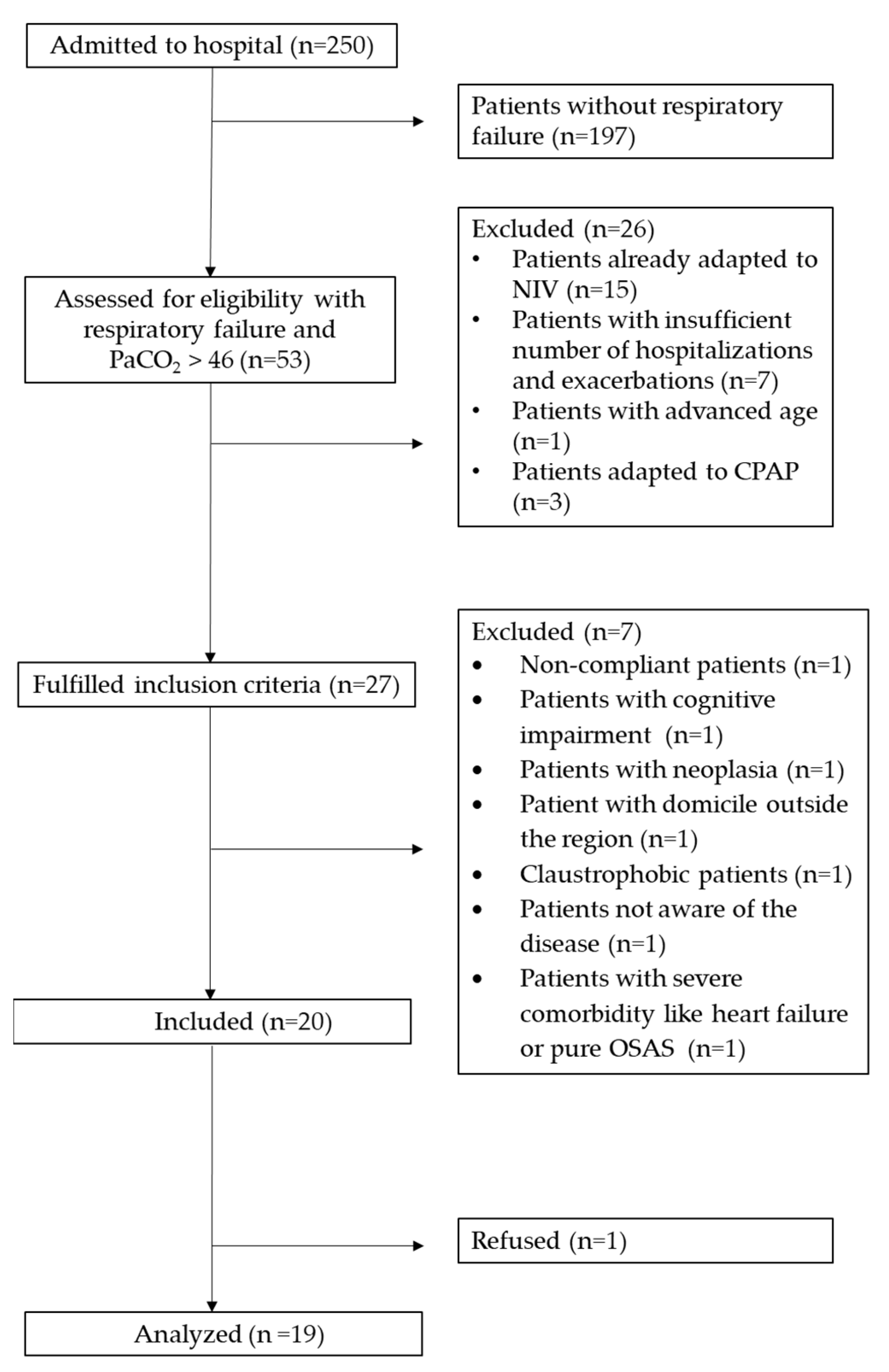
3.1. Recruitment Capability
3.2. Sample Characteristics
3.3. Procedures
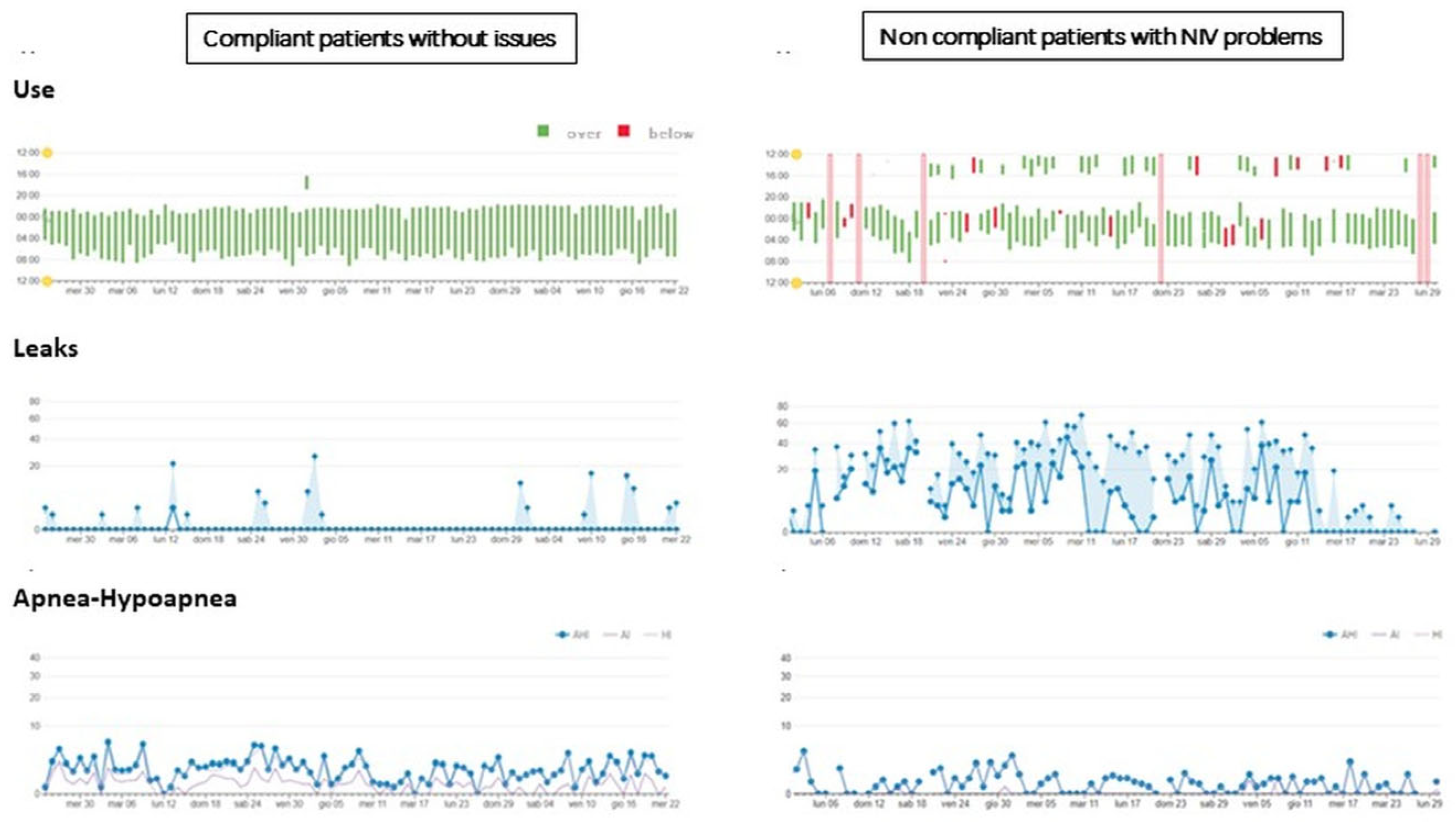
3.4. Acceptability
3.5. Suitability of Procedures
3.6. Resource to Manage
Secondary Outcomes
4. Discussion
4.1. Sample Characteristics
4.2. Recruitment Capability
4.3. Procedures
4.4. Acceptability
4.5. Evaluation of Resources
4.6. Outcome Measures
4.7. Limitations
4.8. Clinical Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ergan, B.; Oczkowski, S.; Rochwerg, B.; Carlucci, A.; Chatwin, M.; Clini, E.; Elliott, M.; Gonzalez-Bermejo, J.; Hart, N.; Lujan, M.; et al. European Respiratory Society guidelines on long-term home non-invasive ventilation for management of COPD. Eur. Respir. J. 2019, 54, 1901003. [Google Scholar] [CrossRef] [PubMed]
- Dretzke, J.; Wang, J.; Yao, M.; Guan, N.; Ling, M.; Zhang, E.; Mukherjee, D.; Hall, J.; Jowett, S.; Mukherjee, R.; et al. Home Non-Invasive Ventilation in COPD: A Global Systematic Review. Chronic Obstr. Pulm. Dis. 2022, 9, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Kampelmacher, M.J. Moving from Inpatient to Outpatient or Home Initiation of Non-Invasive Home Mechanical Ventilation. J. Clin. Med. 2023, 12, 2981. [Google Scholar] [CrossRef] [PubMed]
- Majorski, D.S.; Duiverman, M.L.; Windisch, W.; Schwarz, S.B. Long-term Non-Invasive ventilation in COPD: Current evidence and future directions. Expert. Rev. Respir. Med. 2021, 15, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Ackrivo, J.; Elman, L.; Hansen-Flaschen, J. Telemonitoring for Home-assisted Ventilation: A Narrative Review. Ann. Am. Thorac. Soc. 2021, 18, 1761–1772. [Google Scholar] [CrossRef]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; Van Der Grinten, C.P.M.; ATS/ERS Task Force; et al. Standardization of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef] [PubMed]
