Assessment of the Clinical and Functional Health Status of Patients with Amyotrophic Lateral Sclerosis during the COVID-19 Pandemic in Brazil Using Telemedicine
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Type and Participants
2.2. Patient and Public Involvement
2.3. Study Design
2.4. Pulmonary Function and Respiratory Muscle Strength
2.5. Functionality and Rate of Disease Progression
2.6. Disease Staging
2.7. Clinical Evaluation and Use of NIV
2.8. Adjustment and Prescription of NIV Support
2.9. Statistical Analysis
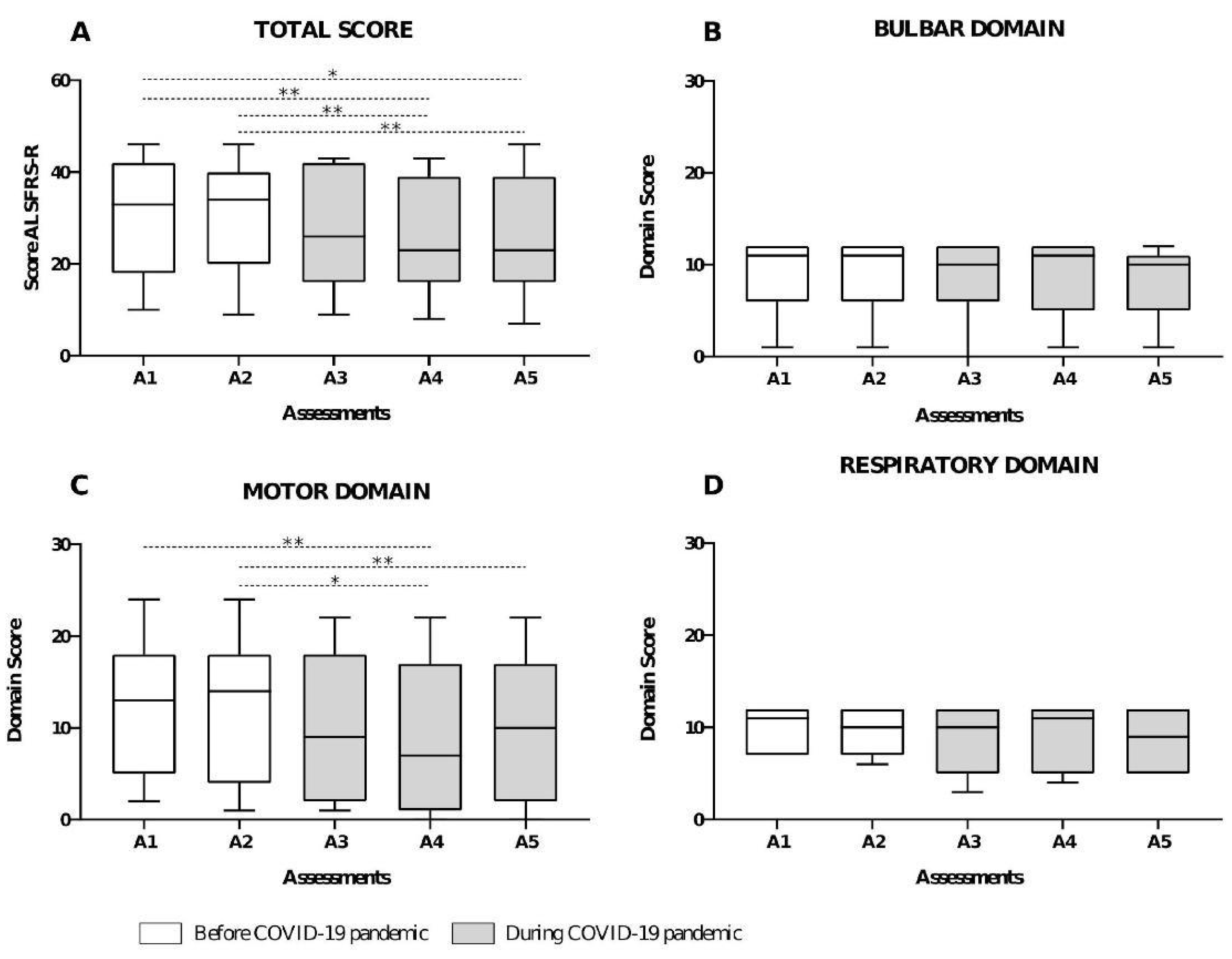
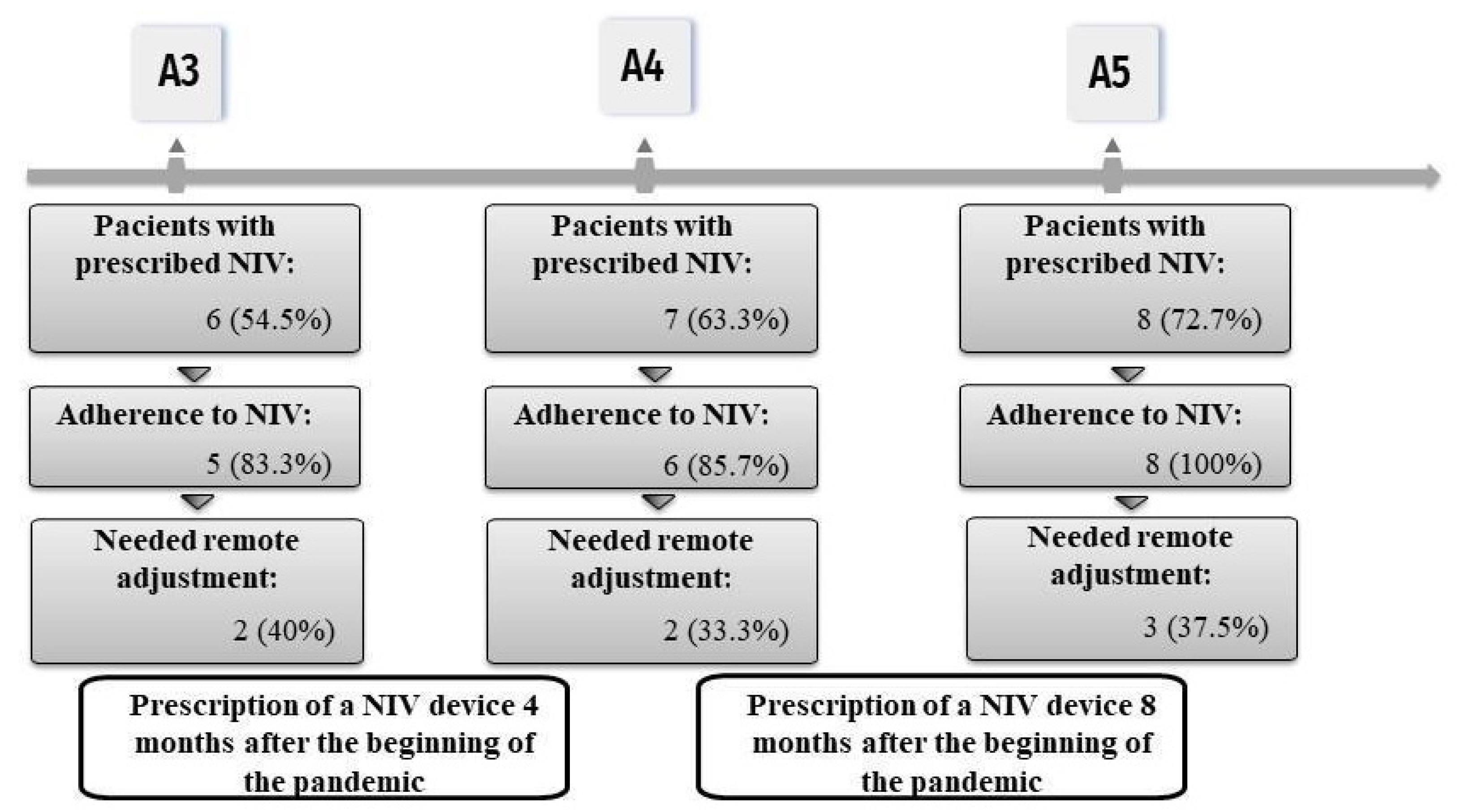
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van Es, M.A.; Hardiman, O.; Chio, A.; Al-Chalabi, A.; Pasterkamp, R.J.; Veldink, J.H.; van den Berg, L.H. Amyotrophic lateral sclerosis. Lancet 2017, 390, 2084–2098. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.P.R.; Júnior, J.B.S.; dos Santos, E.L.; de Carvalho, F.O.; Costa, I.M.P.d.F.; de Mendonça, D.M.F. Quality of life and functional independence in amyotrophic lateral sclerosis: A systematic review. Neurosci. Biobehav. Rev. 2020, 111, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Benditt, J.O.; Boitano, L.J. Pulmonary issues in patients with chronic neuromuscular disease. Am. J. Respir. Crit. Care Med. 2013, 187, 1046–1055. [Google Scholar] [CrossRef]