- Epstein, L.J.; Kristo, D.; Strollo, P.J., Jr.; Friedman, N.; Malhotra, A.; Patil, S.P.; Adult Obstructive Sleep Apnea Task Force of the American Academy of Sleep Medicine. Clinical guidelines for the evaluation, management, and long-term care of obstructive sleep apnea in adults. J. Clin. Sleep. Med. 2009, 5, 263–276. [Google Scholar]
- Mahler, D.A.; Wells, C.K. Evaluation of clinical methods for rating dyspnea. Chest 1988, 93, 580–586. [Google Scholar] [CrossRef]
- Vitacca, M.; Paneroni, M.; Baiardi, P.; De Carolis, V.; Zampogna, E.; Belli, S.; Carone, M.; Spanevello, A.; Balbi, B.; Bertolotti, G.G. Development of a Barthel Index based on dyspnea for patients with respiratory diseases. Int. J. Chron. Obs. Pulmon Dis. 2016, 11, 1199–1206. [Google Scholar] [CrossRef]
- Mahoney, F.I.; Barthel, D. Functional evaluation: The Barthel Index. Md. State Med. J. 1965, 14, 56–61. [Google Scholar]
- Bernabeu-Mora, R.; Medina-Mirapeix, F.; Llamazares-Herrán, E.; García-Guillamón, G.; Giménez-Giménez, L.M.; Sánchez-Nieto, J.M. The Short Physical Performance Battery is a discriminative tool for identifying patients with COPD at risk of disability. Int. J. Chron. Obs. Pulmon Dis. 2015, 10, 2619–2626, Erratum in: Int. J. Chron. Obs. Pulmon Dis. 2016, 11, 623. [Google Scholar] [CrossRef][Green Version]