- Braun, A.T.; Caballero-Eraso, C.; Lechtzin, N. Amyotrophic Lateral Sclerosis and the Respiratory System. Clin. Chest Med. 2018, 39, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Suárez, A.A.; Pessolano, F.A.; Monteiro, S.G.; Ferreyra, G.; Capria, M.E.; Mesa, L.; Dubrovsky, A.; De Vito, E.L. Peak flow and peak cough flow in the evaluation of expiratory muscle weakness and bulbar impairment in patients with neuromuscular disease. Am. J. Phys. Med. Rehabil. 2002, 81, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Chiò, A.; Logroscino, G.; Traynor, B.; Collins, J.; Simeone, J.; Goldstein, L.; White, L. Global epidemiology of amyotrophic lateral sclerosis: A systematic review of the published literature. Neuroepidemiology 2013, 41, 118–130. [Google Scholar] [CrossRef]
- Štětkářová, I.; Ehler, E. Diagnostics of Amyotrophic Lateral Sclerosis: Up to Date. Diagnostics 2021, 11, 231. [Google Scholar] [CrossRef] [PubMed]
- Boentert, M. Sleep and Sleep Disruption in Amyotrophic Lateral Sclerosis. Curr. Neurol. Neurosci. Rep. 2020, 20, 25. [Google Scholar] [CrossRef]
- Morelot-Panzini, C.; Bruneteau, G.; Gonzalez-Bermejo, J. NIV in amyotrophic lateral sclerosis: The ‘when’ and ‘how’ of the matter. Respirology 2019, 24, 521–530. [Google Scholar] [CrossRef]
- Howard, I.M.; Kaufman, M.S. Telehealth Applications for Outpatients With. Muscle Nerve 2018, 58, 475–485. [Google Scholar] [CrossRef]
- Bombaci, A.; Abbadessa, G.; Trojsi, F.; Leocani, L.; Bonavita, S.; Lavorgna, L.; Tedeschi, G.; Mancardi, G.; Padovani, A.; Clerico, M.; et al. Telemedicine for management of patients with amyotrophic lateral sclerosis through COVID-19 tail. Neurol. Sci. 2021, 42, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, F.; Magalhães, B. Effects of prolonged interruption of rehabilitation routines in amyotrophic lateral sclerosis patients. Palliat. Support. Care 2021, 20, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Brooks, B.R.; Miller, R.G.; Swash, M.; Munsat, T.L. El Escorial revisited: Revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. 2000, 1, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Guedes, K.; Pereira, C.; Pavan, K.; Valério, B.C.O. Cross-cultural adaptation and validation of ALS Functional Rating Scale-Revised in Portuguese language. Arq. Neuropsiquiatr. 2010, 68, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Roche, J.C.; Rojas-Garcia, R.; Scott, K.M.; Scotton, W.; Ellis, C.E.; Burman, R.; Wijesekera, L.; Turner, M.R.; Leigh, P.N.; Shaw, C.E.; et al. A proposed staging system for amyotrophic lateral sclerosis. Brain 2012, 135, 847–852. [Google Scholar] [CrossRef] [PubMed]
- BRASIL. Ministério da Saúde. Programa Nacional Telessaúde Brasil Redes. Bibl Virtual em Saúde Published Online First. 2021. Available online: https://aps.bvs.br/programa-nacional-telessaude-brasil-redes/ (accessed on 13 May 2021).
- BRASIL. Ministério da Saúde. Gabinete do Ministro. Portaria nº 467, de 20 de Março de 2020. Brasília. 2020. Available online: https://www.planalto.gov.br/ccivil_03/portaria/prt/portaria%20n%C2%BA%20467-20-ms.htm (accessed on 13 May 2021).