- Logan, S.L.; Gottlieb, B.H.; Maitland, S.B.; Meegan, D.; Spriet, L.L. The Physical Activity Scale for the Elderly (PASE) questionnaire; does it predict physical health? Int. J. Environ. Res. Public. Health 2013, 10, 3967–3986. [Google Scholar] [CrossRef] [PubMed]
- Holland, A.E.; Spruit, M.A.; Troosters, T.; Puhan, M.A.; Pepin, V.; Saey, D.; McCormack, M.C.; Carlin, B.W.; Sciurba, F.C.; Pitta, F.; et al. An official European Respiratory Society/American Thoracic Society technical standard: Field walking tests in chronic respiratory disease. Eur. Respir. J. 2016, 44, 1428–1446. [Google Scholar] [CrossRef] [PubMed]
- Ware, J., Jr.; Kosinski, M.; Keller, S.D. A 12-Item Short-Form Health Survey: Construction of scales and preliminary tests of reliability and validity. Med. Care 1996, 34, 220–233. [Google Scholar] [CrossRef]
- Pépin, J.L.; Tamisier, R.; Hwang, D.; Mereddy, S.; Parthasarathy, S. Does remote monitoring change OSA management and CPAP adherence? Respirology 2017, 22, 1508–1517. [Google Scholar] [CrossRef] [PubMed]
- Windisch, W.; Geiseler, J.; Simon, K.; Walterspacher, S.; Dreher, M.; on behalf of the Guideline Commission. German National Guideline for Treating Chronic Respiratory Failure with Invasive and Non-Invasive Ventilation: Revised Edition 2017—Part 1. Respiration 2018, 96, 66–97. [Google Scholar] [CrossRef] [PubMed]
- Hill, N.S.; Criner, G.J.; Branson, R.D.; Celli, B.R.; MacIntyre, N.R.; Sergew, A.; ONMAP Technical Expert Panel. Optimal NIV Medicare Access Promotion: Patients with COPD: A Technical Expert Panel Report from the American College of Chest Physicians, the American Association for Respiratory Care, the American Academy of Sleep Medicine, and the American Thoracic Society. Chest 2021, 160, e389–e397. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, C.; Vieira, A.L.; Pamplona, P.; Drummond, M.; Seabra, B.; Ferreira, D.; Liberato, H.; Carreiro, A.; Vicente, I.; Castro, L.; et al. Current Practices in Home Mechanical Ventilation for Chronic Obstructive Pulmonary Disease: A Real-Life Cross-Sectional Multicentric Study. Int. J. Chron. Obs. Pulmon Dis. 2021, 16, 2217–2226. [Google Scholar] [CrossRef]
- Bertella, E.; Banfi, P.; Paneroni, M.; Grilli, S.; Bianchi, L.; Volpato, E.; Vitacca, M. Early initiation of night-time NIV in an outpatient setting: A randomized non-inferiority study in ALS patients. Eur. J. Phys. Rehabil. Med. 2017, 53, 892–899. [Google Scholar] [CrossRef]
- Orr, J.E.; Chen, K.; Vaida, F.; Schmickl, C.N.; Laverty, C.G.; Ravits, J.; Lesser, D.; Bhattacharjee, R.; Malhotra, A.; Owens, R.L. Effectiveness of long-term Non-Invasive ventilation measured by remote monitoring in neuromuscular disease. ERJ Open Res. 2023, 9, 00163–02023. [Google Scholar] [CrossRef]
- Van Den Biggelaar, R.; Hazenberg, A.; Duiverman, M.L. The role of telemonitoring in patients on home mechanical ventilation. Eur. Respir. Rev. 2023, 32, 220207. [Google Scholar] [CrossRef] [PubMed]
- Hazenberg, A.; Kerstjens, H.A.; Prins, S.C.; Vermeulen, K.M.; Wijkstra, P.J. Initiation of home mechanical ventilation at home: A randomized controlled trial of efficacy, feasibility, and costs. Respir. Med. 2014, 108, 1387–1395. [Google Scholar] [CrossRef] [PubMed]
- Duiverman, M.L.; Vonk, J.M.; Bladder, G.; van Melle, J.P.; Nieuwenhuis, J.; Hazenberg, A.; Kerstjens, H.A.M.; van Boven, J.F.M.; Wijkstra, P.J. Home initiation of chronic non-invasive ventilation in COPD patients with chronic hypercapnic respiratory failure: A randomized controlled trial. Thorax 2020, 75, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Leger, P.; Laier-Groeneveld, G. Infrastructure, funding and follow-up in a program of Non-Invasive ventilation. Eur. Respir. J. 2002, 20, 1573–1578. [Google Scholar] [CrossRef] [PubMed]
- Crimi, C.; Noto, A.; Princi, P.; Cuvelier, A.; Masa, J.F.; Simonds, A.; Elliott, M.W.; Wijkstra, P.; Windisch, W.; Nava, S. Domiciliary Non-invasive Ventilation in COPD: An International Survey of Indications and Practices. COPD 2016, 13, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Stuart, M.; Weinrich, M. Integrated health system for chronic disease management: Lessons learned from France. Chest 2004, 125, 695–703. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Guber, A.; Morris, E.; Chen, B.; Israeli, S. First experience with the home-care management system for respiratory patients in Israel. Isr. Med. Assoc. J. 2002, 4, 418–420. [Google Scholar]


| Patients, number | 19 |
| Age, years | 71.0 (67.0; 76.0) |
| Gender, number (%) | |
| Male | 8 (42.1) |
| Female | 11 (57.9) |
| Diagnosis, number (%) | |
| COPD with emphysematous phenotype | 8 (42.1) |
| Bronchiectasis | 3 (15.8) |
| Overlap Syndrome | 5 (26.3) |
| Restricted dysventilation + OSAS | 3 (15.8) |
| BMI, score | 20.5 (18.0; 25.0) |
| Hb, g/dL | 12.2 (8.6; 14.5) |
| CIRS S.I., score | 1.8 (1.6; 2.4) |
| CIRS C.I., score | 3.0 (2.0; 6.5) |
| FEV1, % prd | 30.0 (19.0; 38.5) |
| FVC, % prd | 52.0 (43.5; 66.5) |
| FEV1/FVC, score | 35.6 (31.9; 63.3) |
| RV, % prd (n = 16) | 195.0 (153.0; 247.0) |
| Patients hospitalized in the previous year, number (%) | 12 (63.2) |