- American Thoracic Society/European Respiratory Society ATS/ERS Statement on Respiratory Muscle Testing. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, March 2001 and by the ERS executive committee, in June 2001. Am. J. Respir. Crit. Care Med. 2002, 166, 518–624. [Google Scholar] [CrossRef]
- García-Río, F.; Calle, M.; Burgos, F.; Casan, P.; del Campo, F.; Galdiz, J.B.; Giner, J.; González-Mangado, N.; Ortega, F.; Maestu, L.P. Espirometria. Arch. Bronconeumol. 2013, 49, 388–401. [Google Scholar] [CrossRef] [PubMed]
- Araújo, P.R.S.; Resqueti, V.R.; Nascimento, J., Jr.; Carvalho, L.D.A.; Cavalcanti, A.G.L.; Silva, V.C.; Silva, E.; Moreno, M.A.; Andrade, A.D.F.D.D.; Fregonezi, G.A.D.F. Reference values for sniff nasal inspiratory pressure in healthy subjects in Brazil: A multicenter study. J. Bras. Pneumol. 2012, 38, 700–707. [Google Scholar] [CrossRef]
- Neder, J.; Andreoni, S.; Lerario, M.; Nery, L. Reference values for lung function tests: II. Maximal respiratory pressures and voluntary ventilation. Braz. J. Med. Biol. Res. 1999, 32, 719–727. [Google Scholar] [CrossRef]
- Maier, A.; Holm, T.; Wicks, P.; Steinfurth, L.; Linke, P.; Münch, C.; Meyer, R.; Meyer, T. Online assessment of ALS functional rating scale compares well to in-clinic evaluation: A prospective trial. Amyotroph. Lateral Scler. 2012, 13, 210. [Google Scholar] [CrossRef]
- Balendra, R.; Jones, A.; Jivraj, N.; Knights, C.; Ellis, C.M.; Burman, R.; Turner, M.R.; Leigh, P.N.; Shaw, C.E.; Al-Chalabi, A. Estimating clinical stage of amyotrophic lateral sclerosis from the ALS Functional Rating Scale. Amyotroph. Lateral Scler. Front. Degener. 2014, 15, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Masrori, P.; Van Damme, P. Amyotrophic lateral sclerosis: A clinical review. Eur. J. Neurol. 2020, 27, 1918–1929. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wei, Q.-Q.; Chen, Y.; Cao, B.; Ou, R.; Hou, Y.; Yuan, X.; Zhang, L.; Liu, H.; Shang, H. Clinical staging of amyotrophic lateral sclerosis in Chinese patients. Front. Neurol. 2018, 9, 442. [Google Scholar] [CrossRef] [PubMed]
- Al Khleifat, A.; Balendra, R.; Fang, T.; Al-Chalabi, A. Intuitive Staging Correlates with King’s Clinical Stage. Amyotroph. Lateral Scler. Front. Degener. 2021, 22, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Manera, U.; Cabras, S.; Daviddi, M.; Vasta, R.; Torrieri, M.C.; Palumbo, F.; Bombaci, A.; Grassano, M.; Solero, L.; Peotta, L.; et al. Validation of the Italian version of self-administered ALSFRS-R scale. Amyotroph. Lateral Scler. Front. Degener. 2021, 22, 151–153. [Google Scholar] [CrossRef] [PubMed]
- van Eijk, R.P.A.; Bakers, J.N.E.; Bunte, T.M.; de Fockert, A.J.; Eijkemans, M.J.C.; Berg, L.H.v.D. Accelerometry for remote monitoring of physical activity in amyotrophic lateral sclerosis: A longitudinal cohort study. J. Neurol. 2019, 266, 2387–2395. [Google Scholar] [CrossRef]
- Calzada, N.G.; Soro, E.P.; Gomez, L.M.; Bulta, E.G.; Izquierdo, A.C.; Panades, M.P.; Sargatal, J.D.; Muñoz, E.F. Factors predicting survival in amyotrophic lateral sclerosis patients on non-invasive ventilation. Amyotroph. Lateral Scler. Front. Degener. 2016, 17, 337–342. [Google Scholar] [CrossRef]
- De Marchi, F.; Sarnelli, M.F.; Serioli, M.; De Marchi, I.; Zani, E.; Bottone, N.; Ambrosini, S.; Garone, R.; Cantello, R.; Mazzini, L.; et al. Telehealth approach for amyotrophic lateral sclerosis patients: The experience during COVID-19 pandemic. Acta Neurol. Scand. 2020, 143, 489–496. [Google Scholar] [CrossRef]