| Patients relapsed in the previous year, number (%) | 19 (100) |
| Number of relapses in the previous year, number | 2.0 (1.0; 2.0) |
| History of ICU admission, number (%) | 4 (21.0) |
| Recent relapse within 3 months, number (%) | 6 (31.5) |
| Drug therapy, number (%) | |
| LABA + ICS | 1 (5.3) |
| LABA + LAMA | 2 (10.5) |
| TRIPLE | 15 (78.9) |
| SABA + ICS | 1 (5.30) |
| Oxygen, L/min | 1.0 (1.0; 2.0) |
| PaCO2, mmHg | 55.3 (51.0; 61.9) |
| pH, score | 7.4 (7.4; 7.4) |
| PaO2/FiO2 | 239.0 (210.0; 269.0) |
| HCO3−, mEq/L | 31.9 (29.2; 34.2) |
| AHI/h, number (n = 16) | 2.2 (0.7; 6.2) |
| ODI/h, number | 4.1 (1.2; 6.7) |
| Mean SatO2, % | 94.0 (87.8; 96.5) |
| T90, % | 2.4 (0.0; 83,1) |
| Barthel dyspnea, score | 31.0 (16.5; 40.0) |
| MRC, score | 4.0 (3.0; 4.0) |
| Barthel index, score | 100.0 (91.5; 100.0) |
| SPPB, score | 8.0 (6.5; 10.0) |
| MCS-12, score | 47.7 (42.2; 57.1) |
| PCS-12, score | 30.2 (26.3; 35.6) |
| PASE, score | 136.0 (55.0; 157.9) |
| 6MWT, meters | 212.5 (165.0; 288.7) |
| Steps/day, number | 1356.0 (634.7; 2873.5) |
| Time of inactivity, % | 79.5 (69.0; 87.2) |
| Initial NIV setting IPAP, mmHg | 16.0 (13.0; 17.5) |
| Initial NIV setting EPAP, mmHg | 7.0 (5.0; 8.0) |
| Initial NIV setting Respiratory rate, a/m | 12.0 (12.0; 12.0) |
| ∆T3–T0 (CI 95%) | p-Value (Wilcoxon Test) | |
|---|---|---|
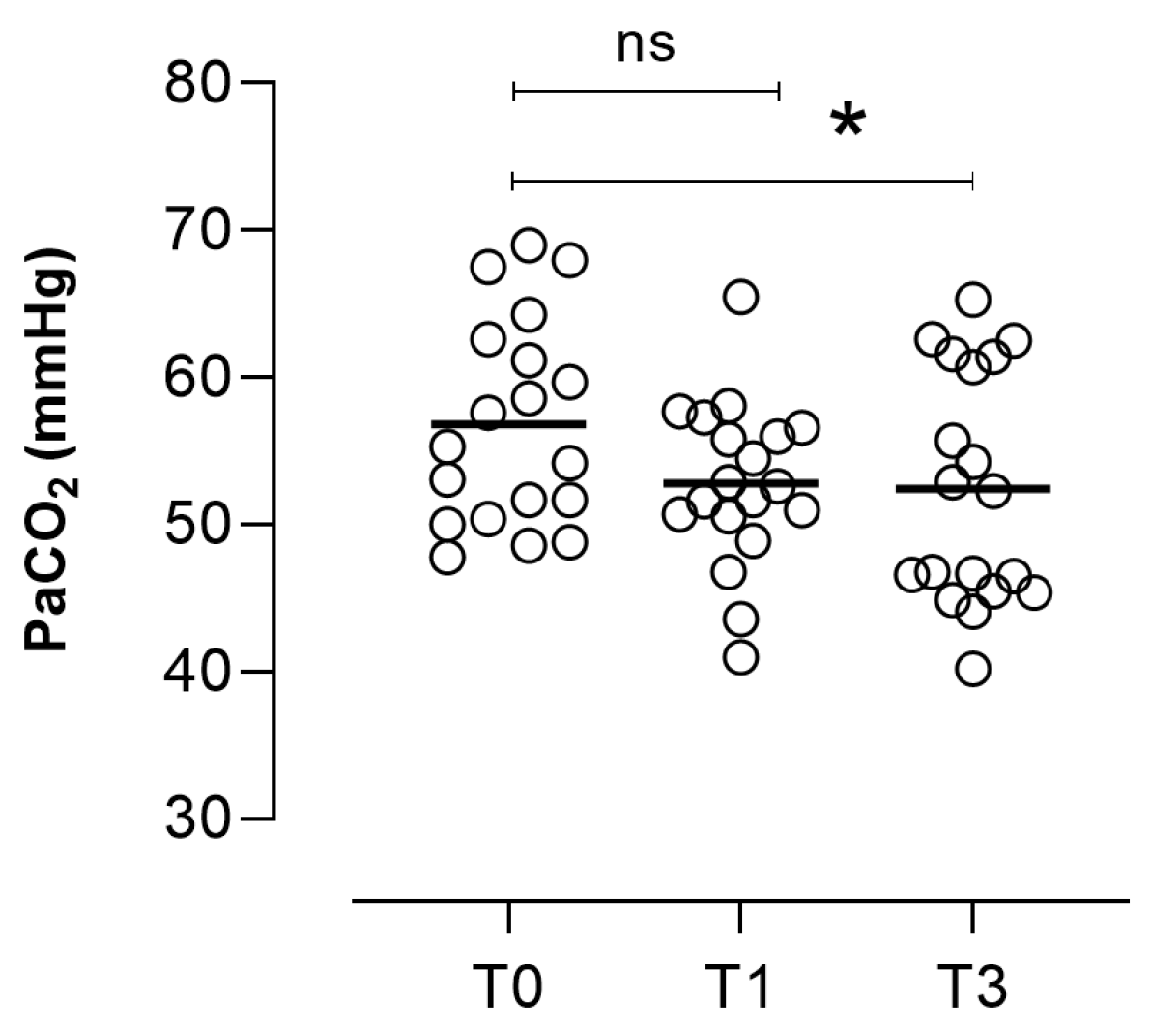
| PaCO2, mmHg (n = 18) | −4.5 (−8.1; −0.7) | 0.0235 |
| PaO2/FiO2 (n = 18) | 21.0 (−34.0; 59.5) | 0.2145 |
| HCO3−, mEq/L (n = 18) | 1.1(−3.3; 4.2) | 0.6012 |
| AHI/h, number (n = 14) | −0.6 (−3.6; 0.3) | 0.1317 |
| Average SatO2, % (n = 11) | 2.3 (0.7; 8.3) | 0.0553 |