| Variables | All Patients (n = 11) | Spinal ALS (n = 8) | Bulbar ALS (n = 3) |
|---|---|---|---|
| Sex | |||
| Male | 8 (72.7%) | 6 (75%) | 2 (66.6%) |
| Female | 3 (27.7%) | 2 (25%) | 1 (33.3%) |
| Age [years] | 51 (43–55) | 47.5 (41.5–51.7) | 64 (55–73) |
| El Escorial | |||
| Possible | - | - | - |
| Probable | 3 (27%) | 2 (25%) | 1 (50%) |
| Confirmed | 8 (72.7%) | 6 (75%) | 1 (50%) |
| Origin | |||
| State capital | 3 (27.7%) | 2 (22.2%) | 1 (50%) |
| Inland cities | 8 (72.2%) | 7 (77.7%) | 1 (50%) |
| FVC [L] | 3.1 (2.1–3.9) | 3.4 (2.7–4.4) | 2.3 (1.8–2.6) |
| FVC [%Pred] | 69.6 (56.5–96.7) | 69.6 (60–97.8) | 65.9 (41.1–90.8) |
| FEV1 [L] | 2.3 (1.6–3.2) | 2.8 (2–3.5) | 1.6 (1.6–2.2) |
| FEV1 [%Pred] | 74 (55.8–89.2) | 81.2 (63.9–92.2) | 65.4 (44.1–80.2) |
| FEF25–75% [L/s] | 2.7 (1.5–3.5) | 3.4 (1.5–3.6) | 1.7 (1.3–2.7) |
| FEF25–75% [%Pred] | 68.4 (56.8–90.9) | 77.4 (58.6–96.2) | 68.4 (49.5–84) |
| PEF [L/s] | 6.0 (1.7–6.6) | 6.1 (3.5–6.7) | 1.9 (1.6–6.4) |
| MIP [cmH2O] | 67 (27.7–86.5) | 54.5 (24.7–99.2) | 69 (30.4–82) |
| MIP [%Pred] | 60 (28.9–81.7) | 46.4 (28.1–84.3) | 67.2 (27.8–92.3) |
| MEP [cmH2O] | 59 (35.5–81.5) | 60.5 (40.2–98.7) | 59 (25–85) |
| MEP [%Pred] | 59 (42.2–70.4) | 62.2 (44.8–85.1) | 52.4 (19.9–69.9) |
| SNIP [cmH2O] | 72 (44–79.5) | 74 (39.2–87.2) | 68 (46–72) |
| SNIP [%Pred] | 64.5 (42.6–85.9) | 65.3 (41.7–92.8) | 61.9 (40.8–85.8) |
| Variables | A3 | A4 | A5 |
|---|---|---|---|
| Dyspnea | 5 (45.4%) | 5 (45.4%) | 4 (36.3%) |
| Fatigue | 5 (45.4%) | 8 (72.7%) | 6 (54.4%) |
| Fever | 2 (25%) | 1 (9.0%) | - |
| Sore throat | - | 1 (9.0%) | 2 (18.1%) |
| Runny nose | 2 (25%) | 3 (27.2%) | 2 (18.1%) |
| Nasal Obstruction | 4 (50%) | 6 (54.4%) | 5 (45.%) |
| Cough | |||
| Effective | 6 (54.5%) | 5 (45.4%) | 5 (45.4%) |
| Ineffective | 5 (45.4%) | 6 (54.5%) | 6 (54.5%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brito, O.; Fregonezi, G.; Pondofe, K.; Vieira, R.G.d.S.; Ribeiro, T.; Dourado Júnior, M.E.; Fidelix, E.C.; Nagem, D.; Valentim, R.; Sarmento, A.; et al. Assessment of the Clinical and Functional Health Status of Patients with Amyotrophic Lateral Sclerosis during the COVID-19 Pandemic in Brazil Using Telemedicine. Healthcare 2024, 12, 627. https://doi.org/10.3390/healthcare12060627
Brito O, Fregonezi G, Pondofe K, Vieira RGdS, Ribeiro T, Dourado Júnior ME, Fidelix EC, Nagem D, Valentim R, Sarmento A, et al. Assessment of the Clinical and Functional Health Status of Patients with Amyotrophic Lateral Sclerosis during the COVID-19 Pandemic in Brazil Using Telemedicine. Healthcare. 2024; 12(6):627. https://doi.org/10.3390/healthcare12060627
Chicago/Turabian StyleBrito, Ozana, Guilherme Fregonezi, Karen Pondofe, Rayane Grayce da Silva Vieira, Tatiana Ribeiro, Mário Emílio Dourado Júnior, Emanuela Coriolano Fidelix, Danilo Nagem, Ricardo Valentim, Antonio Sarmento, and et al. 2024. "Assessment of the Clinical and Functional Health Status of Patients with Amyotrophic Lateral Sclerosis during the COVID-19 Pandemic in Brazil Using Telemedicine" Healthcare 12, no. 6: 627. https://doi.org/10.3390/healthcare12060627
APA StyleBrito, O., Fregonezi, G., Pondofe, K., Vieira, R. G. d. S., Ribeiro, T., Dourado Júnior, M. E., Fidelix, E. C., Nagem, D., Valentim, R., Sarmento, A., & Resqueti, V. (2024). Assessment of the Clinical and Functional Health Status of Patients with Amyotrophic Lateral Sclerosis during the COVID-19 Pandemic in Brazil Using Telemedicine. Healthcare, 12(6), 627. https://doi.org/10.3390/healthcare12060627