| T90, % | −1.2 (−89.1; 0.9) | 0.0893 |
| Barthel dyspnea, score | 0 (−12.0; 11.0) | 0.8245 |
| MRC, score | −1.0 (−1–0; 0.0) | 0.0017 |
| Barthel index, score | 0.0 (0.0; 1.0) | 0.4037 |
| SPPB, score | 2.0 (0.0; 2.5) | 0.0185 |
| MCS-12, score | 3.2 (−5.8; 9.2) | 0.3144 |
| PCS-12, score | −1.2 (−3.2; 2.8) | 0.8092 |
| PASE, score | −5.0 (−24.4; 27.9) | 0.9679 |
| 6MWT, meters (n = 17) | 70.0 (30.0; 110.0) | 0.0021 |
| Steps/day, number (n = 17) | −168.0 (−415.0; 44.0) | 0.1024 |
| Time of inactivity, % (n = 17) | 0.0 (−4.0; 3.0) | 0.9621 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitacca, M.; Asti, G.; Fiorenza, D.; Steinhilber, G.; Salvi, B.; Paneroni, M. Hospital–Provider Company Network for Home Non-Invasive Ventilation: A Feasibility Pilot Study. Healthcare 2024, 12, 328. https://doi.org/10.3390/healthcare12030328
Vitacca M, Asti G, Fiorenza D, Steinhilber G, Salvi B, Paneroni M. Hospital–Provider Company Network for Home Non-Invasive Ventilation: A Feasibility Pilot Study. Healthcare. 2024; 12(3):328. https://doi.org/10.3390/healthcare12030328
Chicago/Turabian StyleVitacca, Michele, Giada Asti, Domenico Fiorenza, Gundi Steinhilber, Beatrice Salvi, and Mara Paneroni. 2024. "Hospital–Provider Company Network for Home Non-Invasive Ventilation: A Feasibility Pilot Study" Healthcare 12, no. 3: 328. https://doi.org/10.3390/healthcare12030328
APA StyleVitacca, M., Asti, G., Fiorenza, D., Steinhilber, G., Salvi, B., & Paneroni, M. (2024). Hospital–Provider Company Network for Home Non-Invasive Ventilation: A Feasibility Pilot Study. Healthcare, 12(3), 328. https://doi.org/10.3390/healthcare12030328






